Abstract
Free full text

Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme.
Abstract
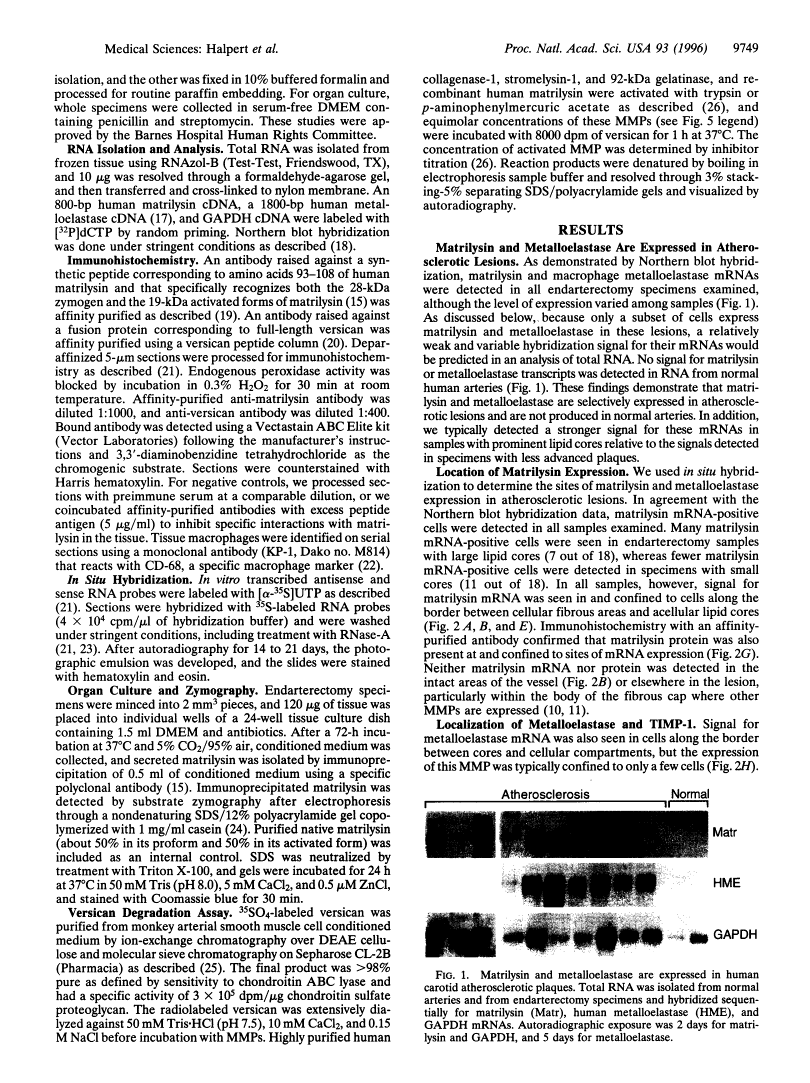
Certain matrix metalloproteinases (MMP) are expressed within the fibrous areas surrounding acellular lipid cores of atherosclerotic plaques, suggesting that these proteinases degrade matrix proteins within these areas and weaken the structural integrity of the lesion. We report that matrilysin and macrophage metalloelastase, two broad-acting MMPs, were expressed in human atherosclerotic lesions in carotid endarterectomy samples (n = 18) but were not expressed in normal arteries (n = 7). In situ hybridization and immunohistochemistry revealed prominent expression of matrilysin in cells confined to the border between acellular lipid cores and overlying fibrous areas, a distribution distinct from other MMPs found in similar lesions. Metalloelastase was expressed in these same border areas. Matrilysin was present in lipid-laden macrophages, identified by staining with anti-CD-68 antibody. Furthermore, endarterectomy tissue in organ culture released matrilysin. Staining for versican demonstrated that this vascular proteoglycan was present at sites of matrilysin expression. Biochemical studies showed that matrilysin degraded versican much more efficiently than other MMPs present in atherosclerotic lesions. Our findings suggest that matrilysin, specifically expressed in atherosclerotic lesions, could cleave structural proteoglycans and other matrix components, potentially leading to separation of caps and shoulders from lipid cores.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
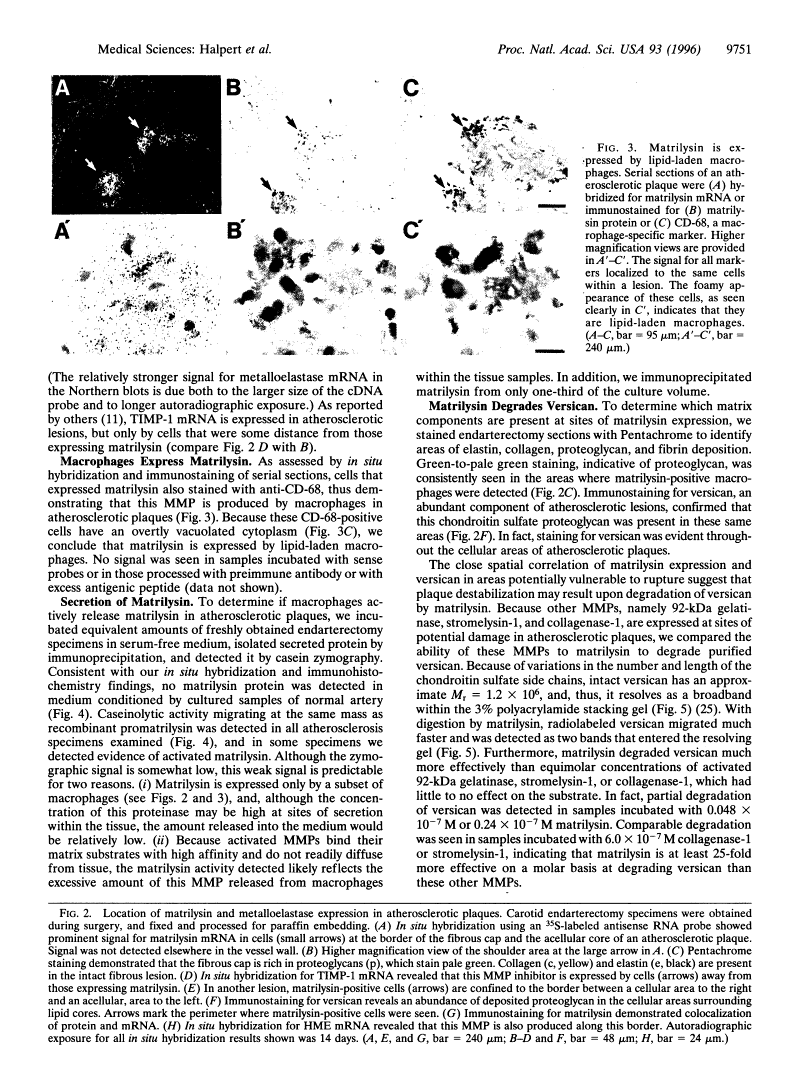
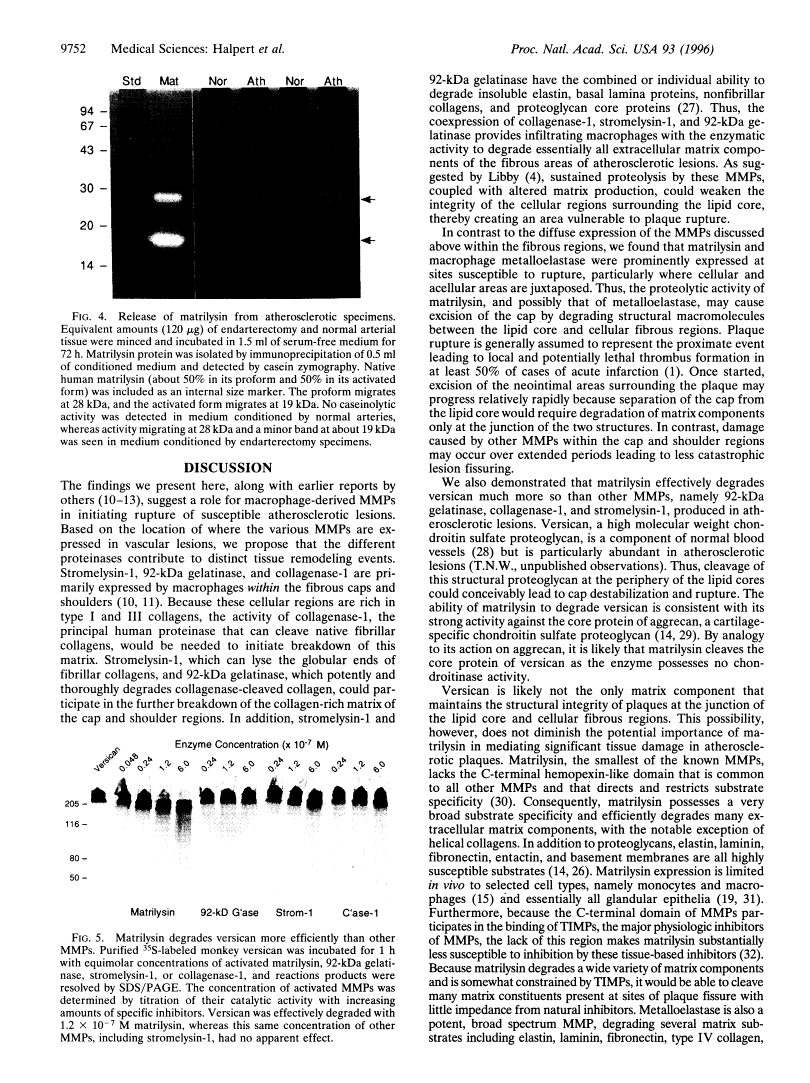
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. [Abstract] [Google Scholar]
- Sanders M. Molecular and cellular concepts in atherosclerosis. Pharmacol Ther. 1994;61(1-2):109–153. [Abstract] [Google Scholar]
- Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995 Jun 1;91(11):2844–2850. [Abstract] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994 May;89(5):2462–2478. [Abstract] [Google Scholar]
- Davies MJ, Thomas AC. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985 Apr;53(4):363–373. [Europe PMC free article] [Abstract] [Google Scholar]
- Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J. 1983 Aug;50(2):127–134. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994 Jan;89(1):36–44. [Abstract] [Google Scholar]
- Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991 Mar;87(1):87–90. [Abstract] [Google Scholar]
