Abstract
Free full text

Seasonal Effects of UCP1 Gene Polymorphism on Visceral Fat Accumulation in Japanese Adults
Associated Data
Abstract
Uncoupling protein 1 (UCP1) and β3 adrenergic receptor (ADRB3) genes play central roles in the thermogenesis of brown adipose tissue (BAT) in adult humans. However, the importance of single-nucleotide polymorphisms (SNPs) in both genes during the development of obesity is controversial. Although active BAT in adult humans is frequently observed in the winter season, the effects of sampling season have not been taken into consideration in previous association studies. Here, we tested the associations of UCP1 -3826A/G and ADRB3 Trp64Arg with body mass index (BMI) and visceral fat area (VFA) in 3013 Japanese adults sampled during different seasons. Association between SNPs and the obesity-related traits were assessed using multiple linear regression models, including sex, age, physical activity, and genotypes. Both SNPs did not show significant associations in the models based on the entire cohort. However, in subsets comprising individuals mainly sampled from winter to spring, UCP1 showed significant associations with VFA (P =
= 0.0098) and VFA adjusted for BMI (P
0.0098) and VFA adjusted for BMI (P =
= 0.0128). Moreover, the effects of UCP1 on VFA were strongly negatively correlated with outdoor temperature (P
0.0128). Moreover, the effects of UCP1 on VFA were strongly negatively correlated with outdoor temperature (P =
= 0.00011), but not with night length (P
0.00011), but not with night length (P =
= 0.039). ADRB3 did not show these associations, but an additive effect with UCP1 was observed for VFA adjusted for BMI (P
0.039). ADRB3 did not show these associations, but an additive effect with UCP1 was observed for VFA adjusted for BMI (P =
= 0.0067). Subsets sampled in the hot season did not show significant associations for both SNPs. The season-specific effects of UCP1 on VFA were consistent with a previous finding that active BAT was more frequently found in winter than in summer, and supported the importance of cold stress in BAT activation and the significance of BAT in the development of obesity in adult humans.
0.0067). Subsets sampled in the hot season did not show significant associations for both SNPs. The season-specific effects of UCP1 on VFA were consistent with a previous finding that active BAT was more frequently found in winter than in summer, and supported the importance of cold stress in BAT activation and the significance of BAT in the development of obesity in adult humans.
Introduction
Brown adipose tissue (BAT) plays an important role in non-shivering thermogenesis in mammals and has received much attention as a potential target in the treatment of obesity. The presence of active BAT in neonatal humans is well documented, but active BAT was believed to be absent or to merely contribute to energy and thermoregulation in adult humans. Recent studies have provided evidence for the presence of active BAT in adult humans under cold-stress conditions [1]–[4]. The activity of BAT in adult humans is reduced with age, and inactive BAT is thought to be linked to reduced energy expenditure and age-induced obesity [5].
Uncoupling protein 1 (UCP1) and β3 adrenergic receptor (ADRB3) are key molecules involved in the thermogenesis of BAT. UCP1 is a mitochondrial membrane protein that mediates heat generation by enhancing proton conductivity of the inner lumen. The expression of UCP1 in BAT is regulated by the sympathetic nervous system and by ADRB3. Genes encoding UCP1 (UCP1) and ADRB3 (ADRB3) have been studied to elucidate genetic variations accounting for the susceptibility to obesity. Most association studies have focused on a regulatory single-nucleotide polymorphism (SNP) of UCP1 (-3826A/G) and/or a coding SNP in ADRB3 (Trp64Arg). Significant associations with various obesity-related traits, primarily body mass index (BMI), have been reported, but the results in several studies were controversial [6], [7]. Yoneshiro et al. recently revealed that these 2 SNPs are associated with age-related decreases in BAT activity in Japanese adults [8]; however, the roles of variations in UCP1 and ADRB3 on the development of obesity are still unclear.
