Abstract
Free full text

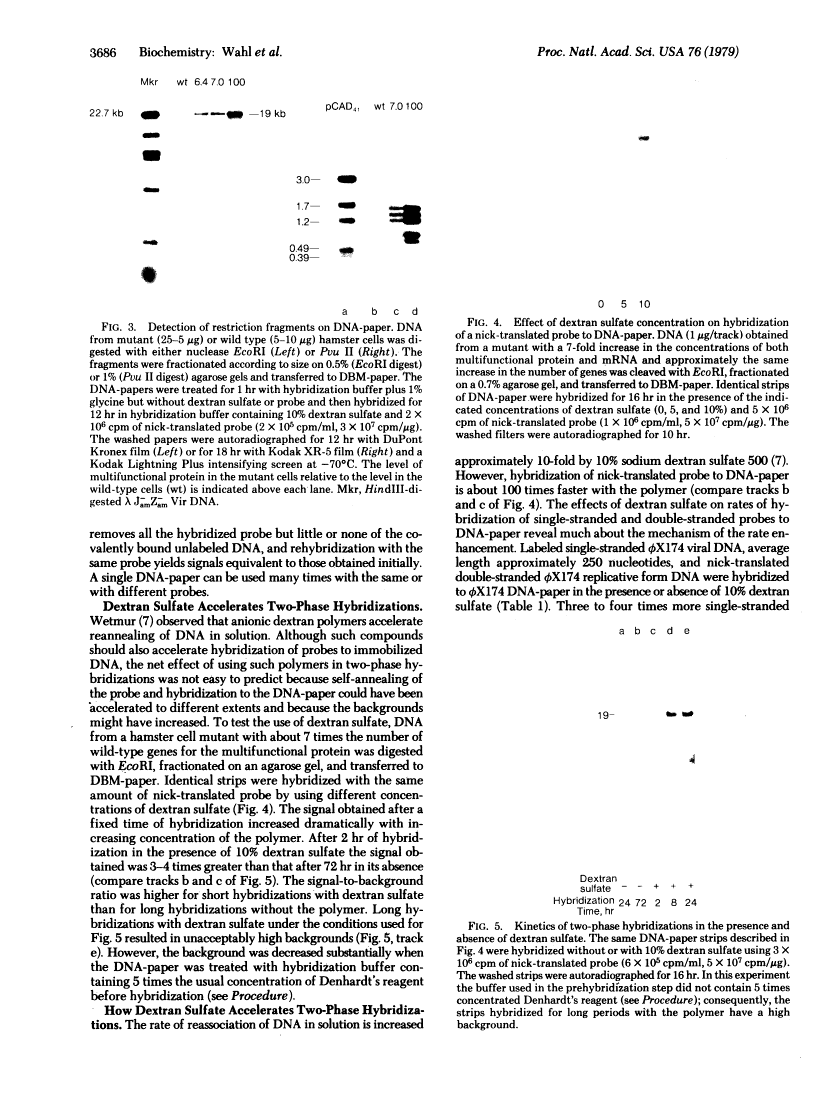
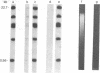
Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate.
