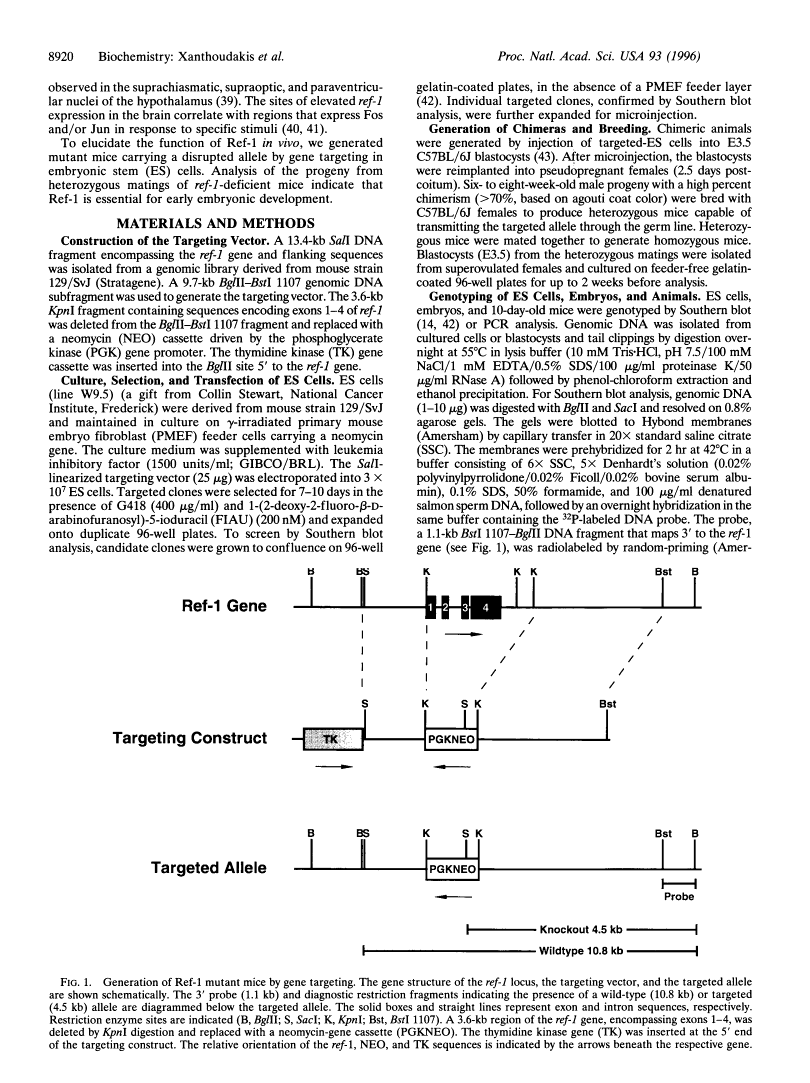
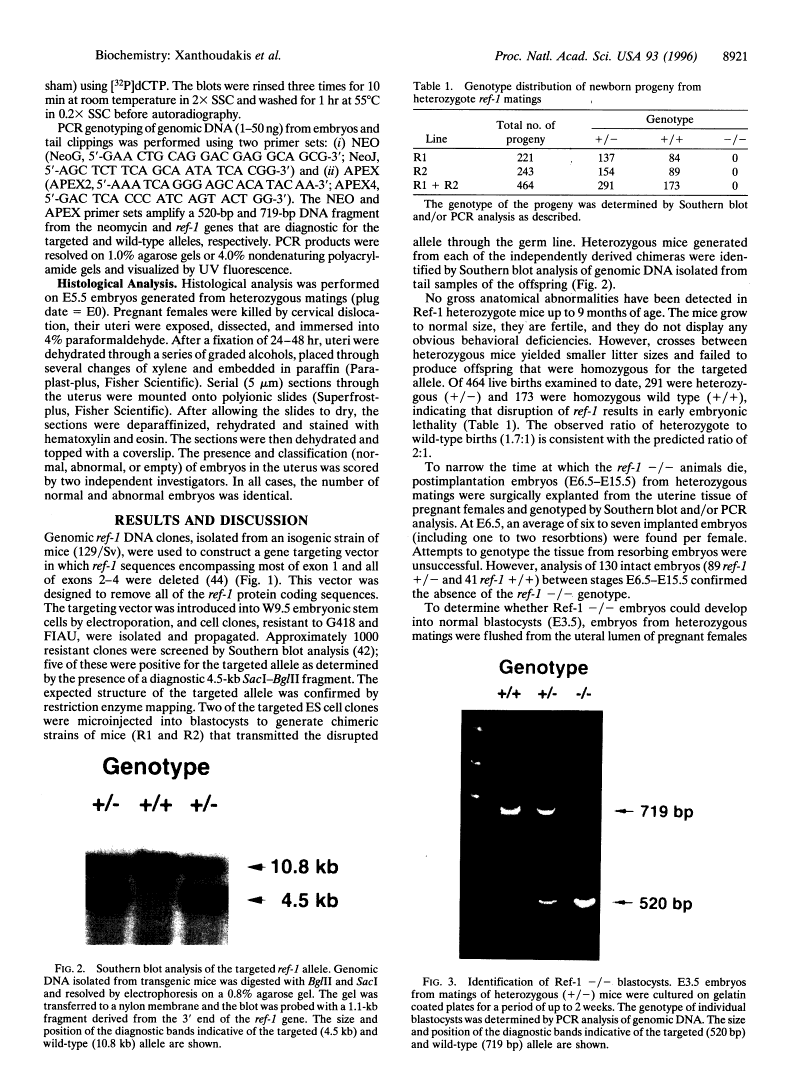
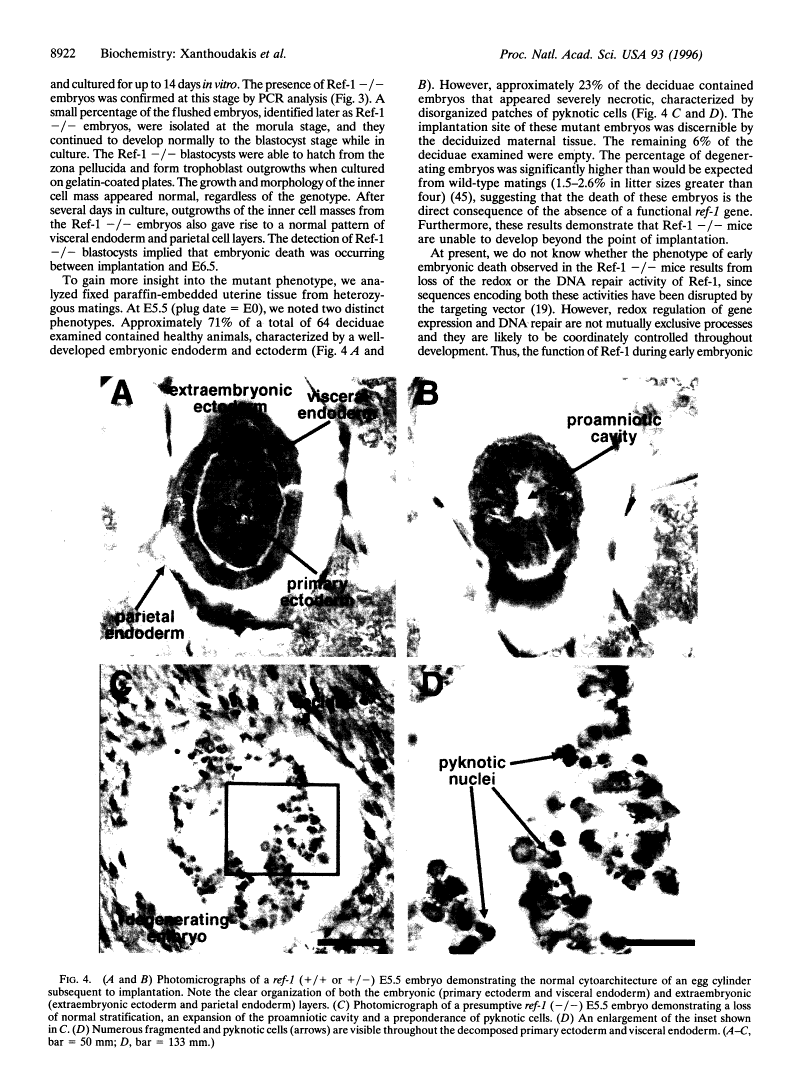
Abstract
Free full text

The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice.