The detection rate of BAT is influenced by season in addition to sex, age, and obesity status of individuals; BAT is more frequently detected in examinations conducted in the winter than those conducted in the summer, even in the same individual [4]. Nevertheless, previous association studies of UCP1 and ADRB3 did not take the effect of sampling season into consideration. Moreover, BMI may not be an optimal indicator of obesity since BMI is influenced by the weight of organs other than adipose tissues. Visceral fat has high lipogenic/lipolytic activities and can act as an energy reservoir, responding to relatively short-term energy balance. In fact, short-duration, moderate-intensity exercises could effectively suppress the accumulation of visceral fat, but not subcutaneous fat, in adult humans [9]. Therefore, visceral fat area, rather than BMI, was thought to be the superior phenotype for testing the associations of UCP1/ADRB3 and obesity.
In the present study, we tested associations of these 2 SNPs with visceral fat accumulation in Japanese adults sampled during various seasons.
Materials and Methods
The design of the present study was approved by the Ethics Committee of Jichi Medical University. The recruited individuals provided written informed consent. A total of 3013 Japanese individuals who attended general health checkups at the Jichi Medical University Hospital from January 2009 to March 2011 were included. Visceral fat area (VFA) was measured using the bioelectrical impedance analysis (BIA) method [10]. Consistency between the results obtained with the BIA and conventional computed tomography methods has been confirmed [11], [12]. The demographics and characteristics of the studied population are summarized in Table 1. Other details about the studied population were described in our previous paper [11].
Table 1
| Entire Cohort | January–April | July–October | ||
| Numbers of individuals | 3013 | 1080 | 979 | |
| Percentage of males1 | 53.8 | 52.1 | 53.6 | P>0.05 |
| Age2 | 51.7±0.2 | 51.7±0.3 | 52.0±0.3 | P>0.05 |
| Body mass index (kg/m2)2 | 23.5±0.1 | 23.5±0.1 | 23.4±0.1 | P>0.05 |
| Waist circumstance (cm)2 | 85.0±0.2 | 84.9±0.3 | 84.7±0.3 | P>0.05 |
| Visceral fat area (cm2)2 | 90.5±0.8 | 89.4±1.3 | 89.6±1.4 | P>0.05 |
Genotyping of UCP1 -3826A/G (rs1800592) and ADRB3 Trp64Arg (rs4994) was performed using the TaqMan method. The Hardy-Weinberg equilibrium of the genotype was tested using the chi-square goodness of fit test. These SNPs were tested for associations with BMI, VFA, and VFA adjusted for BMI using multiple linear regression models. One hundred two individuals who had undergone the abdominal surgery were excluded from the analyses. Genotypes of each SNP were coded as 0, 1, or 2 according to numbers of the obesity-risk UCP1 G allele and ADRB3 Arg allele. Sex, age, and genotype were included as independent variables. To adjust for inter-individual variability of physical activity, speed of walking (slow =
= 0 and fast
0 and fast =
= 1), obtained by including this question in the health checkup questionnaire “Do you walk faster than other people of same sex and about same ages?”, was included as an independent variable. BMI was also included as an independent variable in models adjusted for BMI. Normalization of dependent variables was not applied since the distribution of BMI and VFA was not strongly skewed. Sex-specific multiple linear regression models included genotype, age, and speed of walking as independent variables. The significance level was set at 0.025, which corresponded to 0.05 in 2 independent tests. The summed number of risk alleles of 2 SNPs in each individual, which ranged from 0 to 4, was also used as an independent variable.
1), obtained by including this question in the health checkup questionnaire “Do you walk faster than other people of same sex and about same ages?”, was included as an independent variable. BMI was also included as an independent variable in models adjusted for BMI. Normalization of dependent variables was not applied since the distribution of BMI and VFA was not strongly skewed. Sex-specific multiple linear regression models included genotype, age, and speed of walking as independent variables. The significance level was set at 0.025, which corresponded to 0.05 in 2 independent tests. The summed number of risk alleles of 2 SNPs in each individual, which ranged from 0 to 4, was also used as an independent variable.