Abstract
We describe a technique for transferring electrophoretically separated bands of double-stranded DNA from agarose gels to diazobenzyloxymethyl-paper. Controlled cleavage of the DNA in situ by sequential treatment with dilute acid, which causes partial depurination, and dilute alkali, which causes cleavage and separation of the strands, allows the DNA to leave the gel rapidly and completely, with an efficiency independent of its size. Covalent attachment of DNA to paper prevents losses during subsequent hybridization and washing steps and allows a single paper to be reused many times. Ten percent dextran sulfate, originally found to accelerate DNA hybridization in solution by about 10-fold [J.G. Wetmur (1975) Biopolymers 14, 2517-2524], accelerates the rate of hybridization of randomly cleaved double-stranded DNA probes to immobilized nucleic acids by as much as 100-fold, without increasing the background significantly.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Mears JG, Ramirez F, Leibowitz D, Nakamura F, Bloom A, Konotey-Ahulu F, Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. [Europe PMC free article] [Abstract] [Google Scholar]
- Kan YW, Dozy AM. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. [Europe PMC free article] [Abstract] [Google Scholar]
- Noyes BE, Stark GR. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. [Abstract] [Google Scholar]
- Alwine JC, Kemp DJ, Stark GR. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. [Europe PMC free article] [Abstract] [Google Scholar]
- Reiser J, Renart J, Stark GR. Transfer of small DNA fragments from polyacrylamide gels to diazobenzyloxymethyl-paper and detection by hybridization with DNA probes. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1104–1112. [Abstract] [Google Scholar]
- McDonell MW, Simon MN, Studier FW. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. [Abstract] [Google Scholar]
- Rigby PW, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. [Abstract] [Google Scholar]
- Donoghue DJ, Sharp PA. An improved bacteriophage lambda vector: construction of model recombinants coding for kanamycin resistance. Gene. 1977 May;1(3-4):209–227. [Abstract] [Google Scholar]
- Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Denhardt DT. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. [Abstract] [Google Scholar]
- Shank PR, Hughes SH, Kung HJ, Majors JE, Quintrell N, Guntaka RV, Bishop JM, Varmus HE. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. [Abstract] [Google Scholar]
- Lis JT, Prestidge L, Hogness DS. A novel arrangement of tandemly repeated genes at a major heat shock site in D. melanogaster. Cell. 1978 Aug;14(4):901–919. [Abstract] [Google Scholar]
- Gillespie D, Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. [Abstract] [Google Scholar]
- Wetmur JG, Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. [Abstract] [Google Scholar]
- Stark GR, Williams JG. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. [Europe PMC free article] [Abstract] [Google Scholar]
- Pardue ML, Gall JG. Molecular hybridization of radioactive DNA to the DNA of cytological preparations. Proc Natl Acad Sci U S A. 1969 Oct;64(2):600–604. [Europe PMC free article] [Abstract] [Google Scholar]
- Villarreal LP, Berg P. Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science. 1977 Apr 8;196(4286):183–185. [Abstract] [Google Scholar]
- Benton WD, Davis RW. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. [Abstract] [Google Scholar]
- Grunstein M, Hogness DS. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.76.8.3683
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc383897?pdf=render
Citations & impact
Impact metrics
Article citations
Nna1, Essential for Purkinje Cell Survival, Is also Associated with Emotion and Memory.
Int J Mol Sci, 23(21):12961, 26 Oct 2022
Cited by: 3 articles | PMID: 36361749 | PMCID: PMC9654422
Advances in endogenous RNA pull-down: A straightforward dextran sulfate-based method enhancing RNA recovery.
Front Mol Biosci, 9:1004746, 19 Oct 2022
Cited by: 4 articles | PMID: 36339717 | PMCID: PMC9629853
Transposons-Based Clonal Diversity in Trematode Involves Parts of CR1 (LINE) in Eu- and Heterochromatin.
Genes (Basel), 12(8):1129, 25 Jul 2021
Cited by: 2 articles | PMID: 34440303 | PMCID: PMC8392823
Using the zebrafish to understand tendon development and repair.
Methods Cell Biol, 138:299-320, 09 Nov 2016
Cited by: 7 articles | PMID: 28129848 | PMCID: PMC5633932
Review Free full text in Europe PMC
From synthetic DNA to PCR product: detection of fungal infections using SERS.
Faraday Discuss, 187:461-472, 01 Jun 2016
Cited by: 5 articles | PMID: 27034997
Go to all (1,854) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes.
Proc Natl Acad Sci U S A, 74(12):5350-5354, 01 Dec 1977
Cited by: 955 articles | PMID: 414220 | PMCID: PMC431715
Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper.
Methods Enzymol, 68:220-242, 01 Jan 1979
Cited by: 326 articles | PMID: 94421
Acid depurination after field inversion agarose gel electrophoresis reduces transfer of large DNA molecules.
Appl Theor Electrophor, 1(4):189-192, 01 Jan 1990
Cited by: 2 articles | PMID: 1711375
Isolation and purification of large DNA restriction fragments from agarose gels.
Curr Protoc Immunol, Chapter 10:Unit 10.5, 01 May 2001
Cited by: 2 articles | PMID: 18432696
Review