- Henney AM, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G, Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. [Europe PMC free article] [Abstract] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. [Europe PMC free article] [Abstract] [Google Scholar]
- Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995 Apr 15;91(8):2125–2131. [Abstract] [Google Scholar]
- Nikkari ST, O'Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, Clowes AW. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995 Sep 15;92(6):1393–1398. [Abstract] [Google Scholar]
- Murphy G, Cockett MI, Ward RV, Docherty AJ. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. [Europe PMC free article] [Abstract] [Google Scholar]
- Busiek DF, Ross FP, McDonnell S, Murphy G, Matrisian LM, Welgus HG. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992 May 5;267(13):9087–9092. [Abstract] [Google Scholar]
- Busiek DF, Baragi V, Nehring LC, Parks WC, Welgus HG. Matrilysin expression by human mononuclear phagocytes and its regulation by cytokines and hormones. J Immunol. 1995 Jun 15;154(12):6484–6491. [Abstract] [Google Scholar]
- Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993 Nov 15;268(32):23824–23829. [Abstract] [Google Scholar]
- Parks WC, Secrist H, Wu LC, Mecham RP. Developmental regulation of tropoelastin isoforms. J Biol Chem. 1988 Mar 25;263(9):4416–4423. [Abstract] [Google Scholar]
- Saarialho-Kere UK, Crouch EC, Parks WC. Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol. 1995 Aug;105(2):190–196. [Abstract] [Google Scholar]
- LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992 May 15;267(14):10003–10010. [Abstract] [Google Scholar]
- Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993 Dec;92(6):2858–2866. [Europe PMC free article] [Abstract] [Google Scholar]
- Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. [Europe PMC free article] [Abstract] [Google Scholar]
- Prosser IW, Stenmark KR, Suthar M, Crouch EC, Mecham RP, Parks WC. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [Europe PMC free article] [Abstract] [Google Scholar]
- Marshall BC, Santana A, Xu QP, Petersen MJ, Campbell EJ, Hoidal JR, Welgus HG. Metalloproteinases and tissue inhibitor of metalloproteinases in mesothelial cells. Cellular differentiation influences expression. J Clin Invest. 1993 Apr;91(4):1792–1799. [Europe PMC free article] [Abstract] [Google Scholar]
- Schönherr E, Järveläinen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991 Sep 15;266(26):17640–17647. [Abstract] [Google Scholar]
- Sires UI, Griffin GL, Broekelmann TJ, Mecham RP, Murphy G, Chung AE, Welgus HG, Senior RM. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J Biol Chem. 1993 Jan 25;268(3):2069–2074. [Abstract] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. [Abstract] [Google Scholar]