Correlations between the effects of UCP1 -3826A/G on VFA and environmental factors were assessed using Spearman's rank correlation method. β coefficients obtained in the multiple linear regression models were used as variables representing the effects of UCP1 -3826A/G on VFA. The β coefficients indicated the effect size of SNP on VFA (adjusted for age and sex) and larger positive β coefficients indicated carriers of the G allele that tended to accumulate more visceral fat. Monthly outdoor temperature and night length data measured at the nearest observatory were obtained from the Japan Meteorological Agency. The significance level of the correlation test was set to be P<0.05. The above-described statistical tests were performed using SPSS version 20 (IBM Corporation, NY).
Results
Results of association analyses are summarized in Table 2. β coefficients and P values for independent variables other than genotypes are shown in Table S1. Frequencies of the UCP1 G allele and ADRB3 Arg allele in our population were 0.51 and 0.19, respectively. Both SNPs did not show deviations from the Hardy-Weinberg equilibrium (P>0.1). In multiple linear regression models based on all genotyped individuals, no significant associations with BMI, VFA, or VFA adjusted for BMI were observed for both SNPs (P>0.05). To test the season-dependent effects of UCP1 and ADRB3, 2 subsets were drawn from the population (Tables 1 and and2).2). The first subset consisted of 1080 individuals sampled during the cold season (January through April), and the second subset consisted of 979 individuals sampled during the hot season (June through September). In the first subset, UCP1 showed significant associations with VFA (P =
= 0.0197), and the G allele exhibited an increased VFA (GG genotype showed a 3.39±1.45-cm2 increase in VFA compared to AA genotype). UCP1 was also associated with VFA adjusted for BMI (P
0.0197), and the G allele exhibited an increased VFA (GG genotype showed a 3.39±1.45-cm2 increase in VFA compared to AA genotype). UCP1 was also associated with VFA adjusted for BMI (P =
= 0.0246), but did not associate with BMI (P
0.0246), but did not associate with BMI (P =
= 0.203). In the second subset, however, UCP1 did not show association with either VFA or VFA adjusted for BMI (P
0.203). In the second subset, however, UCP1 did not show association with either VFA or VFA adjusted for BMI (P =
= 0.667 and P
0.667 and P =
= 0.954, respectively). ADRB3 did not associate with any trait in both subsets. To know whether the associations with VFA or VFA adjusted for BMI were sex specific in nature, we constructed multiple linear regression models separately for male and female populations. The association between UCP1 and VFA was significant for males in the entire cohort as well as in the cold season; however the association did not remain for females suggesting a sex-specific association (Table S2).
0.954, respectively). ADRB3 did not associate with any trait in both subsets. To know whether the associations with VFA or VFA adjusted for BMI were sex specific in nature, we constructed multiple linear regression models separately for male and female populations. The association between UCP1 and VFA was significant for males in the entire cohort as well as in the cold season; however the association did not remain for females suggesting a sex-specific association (Table S2).
