Abstract
Free full text

A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung.
Abstract
Effective gene therapy for lung tissue requires the use of efficient vehicles to deliver the gene of interest into lung cells. When plasmid DNA encoding chloramphenicol acetyltransferase (CAT) was administered intranasally to BALB/c mice without carrier lipids, CAT activity was detected in mouse lung extracts. Plasmid DNA delivered with optimally formulated commercially available transfection reagents expressed up to 10-fold more CAT activity in lung than observed with naked DNA alone. Liposome formulations consisting of (+/-)-N-(3-aminopropyl)-N,N-dimethyl-2,3-bis (dodecyloxy)-1-propanaminium bromide (GAP-DLRIE) plus the neutral colipid dioleoylphosphatidylethanolamine (DOPE) enhanced CAT expression by more than 100-fold relative to plasmid DNA alone. A single administration of GAP-DLRIE liposome-CAT DNA complexes to mouse lung elicited peak expression at days 1-4 posttransfection, followed by a gradual return to baseline by day 21 postadministration. Readministration of GAP-DLRIE liposome CAT complexes at day 21 led to another transient peak of reporter gene expression. Histological examination of lungs treated with GAP-DLRIE complexed beta-galactosidase DNA revealed that alveolar epithelial cells were the primary locus of expression and that up to 1% of all alveoli contained epithelial cells expressing the transgene.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Tsui LC. Mutations and sequence variations detected in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: a report from the Cystic Fibrosis Genetic Analysis Consortium. Hum Mutat. 1992;1(3):197–203. [Abstract] [Google Scholar]
- Felgner PL, Rhodes G. Gene therapeutics. Nature. 1991 Jan 24;349(6307):351–352. [Abstract] [Google Scholar]
- Rosenfeld MA, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier LE, Päkkö PK, Gilardi P, Stratford-Perricaudet LD, Perricaudet M, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. [Abstract] [Google Scholar]
- Crystal RG, McElvaney NG, Rosenfeld MA, Chu CS, Mastrangeli A, Hay JG, Brody SL, Jaffe HA, Eissa NT, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994 Sep;8(1):42–51. [Abstract] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Gönczöl E, Engelhardt JF, Wilson JM. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. [Abstract] [Google Scholar]
- Knowles MR, Hohneker KW, Zhou Z, Olsen JC, Noah TL, Hu PC, Leigh MW, Engelhardt JF, Edwards LJ, Jones KR, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med. 1995 Sep 28;333(13):823–831. [Abstract] [Google Scholar]
- Alton EW, Geddes DM. Gene therapy for cystic fibrosis: a clinical perspective. Gene Ther. 1995 Mar;2(2):88–95. [Abstract] [Google Scholar]
- Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J, Cheng SH. Improved cationic lipid formulations for in vivo gene therapy. Ann N Y Acad Sci. 1995 Nov 27;772:126–139. [Abstract] [Google Scholar]
- Alton EW, Middleton PG, Caplen NJ, Smith SN, Steel DM, Munkonge FM, Jeffery PK, Geddes DM, Hart SL, Williamson R, et al. Non-invasive liposome-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet. 1993 Oct;5(2):135–142. [Abstract] [Google Scholar]
- Hyde SC, Gill DR, Higgins CF, Trezise AE, MacVinish LJ, Cuthbert AW, Ratcliff R, Evans MJ, Colledge WH. Correction of the ion transport defect in cystic fibrosis transgenic mice by gene therapy. Nature. 1993 Mar 18;362(6417):250–255. [Abstract] [Google Scholar]
- Stribling R, Brunette E, Liggitt D, Gaensler K, Debs R. Aerosol gene delivery in vivo. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11277–11281. [Europe PMC free article] [Abstract] [Google Scholar]