Abstract
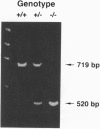
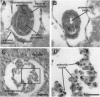
The DNA-binding activity of AP-1 proteins is modulated, in vitro, by a posttranslational mechanism involving reduction oxidation. This mode of regulation has been proposed to control both the transcriptional activity and the oncogenic potential of Fos and Jun. Previous studies revealed that reduction of oxidized Fos and Jun by a cellular protein, Ref-1, stimulates sequence-specific AP-1 DNA-binding activity. Ref-1, a bifunctional protein, is also capable of initiating the repair of apurinic/apyrymidinic sites in damaged DNA. The relationship between the redox and DNA repair activities of Ref-1 is intriguing; both activities have been suggested to play an important role in the cellular response to oxidative stress. To investigate the physiological function of Ref-1, we used a gene targeting strategy to generate mice lacking a functional ref-1 gene. We report here that heterozygous mutant mice develop into adulthood without any apparent abnormalities. In contrast, homozygous mutant mice, lacking a functional ref-1 gene, die during embryonic development. Detailed analysis indicates that death occurs following blastocyst formation, shortly after the time of implantation. Degeneration of the mutant embryos is clearly evident at embryonic day 5.5. These findings demonstrate that Ref-1 is essential for early embryonic development.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Seroz T, Hwang JR, Moncollin V, Egly JM. TFIIH: a link between transcription, DNA repair and cell cycle regulation. Curr Opin Genet Dev. 1995 Apr;5(2):217–221. [Abstract] [Google Scholar]
- Drapkin R, Sancar A, Reinberg D. Where transcription meets repair. Cell. 1994 Apr 8;77(1):9–12. [Abstract] [Google Scholar]
- Hanawalt PC, Donahue BA, Sweder KS. Repair and transcription. Collision or collusion? Curr Biol. 1994 Jun 1;4(6):518–521. [Abstract] [Google Scholar]
- Aboussekhra A, Wood RD. Repair of UV-damaged DNA by mammalian cells and Saccharomyces cerevisiae. Curr Opin Genet Dev. 1994 Apr;4(2):212–220. [Abstract] [Google Scholar]
- Drapkin R, Reardon JT, Ansari A, Huang JC, Zawel L, Ahn K, Sancar A, Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994 Apr 21;368(6473):769–772. [Abstract] [Google Scholar]
- Hanawalt PC. Transcription-coupled repair and human disease. Science. 1994 Dec 23;266(5193):1957–1958. [Abstract] [Google Scholar]
- Taylor AM, McConville CM, Byrd PJ. Cancer and DNA processing disorders. Br Med Bull. 1994 Jul;50(3):708–717. [Abstract] [Google Scholar]
- Powell SN, Abraham EH. The biology of radioresistance: similarities, differences and interactions with drug resistance. Cytotechnology. 1993;12(1-3):325–345. [Abstract] [Google Scholar]
- Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab Invest. 1993 Sep;69(3):261–274. [Abstract] [Google Scholar]
- Holbrook NJ, Fornace AJ., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [Abstract] [Google Scholar]
- Robson CN, Hickson ID. Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991 Oct 25;19(20):5519–5523. [Europe PMC free article] [Abstract] [Google Scholar]
- Demple B, Herman T, Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11450–11454. [Europe PMC free article] [Abstract] [Google Scholar]
- Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. [Europe PMC free article] [Abstract] [Google Scholar]
- Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. [Europe PMC free article] [Abstract] [Google Scholar]
- Seki S, Akiyama K, Watanabe S, Hatsushika M, Ikeda S, Tsutsui K. cDNA and deduced amino acid sequence of a mouse DNA repair enzyme (APEX nuclease) with significant homology to Escherichia coli exonuclease III. J Biol Chem. 1991 Nov 5;266(31):20797–20802. [Abstract] [Google Scholar]
- Babiychuk E, Kushnir S, Van Montagu M, Inzé D. The Arabidopsis thaliana apurinic endonuclease Arp reduces human transcription factors Fos and Jun. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3299–3303. [Europe PMC free article] [Abstract] [Google Scholar]
- Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. [Abstract] [Google Scholar]
- Walker LJ, Robson CN, Black E, Gillespie D, Hickson ID. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993 Sep;13(9):5370–5376. [Europe PMC free article] [Abstract] [Google Scholar]
- Xanthoudakis S, Miao GG, Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):23–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. [Abstract] [Google Scholar]
- Huang RP, Adamson ED. Characterization of the DNA-binding properties of the early growth response-1 (Egr-1) transcription factor: evidence for modulation by a redox mechanism. DNA Cell Biol. 1993 Apr;12(3):265–273. [Abstract] [Google Scholar]
- Hainaut P, Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993 Oct 1;53(19):4469–4473. [Abstract] [Google Scholar]
- Hainaut P. The tumor suppressor protein p53: a receptor to genotoxic stress that controls cell growth and survival. Curr Opin Oncol. 1995 Jan;7(1):76–82. [Abstract] [Google Scholar]
- Lane DP, Midgley CA, Hupp TR, Lu X, Vojtesek B, Picksley SM. On the regulation of the p53 tumour suppressor, and its role in the cellular response to DNA damage. Philos Trans R Soc Lond B Biol Sci. 1995 Jan 30;347(1319):83–87. [Abstract] [Google Scholar]
- Kaufmann WK. Cell cycle checkpoints and DNA repair preserve the stability of the human genome. Cancer Metastasis Rev. 1995 Mar;14(1):31–41. [Abstract] [Google Scholar]
- Gujuluva CN, Baek JH, Shin KH, Cherrick HM, Park NH. Effect of UV-irradiation on cell cycle, viability and the expression of p53, gadd153 and gadd45 genes in normal and HPV-immortalized human oral keratinocytes. Oncogene. 1994 Jul;9(7):1819–1827. [Abstract] [Google Scholar]
- Guillouf C, Rosselli F, Krishnaraju K, Moustacchi E, Hoffman B, Liebermann DA. p53 involvement in control of G2 exit of the cell cycle: role in DNA damage-induced apoptosis. Oncogene. 1995 Jun 1;10(11):2263–2270. [Abstract] [Google Scholar]
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. [Abstract] [Google Scholar]
- Doetsch PW, Cunningham RP. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. [Abstract] [Google Scholar]
- Levin JD, Demple B. Analysis of class II (hydrolytic) and class I (beta-lyase) apurinic/apyrimidinic endonucleases with a synthetic DNA substrate. Nucleic Acids Res. 1990 Sep 11;18(17):5069–5075. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen DS, Herman T, Demple B. Two distinct human DNA diesterases that hydrolyze 3'-blocking deoxyribose fragments from oxidized DNA. Nucleic Acids Res. 1991 Nov 11;19(21):5907–5914. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramotar D, Popoff SC, Demple B. Complementation of DNA repair-deficient Escherichia coli by the yeast Apn1 apurinic/apyrimidinic endonuclease gene. Mol Microbiol. 1991 Jan;5(1):149–155. [Abstract] [Google Scholar]
- Ramotar D, Popoff SC, Gralla EB, Demple B. Cellular role of yeast Apn1 apurinic endonuclease/3'-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol. 1991 Sep;11(9):4537–4544. [Europe PMC free article] [Abstract] [Google Scholar]
- de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995 Jul 28;82(2):321–330. [Abstract] [Google Scholar]
- Baker SM, Bronner CE, Zhang L, Plug AW, Robatzek M, Warren G, Elliott EA, Yu J, Ashley T, Arnheim N, et al. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995 Jul 28;82(2):309–319. [Abstract] [Google Scholar]
- Yao KS, Xanthoudakis S, Curran T, O'Dwyer PJ. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol Cell Biol. 1994 Sep;14(9):5997–6003. [Europe PMC free article] [Abstract] [Google Scholar]
- Harrison L, Galanopoulos T, Ascione AG, Antoniades HN, Demple B. Regulated expression of APE apurinic endonuclease mRNA during wound healing in porcine epidermis. Carcinogenesis. 1996 Feb;17(2):377–381. [Abstract] [Google Scholar]
- Ono Y, Watanabe M, Inoue Y, Ohmoto T, Akiyama K, Tsutsui K, Seki S. Developmental expression of APEX nuclease, a multifunctional DNA repair enzyme, in mouse brains. Brain Res Dev Brain Res. 1995 May 26;86(1-2):1–6. [Abstract] [Google Scholar]