Table 2
| BMI | VFA | a-VFA | ||||
| β(S.E.) | P | β(S.E.) | P | β(S.E.) | P | |
Entire cohort (F = = 1339, M 1339, M = = 1572) 1572) | ||||||
| UCP1 -3826 G (0.51) | 0.011(0.018) | 0.551 | 0.022(0.014) | 0.106 | 0.015(0.008) | 0.051 |
| ADRB3 64Arg (0.19) | −0.037(0.018) | 0.038 | −0.018(0.014) | 0.189 | 0.005(0.008) | 0.521 |
January–April (F = = 517, M 517, M = = 563) 563) | ||||||
| UCP1 -3826G (0.50) | 0.037(0.029) | 0.203 | 0.053(0.023) | 0.0197* | 0.029(0.013) | 0.0246* |
| ADRB3 64Arg (0.20) | −0.033(0.029) | 0.260 | −0.006(0.023) | 0.801 | 0.015(0.013) | 0.242 |
July–October (F = = 454, M 454, M = = 525) 525) | ||||||
| UCP1 -3826G (0.52) | 0.018 (0.031) | 0.667 | 0.010(0.024) | 0.667 | −0.001(0.014) | 0.954 |
| ADRB3 64Arg (0.19) | −0.066(0.031) | 0.032 | −0.045(0.023) | 0.057 | −0.004(0.014) | 0.763 |
To more precisely depict the season-dependent effect of UCP1 on the VFA, associations with VFA were tested in 12 subsets, which each consisted of individuals sampled during overlapping continuous 3-month periods (Figure 1). UCP1 was significantly associated with VFA in the subsets sampled from February to April and March to May (P =
= 0.0098 and 0.0128, respectively). Association in the subset January to March was marginal (P
0.0098 and 0.0128, respectively). Association in the subset January to March was marginal (P =
= 0.056). Moreover, significant associations with VFA adjusted for BMI were observed in subsets sampled from January to March and February to April (P
0.056). Moreover, significant associations with VFA adjusted for BMI were observed in subsets sampled from January to March and February to April (P =