- Canonico AE, Conary JT, Meyrick BO, Brigham KL. Aerosol and intravenous transfection of human alpha 1-antitrypsin gene to lungs of rabbits. Am J Respir Cell Mol Biol. 1994 Jan;10(1):24–29. [Abstract] [Google Scholar]
- Logan JJ, Bebok Z, Walker LC, Peng S, Felgner PL, Siegal GP, Frizzell RA, Dong J, Howard M, Matalon, et al. Cationic lipids for reporter gene and CFTR transfer to rat pulmonary epithelium. Gene Ther. 1995 Jan;2(1):38–49. [Abstract] [Google Scholar]
- Sorscher Eric J, Logan James J, Frizzell Raymond A, Lyrene Raymond K, Bebok Zsuzsa, Dong JY, Duvall MD, Felgner PL, Matalon Sadis, Walker Lynn, et al. Informed consent to participate in a research study -- gene therapy for cystic fibrosis using cationic liposome mediated gene transfer: a phase I trial of safety and efficacy in the nasal airway. Hum Gene Ther. 1994 Oct;5(10):1271–1277. [Abstract] [Google Scholar]
- Canonico AE, Plitman JD, Conary JT, Meyrick BO, Brigham KL. No lung toxicity after repeated aerosol or intravenous delivery of plasmid-cationic liposome complexes. J Appl Physiol (1985) 1994 Jul;77(1):415–419. [Abstract] [Google Scholar]
- San H, Yang ZY, Pompili VJ, Jaffe ML, Plautz GE, Xu L, Felgner JH, Wheeler CJ, Felgner PL, Gao X, et al. Safety and short-term toxicity of a novel cationic lipid formulation for human gene therapy. Hum Gene Ther. 1993 Dec;4(6):781–788. [Abstract] [Google Scholar]
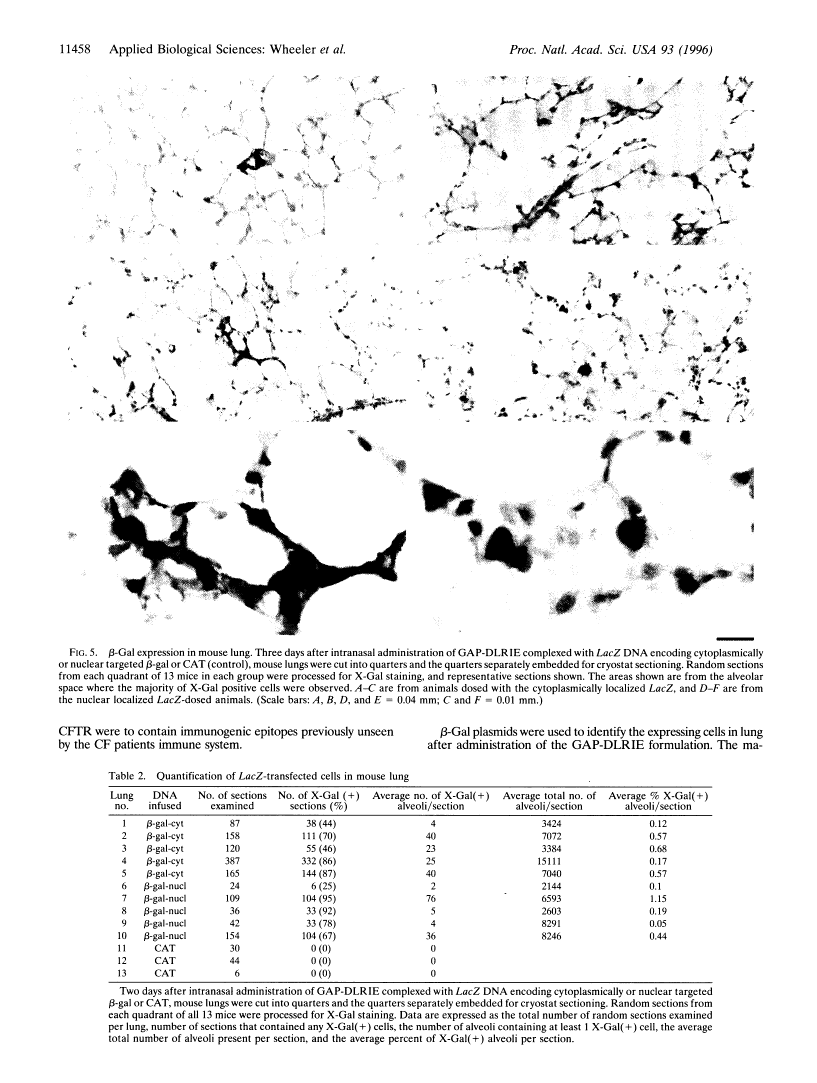
- Parker SE, Vahlsing HL, Serfilippi LM, Franklin CL, Doh SG, Gromkowski SH, Lew D, Manthorpe M, Norman J. Cancer gene therapy using plasmid DNA: safety evaluation in rodents and non-human primates. Hum Gene Ther. 1995 May;6(5):575–590. [Abstract] [Google Scholar]
- Meyer KB, Thompson MM, Levy MY, Barron LG, Szoka FC., Jr Intratracheal gene delivery to the mouse airway: characterization of plasmid DNA expression and pharmacokinetics. Gene Ther. 1995 Sep;2(7):450–460. [Abstract] [Google Scholar]
- Tsan MF, White JE, Shepard B. Lung-specific direct in vivo gene transfer with recombinant plasmid DNA. Am J Physiol. 1995 Jun;268(6 Pt 1):L1052–L1056. [Abstract] [Google Scholar]
- Montgomery DL, Shiver JW, Leander KR, Perry HC, Friedman A, Martinez D, Ulmer JB, Donnelly JJ, Liu MA. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993 Nov;12(9):777–783. [Abstract] [Google Scholar]
- Hartikka J, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing HL, Meek J, Marquet M, Hobart P, et al. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum Gene Ther. 1996 Jun 20;7(10):1205–1217. [Abstract] [Google Scholar]
- Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994 Jan 28;269(4):2550–2561. [Abstract] [Google Scholar]
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. [Europe PMC free article] [Abstract] [Google Scholar]
- Haies DM, Gil J, Weibel ER. Morphometric study of rat lung cells. I. Numerical and dimensional characteristics of parenchymal cell population. Am Rev Respir Dis. 1981 May;123(5):533–541. [Abstract] [Google Scholar]
- Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982 Aug;126(2):332–337. [Abstract] [Google Scholar]
- Lin H, Parmacek MS, Morle G, Bolling S, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. [Abstract] [Google Scholar]
- Hickman MA, Malone RW, Lehmann-Bruinsma K, Sih TR, Knoell D, Szoka FC, Walzem R, Carlson DM, Powell JS. Gene expression following direct injection of DNA into liver. Hum Gene Ther. 1994 Dec;5(12):1477–1483. [Abstract] [Google Scholar]
- Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9519–9523. [Europe PMC free article] [Abstract] [Google Scholar]
- Nabel EG, Plautz G, Nabel GJ. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. [Abstract] [Google Scholar]
- Vile RG, Hart IR. Use of tissue-specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Res. 1993 Sep 1;53(17):3860–3864. [Abstract] [Google Scholar]