- Yao LY, Moody C, Schönherr E, Wight TN, Sandell LJ. Identification of the proteoglycan versican in aorta and smooth muscle cells by DNA sequence analysis, in situ hybridization and immunohistochemistry. Matrix Biol. 1994 Apr;14(3):213–225. [Abstract] [Google Scholar]
- Sellers A, Woessner JF., Jr The extraction of a neutral metalloproteinase from the involuting rat uterus, and its action on cartilage proteoglycan. Biochem J. 1980 Sep 1;189(3):521–531. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996 Feb;28(2):123–136. [Abstract] [Google Scholar]
- Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995 Jul;6(7):851–869. [Europe PMC free article] [Abstract] [Google Scholar]
- Baragi VM, Fliszar CJ, Conroy MC, Ye QZ, Shipley JM, Welgus HG. Contribution of the C-terminal domain of metalloproteinases to binding by tissue inhibitor of metalloproteinases. C-terminal truncated stromelysin and matrilysin exhibit equally compromised binding affinities as compared to full-length stromelysin. J Biol Chem. 1994 Apr 29;269(17):12692–12697. [Abstract] [Google Scholar]
- Marti HP, McNeil L, Thomas G, Davies M, Lovett DH. Molecular characterization of a low-molecular-mass matrix metalloproteinase secreted by glomerular mesangial cells as PUMP-1. Biochem J. 1992 Aug 1;285(Pt 3):899–905. [Europe PMC free article] [Abstract] [Google Scholar]
- Gaire M, Magbanua Z, McDonnell S, McNeil L, Lovett DH, Matrisian LM. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J Biol Chem. 1994 Jan 21;269(3):2032–2040. [Abstract] [Google Scholar]
- Shapiro SD, Campbell EJ, Kobayashi DK, Welgus HG. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990 Oct;86(4):1204–1210. [Europe PMC free article] [Abstract] [Google Scholar]
- Shapiro SD, Kobayashi DK, Pentland AP, Welgus HG. Induction of macrophage metalloproteinases by extracellular matrix. Evidence for enzyme- and substrate-specific responses involving prostaglandin-dependent mechanisms. J Biol Chem. 1993 Apr 15;268(11):8170–8175. [Abstract] [Google Scholar]
- Mauch C, Adelmann-Grill B, Hatamochi A, Krieg T. Collagenase gene expression in fibroblasts is regulated by a three-dimensional contact with collagen. FEBS Lett. 1989 Jul 3;250(2):301–305. [Abstract] [Google Scholar]
- Kanemoto T, Reich R, Royce L, Greatorex D, Adler SH, Shiraishi N, Martin GR, Yamada Y, Kleinman HK. Identification of an amino acid sequence from the laminin A chain that stimulates metastasis and collagenase IV production. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2279–2283. [Europe PMC free article] [Abstract] [Google Scholar]