= 0.0248 and 0.0116, respectively). Subjects sampled from winter to spring generally showed larger VFAs (and VFAs adjusted for BMIs) in comparison with subjects sampled from summer to autumn. Furthermore, the G allele of UCP1 showed an obvious copy number-dependent increase in VFA among individuals sampled from January to May (Figure 2). For ADRB3, the Arg allele tended to associate with a decreased VFA in several subsets, but the association did not survive the multiple testing correction (P>0.025). We also tested summed numbers of risk alleles of UCP1 and ADRB3 and observed strong associations with VFA adjusted for BMI in subsets sampled from January to May (P
0.0248 and 0.0116, respectively). Subjects sampled from winter to spring generally showed larger VFAs (and VFAs adjusted for BMIs) in comparison with subjects sampled from summer to autumn. Furthermore, the G allele of UCP1 showed an obvious copy number-dependent increase in VFA among individuals sampled from January to May (Figure 2). For ADRB3, the Arg allele tended to associate with a decreased VFA in several subsets, but the association did not survive the multiple testing correction (P>0.025). We also tested summed numbers of risk alleles of UCP1 and ADRB3 and observed strong associations with VFA adjusted for BMI in subsets sampled from January to May (P =
= 0.0067 ~ 0.010). ADRB3 and UCP1 did not show associations with BMI in all subsets (P>0.025).
0.0067 ~ 0.010). ADRB3 and UCP1 did not show associations with BMI in all subsets (P>0.025).
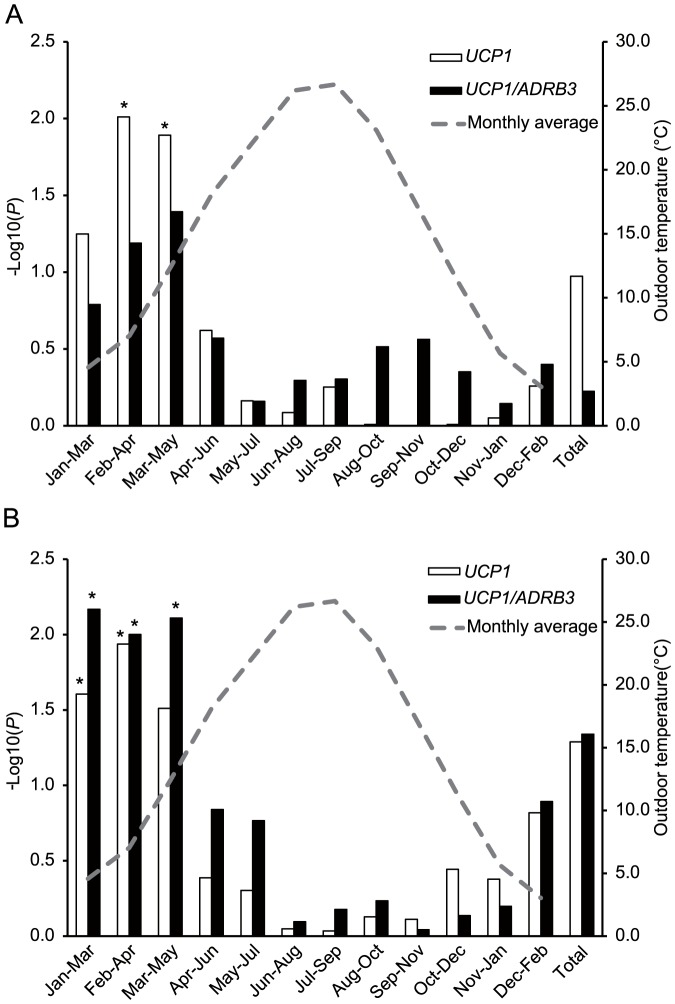
The –log of P-values for UCP1 and UCP1/ADRB3 in individuals sampled in windows of successive 3 months. Average outdoor temperatures in the second month of each 3-month window are shown with a gray broken line. *: P<0.05 after applying Bonferroni correction.
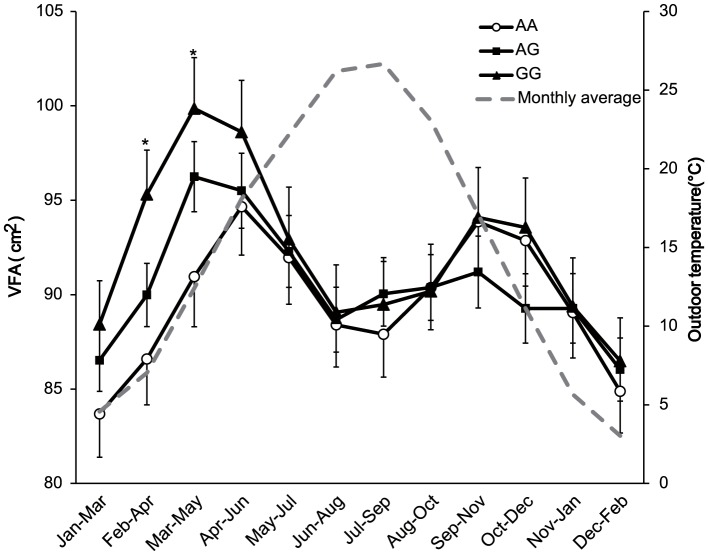
For each 3-month window, the means and standard errors of the VFA in the 3 genotype groups are shown. Average outdoor temperatures in the second month of each 3-month window are shown with a gray broken line. Asterisks indicate 3-month windows in which the UCP1 genotype showed significant associations with VFA.