- Liu Y, Liggitt D, Zhong W, Tu G, Gaensler K, Debs R. Cationic liposome-mediated intravenous gene delivery. J Biol Chem. 1995 Oct 20;270(42):24864–24870. [Abstract] [Google Scholar]
- Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffery PK, Hodson ME, Coutelle C, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995 Jan;1(1):39–46. [Abstract] [Google Scholar]
- McLachlan G, Davidson DJ, Stevenson BJ, Dickinson P, Davidson-Smith H, Dorin JR, Porteous DJ. Evaluation in vitro and in vivo of cationic liposome-expression construct complexes for cystic fibrosis gene therapy. Gene Ther. 1995 Nov;2(9):614–622. [Abstract] [Google Scholar]
- Engelhardt JF, Yang Y, Stratford-Perricaudet LD, Allen ED, Kozarsky K, Perricaudet M, Yankaskas JR, Wilson JM. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993 May;4(1):27–34. [Abstract] [Google Scholar]
- Yang Y, Raper SE, Cohn JA, Engelhardt JF, Wilson JM. An approach for treating the hepatobiliary disease of cystic fibrosis by somatic gene transfer. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4601–4605. [Europe PMC free article] [Abstract] [Google Scholar]
- Scarlata SF, Rosenberg M. Effect of increased lipid packing on the surface charge of micelles and membranes. Biochemistry. 1990 Nov 6;29(44):10233–10240. [Abstract] [Google Scholar]
- Wheeler CJ, Sukhu L, Yang G, Tsai Y, Bustamente C, Felgner P, Norman J, Manthorpe M. Converting an alcohol to an amine in a cationic lipid dramatically alters the co-lipid requirement, cellular transfection activity and the ultrastructure of DNA-cytofectin complexes. Biochim Biophys Acta. 1996 Apr 3;1280(1):1–11. [Abstract] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. [Europe PMC free article] [Abstract] [Google Scholar]
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6077–6081. [Europe PMC free article] [Abstract] [Google Scholar]
- Bennett CF, Chiang MY, Chan H, Shoemaker JE, Mirabelli CK. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1992 Jun;41(6):1023–1033. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.93.21.11454
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc38078?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The 60-year evolution of lipid nanoparticles for nucleic acid delivery.
Nat Rev Drug Discov, 23(9):709-722, 04 Jul 2024
Cited by: 4 articles | PMID: 38965378
Review
Lipoplexes' Structure, Preparation, and Role in Managing Different Diseases.
AAPS PharmSciTech, 25(5):131, 07 Jun 2024
Cited by: 0 articles | PMID: 38849687
Review
Liposome-Based Carriers for CRISPR Genome Editing.
Int J Mol Sci, 24(16):12844, 16 Aug 2023
Cited by: 1 article | PMID: 37629024 | PMCID: PMC10454197
Review Free full text in Europe PMC
The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatments.
J Med Chem, 65(10):6975-7015, 09 May 2022
Cited by: 27 articles | PMID: 35533054 | PMCID: PMC9115888
Review Free full text in Europe PMC
Synthesis and bioactivity of readily hydrolysable novel cationic lipids for potential lung delivery application of mRNAs.
Chem Phys Lipids, 243:105178, 03 Feb 2022
Cited by: 7 articles | PMID: 35122738 | PMCID: PMC9749011
Go to all (152) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Improved cationic lipid formulations for in vivo gene therapy.
Ann N Y Acad Sci, 772:126-139, 01 Nov 1995
Cited by: 112 articles | PMID: 8546385
Review
In vitro and in vivo gene transfer to pulmonary cells mediated by cationic liposomes.
Biochim Biophys Acta, 1306(1):55-62, 01 Apr 1996
Cited by: 37 articles | PMID: 8611625
A new cationic liposome DNA complex enhances the efficiency of arterial gene transfer in vivo.
Hum Gene Ther, 7(15):1803-1812, 01 Oct 1996
Cited by: 70 articles | PMID: 8894672
Gene transfection into fetal sheep airways in utero using guanidinium-cholesterol cationic lipids.
J Gene Med, 6(3):328-336, 01 Mar 2004
Cited by: 10 articles | PMID: 15026994