- Rivkees SA, Kelley MR. Expression of a multifunctional DNA repair enzyme gene, apurinic/apyrimidinic endonuclease (APE; Ref-1) in the suprachiasmatic, supraoptic and paraventricular nuclei. Brain Res. 1994 Dec 12;666(1):137–142. [Abstract] [Google Scholar]
- Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992 Mar 20;255(5051):1581–1584. [Abstract] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. [Abstract] [Google Scholar]
- Ramírez-Solis R, Rivera-Pérez J, Wallace JD, Wims M, Zheng H, Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992 Mar;201(2):331–335. [Abstract] [Google Scholar]
- Takiguchi Y, Chen DJ. Genomic structure of the mouse apurinic/apyrimidinic endonuclease gene. Mamm Genome. 1994 Nov;5(11):717–722. [Abstract] [Google Scholar]
- Gardiner CS, Reed DJ. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol Reprod. 1994 Dec;51(6):1307–1314. [Abstract] [Google Scholar]
- Gardiner CS, Reed DJ. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys. 1995 Apr 1;318(1):30–36. [Abstract] [Google Scholar]
- Winn LM, Wells PG. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryo protection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species-mediated DNA oxidation in the molecular mechanism of phenytoin teratogenicity. Mol Pharmacol. 1995 Jul;48(1):112–120. [Abstract] [Google Scholar]
- Crawford DR, Edbauer-Nechamen CA, Lowry CV, Salmon SL, Kim YK, Davies JM, Davies KJ. Assessing gene expression during oxidative stress. Methods Enzymol. 1994;234:175–217. [Abstract] [Google Scholar]
- Ono Y, Furuta T, Ohmoto T, Akiyama K, Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat Res. 1994 Jul;315(1):55–63. [Abstract] [Google Scholar]
- Walker LJ, Craig RB, Harris AL, Hickson ID. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA damaging agents and hypoxic stress. Nucleic Acids Res. 1994 Nov 25;22(23):4884–4889. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993 Jul;7(7B):1309–1317. [Abstract] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994 Oct 21;266(5184):443–448. [Abstract] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.93.17.8919
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc38569?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.93.17.8919
Article citations
Distinct regulation of ATM signaling by DNA single-strand breaks and APE1.
Nat Commun, 15(1):6517, 07 Aug 2024
Cited by: 0 articles | PMID: 39112456 | PMCID: PMC11306256
Back-Up Base Excision DNA Repair in Human Cells Deficient in the Major AP Endonuclease, APE1.
Int J Mol Sci, 25(1):64, 20 Dec 2023
Cited by: 3 articles | PMID: 38203235 | PMCID: PMC10778768
Altered accumulation of hepatic mitochondrial antioxidant proteins with age and environmental heat stress.
J Appl Physiol (1985), 135(6):1339-1347, 26 Oct 2023
Cited by: 0 articles | PMID: 37881850
Functional importance and divergence of plant apurinic/apyrimidinic endonucleases in somatic and meiotic DNA repair.
Plant Cell, 35(6):2316-2331, 01 May 2023
Cited by: 4 articles | PMID: 36856605
APEX1 Nuclease and Redox Functions are Both Essential for Adult Mouse Hematopoietic Stem and Progenitor Cells.
Stem Cell Rev Rep, 19(6):2052-2072, 02 Jun 2023
Cited by: 0 articles | PMID: 37266894 | PMCID: PMC10390635
Go to all (297) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cysteine 64 of Ref-1 is not essential for redox regulation of AP-1 DNA binding.
Mol Cell Biol, 23(12):4257-4266, 01 Jun 2003
Cited by: 43 articles | PMID: 12773568 | PMCID: PMC156143
The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains.
Proc Natl Acad Sci U S A, 91(1):23-27, 01 Jan 1994
Cited by: 217 articles | PMID: 7506414 | PMCID: PMC42878
A murine AP-endonuclease gene-targeted deficiency with post-implantation embryonic progression and ionizing radiation sensitivity.
Mutat Res, 409(1):17-29, 01 Oct 1998
Cited by: 121 articles | PMID: 9806499
The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target.
Mol Aspects Med, 28(3-4):375-395, 03 May 2007
Cited by: 167 articles | PMID: 17560642
Review