Next, correlations between the effects of UCP1 polymorphisms and environmental factors, which possibly account for the seasonality of BAT activity, were tested (Table 3). A set of β coefficients for UCP1 -3826 G allele, obtained from the linear regression models based on the overlapping 12 subsets (described above), was used as a variable representing temporal difference in the effect of UCP1. The average outdoor temperature and average night length of the first month, second (midst) month, third month, and the previous month of each 3-month period (e.g., February, March, April, and January for February–April subset) were used as variables representing environmental factors. β coefficients for G alleles showed negative correlations with average outdoor temperatures of the first month in each subset (Spearman's rank correlation test, R =
= −0.629, P
−0.629, P =
= 0.028). Moreover, a stronger correlation was observed for β coefficients and the average outdoor temperature of the previous month (R
0.028). Moreover, a stronger correlation was observed for β coefficients and the average outdoor temperature of the previous month (R =
= −0.888, P
−0.888, P =
= 0.00011). β coefficients also showed correlation with the average night length of the third month (R
0.00011). β coefficients also showed correlation with the average night length of the third month (R =
= −0.643, P
−0.643, P =
= 0.024)
0.024)
Table 3
| R | P | |
| Average outdoor temperature | ||
| in previous months | −0.888 | 0.00011 |
| in the first month | −0.629 | 0.028 |
| in the second month | −0.203 | 0.527 |
| in the third month | 0.224 | 0.484 |
| Average night length | ||
| in previous months | 0.573 | 0.051 |
| in the first month | 0.196 | 0.542 |
| in the second month | −0.238 | 0.457 |
| in the third month | −0.643 | 0.024 |
Discussion
Non-shivering thermogenesis by BAT offers resting energy expenditure in adult humans, and therefore, heritable variation of BAT activity is expected to influence the risk for obesity. The UCP1 -3826 G allele was reported to be associated with reduced BAT activity [8], diminished resting energy expenditure, and reduced thermoregulatory sympathetic nervous system activity [13]. In the present study, we demonstrated the season-dependent effects of UCP1 -3826A/G on VFA in Japanese adults, in which individuals sampled in cold months but not in hot months showed significant associations. This observation was consistent with the fact that BAT in adult humans required cold exposure for activation and that active BAT was more frequently found in subjects tested in the winter than in the summer [4]. VFA generally tended to be larger in individuals sampled from winter to spring. This could be explained by the reduced physical exercise and/or increased food intake in cold seasons [14]. -3826 A/G is located in a transcriptional enhancer site of UCP1, and G allele was shown to reduce the transcriptional activity of UCP1 [15]. G allele was associated with the age-related decrease of BAT activity [8]. During the cold season, G allele carriers would be more vulnerable to visceral fat accumulation due to reduced non-shivering thermogenesis. During the hot season, non-shivering thermogenesis merely contributed to energy expenditure, and UCP -3826A/G no longer showed intergenotype differences in VFA. The season-dependent effects of UCP1-3826A/G on VFA were supported by the observation that the metabolic response to cold exposure was higher in the winter than in the summer [16]. Our findings also suggested that inconsistency among the previous association studies [6], [7] may be partly explained by the lack of attention to the sampling season.
Although there is no doubt about the crucial role of cold exposure in the activation of BAT, it was still unclear whether ambient temperature was the true factor influencing the seasonality of BAT activity. Au-Yong reported that the seasonality of BAT activity was more closely correlated with night length than with ambient temperature [17]. In the present study, the effects of UCP1 on VFA were negatively correlated with the average outdoor temperature rather than with night length (Table 3), supporting the predominant role of ambient temperature in activating the BAT. The strongest association was observed in the subsets sampled from February to April. This period did not completely overlap with the coldest months included in the study (Figure 1). Interestingly, the effects of UCP1 showed a stronger correlation with the average outdoor temperature in the previous month rather than that in sampling months (Table 3). Thus, VFA reduction by cold-induced BAT activation may be manifested after a delay of a few months.
UCP1 -3826A/G also showed associations with VFA adjusted for BMI. Moreover, ADRB3 Trp64Arg showed additive effects on the association of UCP1 and VFA adjusted for BMI. Adjustment for BMI had significant effects on the measurement of visceral fat relative to other organs. We recently identified Tribbles homolog 2 gene (TRIB2) as a locus associated with VFA adjusted for BMI in Japanese individuals [11]. Moreover, Fox et al. showed that an intergenic SNP near TRIB2 was strongly associated with pericardial fat mass [18]. VFA adjusted for BMI may be an estimation of the amount of other ectopic fat depots, which are also metabolically active and can exhibit adverse metabolic abnormalities [19]. The roles of UCP1 -3826A/G (and possibly ADRB3 Trp64Arg) on ectopic fat formation should be explored in future studies.