- Noble PW, Lake FR, Henson PM, Riches DW. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J Clin Invest. 1993 Jun;91(6):2368–2377. [Europe PMC free article] [Abstract] [Google Scholar]
- Rouis M, Nigon F, Lafuma C, Hornebeck W, Chapman MJ. Expression of elastase activity by human monocyte-macrophages is modulated by cellular cholesterol content, inflammatory mediators, and phorbol myristate acetate. Arteriosclerosis. 1990 Mar-Apr;10(2):246–255. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.93.18.9748
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc38500?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.93.18.9748
Article citations
Unveiling TIMPs: A Systematic Review of Their Role as Biomarkers in Atherosclerosis and Coronary Artery Disease.
Diseases, 12(8):177, 02 Aug 2024
Cited by: 0 articles | PMID: 39195176 | PMCID: PMC11353419
Review Free full text in Europe PMC
Macrophage profiling in atherosclerosis: understanding the unstable plaque.
Basic Res Cardiol, 119(1):35-56, 20 Jan 2024
Cited by: 5 articles | PMID: 38244055
Review
Matrix metalloproteinases in coronary artery disease and myocardial infarction.
Basic Res Cardiol, 118(1):18, 09 May 2023
Cited by: 12 articles | PMID: 37160529 | PMCID: PMC10169894
Review Free full text in Europe PMC
Pulsatilla decoction suppresses matrix metalloproteinase-7-mediated leukocyte recruitment in dextran sulfate sodium-induced colitis mouse model.
BMC Complement Med Ther, 22(1):211, 06 Aug 2022
Cited by: 2 articles | PMID: 35933374 | PMCID: PMC9356479
Proteolysis: a key post-translational modification regulating proteoglycans.
Am J Physiol Cell Physiol, 323(3):C651-C665, 04 Jul 2022
Cited by: 17 articles | PMID: 35785985 | PMCID: PMC9448339
Review Free full text in Europe PMC
Go to all (226) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Accumulation of matrilysin (MMP-7) and macrophage metalloelastase (MMP-12) in actinic damage.
J Invest Dermatol, 113(4):664-672, 01 Oct 1999
Cited by: 70 articles | PMID: 10504457
Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations.
Am J Pathol, 152(4):1005-1014, 01 Apr 1998
Cited by: 135 articles | PMID: 9546361 | PMCID: PMC1858229
Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett's oesophageal adenocarcinoma.
Br J Cancer, 85(3):383-392, 01 Aug 2001
Cited by: 58 articles | PMID: 11487270 | PMCID: PMC2364078
Regulation of matrilysin in the rat uterus.
Biochem Cell Biol, 74(6):777-784, 01 Jan 1996
Cited by: 17 articles | PMID: 9164647
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (3)
Grant ID: HL48762
Grant ID: HL49250
Grant ID: HL29594