The present findings were obtained from a cross-sectional analysis of single cohort; therefore, a longitudinal analysis and/or a replication study are needed. Interestingly, we found possible sex-specific effects of UCP1 -3826A/G on VFA (Table S2). Imbalance of sample size does not appear to determine the association since the numbers of males and females were almost even for each analysis (Table 2). Testing the male predominant effect of UCP1 on VFA reduction would contribute to a debate on the sexual dimorphisms of prevalence and activity of BAT in adult humans [20]. Inter-individual variation in physical activity and BAT activity should be taken into account in future studies. Additionally, it is still uncertain whether the outdoor temperature was the true factor influencing the association of UCP1 -3826A/G and VFA. Finally, our hypothesis does not consider the role of the UCP1 in diet-induced thermogenesis [21], which may contribute to resting energy expenditure in a season-independent manner.
In conclusion, UCP1 -3826A/G was significantly associated with VFA in a season-dependent manner, supporting the importance of cold stress in the activation of BAT and the significance of BAT in the development of obesity in adult humans. Furthermore, association studies of visceral adiposity in adult humans in a geographic region with distinctive seasons might consider employing controls for the sampling season.
Supporting Information
Table S1
Effect sizes (β) and P values of independent variables in the entire cohort.
(DOCX)
Table S2
Sex-specific nature of the effects of UCP1 -3826 A/G on visceral fat area.
(DOCX)
Acknowledgments
We are grateful to the staff of the Jichi Medical University Health Care Center for their cooperation in the collection of samples. We would like to thank the anonymous reviewers for their valuable suggestions and Editage for providing editorial assistance.
Funding Statement
This study was supported in part by KAKENHI Grant Number 23687036 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
Articles from PLOS ONE are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pone.0074720
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0074720&type=printable
Citations & impact
Impact metrics
Citations of article over time
Article citations
Association between thermogenic brown fat and genes under positive natural selection in circumpolar populations.
J Physiol Anthropol, 43(1):19, 19 Aug 2024
Cited by: 0 articles | PMID: 39160621 | PMCID: PMC11331686
Genetic evidence for involvement of β2-adrenergic receptor in brown adipose tissue thermogenesis in humans.
Int J Obes (Lond), 48(8):1110-1117, 17 Apr 2024
Cited by: 4 articles | PMID: 38632325 | PMCID: PMC11281906
Subcutaneous and Visceral Fat: Relation with Brown Adipose Tissue Activation in Women.
Sports Med Int Open, 8:a21876974, 08 Jan 2024
Cited by: 0 articles | PMID: 38312927 | PMCID: PMC10832575
Thermogenic adipose tissue in energy regulation and metabolic health.
Front Endocrinol (Lausanne), 14:1150059, 20 Mar 2023
Cited by: 8 articles | PMID: 37020585 | PMCID: PMC10067564
Review Free full text in Europe PMC
The Relationships between Leptin, Genotype, and Chinese Medicine Body Constitution for Obesity.
Evid Based Complement Alternat Med, 2021:5510552, 07 May 2021
Cited by: 3 articles | PMID: 34055005 | PMCID: PMC8123988
Go to all (14) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association between accumulation of visceral fat and the combination of β3 adrenergic receptor Trp64Arg, β2 adrenergic receptor Arg16Gly and uncoupling protein 1 -3826A>G polymorphisms detected by Smart Amplification Process 2.
Endocr J, 58(12):1079-1086, 30 Sep 2011
Cited by: 9 articles | PMID: 21959333
Seasonal effects of the UCP3 and the RPTOR gene polymorphisms on obesity traits in Japanese adults.
J Physiol Anthropol, 33:38, 22 Dec 2014
Cited by: 1 article | PMID: 25533680 | PMCID: PMC4347541
Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans.
Int J Obes (Lond), 37(7):993-998, 02 Oct 2012
Cited by: 53 articles | PMID: 23032405
Negative regulators of brown adipose tissue (BAT)-mediated thermogenesis.
J Cell Physiol, 229(12):1901-1907, 01 Dec 2014
Cited by: 31 articles | PMID: 24809334 | PMCID: PMC4149692
Review Free full text in Europe PMC




