Abstract
Free full text

MHC class II–dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies
Abstract
Whether B cells serve as antigen-presenting cells (APCs) for activation of pathogenic T cells in the multiple sclerosis model experimental autoimmune encephalomyelitis (EAE) is unclear. To evaluate their role as APCs, we engineered mice selectively deficient in MHC II on B cells (B–MHC II−/−), and to distinguish this function from antibody production, we created transgenic (Tg) mice that express the myelin oligodendrocyte glycoprotein (MOG)–specific B cell receptor (BCR; IgHMOG-mem) but cannot secrete antibodies. B–MHC II−/− mice were resistant to EAE induced by recombinant human MOG (rhMOG), a T cell– and B cell–dependent autoantigen, and exhibited diminished Th1 and Th17 responses, suggesting a role for B cell APC function. In comparison, selective B cell IL-6 deficiency reduced EAE susceptibility and Th17 responses alone. Administration of MOG-specific antibodies only partially restored EAE susceptibility in B–MHC II−/− mice. In the absence of antibodies, IgHMOG-mem mice, but not mice expressing a BCR of irrelevant specificity, were fully susceptible to acute rhMOG-induced EAE, also demonstrating the importance of BCR specificity. Spontaneous opticospinal EAE and meningeal follicle–like structures were observed in IgHMOG-mem mice crossed with MOG-specific TCR Tg mice. Thus, B cells provide a critical cellular function in pathogenesis of central nervous system autoimmunity independent of their humoral involvement, findings which may be relevant to B cell–targeted therapies.
Evidence supports roles for B cells and antibodies in the pathogenesis of multiple sclerosis (MS), a central nervous system (CNS) inflammatory demyelinating disease (von Büdingen et al., 2011). B cells and plasma cells are frequently observed in active inflammatory MS lesions, and myelin-specific antibodies have been detected in areas of CNS demyelination (Meinl et al., 2006). Identification of oligoclonal antibodies in cerebrospinal fluid is also used to confirm the diagnosis of MS (Blennow et al., 1994). Further, it has been suggested that the meningeal B cell follicles detected in progressive MS may contribute to detrimental humoral immunity (Magliozzi et al., 2007). Although it was the emphasis on humoral autoimmunity that provided the impetus to test B cell depletion in MS, the clinical benefit observed in recent clinical MS trials that tested anti-CD20 agents was not associated with reduction in serum or cerebrospinal fluid Ig titers, and oligoclonal antibodies were unchanged (Cross et al., 2006; Martin et al., 2009). Besides serving as the source for antibody-secreting plasma cells, B cells express MHC class II (MHC II) molecules constitutively, and via up-regulation of co-stimulatory molecules, they can participate as APCs for activation of antigen (Ag)-specific T cells (Lanzavecchia, 1985; van der Veen et al., 1992; Constant et al., 1995b). In this regard, reduction of proinflammatory Th17 cells has been observed in CD20-mediated B cell depletion in MS (Bar-Or et al., 2010). Together, these observations suggest that, independent of their potential humoral participation, there is prominent cellular involvement of B cells in MS pathogenesis.
Distinguishing the separate cellular and humoral contributions of B cells in the pathogenesis of MS, and in its model experimental autoimmune encephalomyelitis (EAE), has been challenging. As myelin-specific T cells are essential for initiation of CNS inflammation and clinical manifestations, EAE is T cell dependent (Zamvil and Steinman, 1990). B cells are not required in many EAE models, in particular those that are induced by encephalitogenic peptides of myelin proteins. In this regard, immunization with myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (p35–55) induces EAE in B cell–deficient mice and does not elicit strong humoral responses in WT mice (Fillatreau et al., 2002; Lyons et al., 2002). In contrast, immunization of WT mice with recombinant human MOG (rhMOG) protein induces B cell activation and production of anti-MOG antibodies, which are pathogenic only in association with T cell–mediated CNS inflammation (Lyons et al., 2002; Oliver et al., 2003; Marta et al., 2005). As B cell–deficient mice are resistant to EAE induced by immunization with rhMOG, this model is considered both T cell and B cell dependent. Therefore, this EAE model is well suited to evaluate the dual cellular and humoral roles of B cells in CNS autoimmunity. It is recognized that B cells are capable of processing native Ags and are very efficient APCs when they recognize the same Ag as the responding T cells (Constant et al., 1995a,b). When MOG-specific BCR knockin (IgHMOG-ki) mice (Litzenburger et al., 1998), which are capable of secreting all Ig isotypes, were crossed to MOG-specific TCR (TCRMOG) mice (Bettelli et al., 2003), the progeny developed a spontaneous form of EAE that affected primarily the optic nerves and spinal cord (Bettelli et al., 2006a; Krishnamoorthy et al., 2006). Spontaneous opticospinal EAE (OSE) was associated with development of MOG-specific Th17 responses and signs of B cell activation, findings suggesting a role for B cell APC function (Bettelli et al., 2006a; Krishnamoorthy et al., 2006). Meningeal B cell aggregates containing germinal center–like structures resembling the ectopic B cell follicles identified in the leptomeninges in secondary progressive MS (SPMS) patients were also observed in mice with spontaneous OSE (Bettelli et al., 2006a). Furthermore, IgG1 was elevated in IgHMOG-ki × TCRMOG mice (Bettelli et al., 2006a; Krishnamoorthy et al., 2006). Thus, similar to studies of MOG protein–induced EAE, the role of B cell APC function was not clearly distinguished from humoral immunity in OSE.
The goal of this investigation was to distinguish the role of APC function by B cells from the production and participation of myelin-specific antibodies in EAE pathogenesis. To this end, we used novel murine models that permitted us to evaluate requirements for B cell MHC II expression, BCR specificity and myelin-specific antibodies. First, we created mice that were selectively MHC II deficient on B cells (B–MHC II−/− mice). These B–MHC II−/− mice were susceptible to MOG p35–55–induced EAE. However, in comparison with mice containing B cells that expressed MHC II (B–MHC II+/+), which were susceptible to EAE induced by rhMOG, B–MHC II−/− mice were completely resistant to clinical or histological EAE, and they exhibited reduced Th1 and Th17 responses. Thus, B cell MHC II expression was required for EAE induction, which provided support for B cell APC function. Furthermore, administration of pathogenic MOG-specific antibodies only partially restored EAE susceptibility. To study B cell APC function in the absence of any possible contribution of antibodies, we created transgenic (Tg) mice containing B cells that expressed cell surface MOG-specific BCR but could not secrete antibodies. These MOG-specific “membrane-only” Tg (IgHMOG-mem)/B cell–deficient (JHT; IgHMOG-mem/JHT) mice were fully susceptible to acute EAE induced by rhMOG, although their chronic course was slightly milder than that in either WT or IgHMOG-ki mice. Spontaneous OSE was observed in the absence of MOG-specific antibodies when IgHMOG-mem/JHT mice were crossed to MOG-specific TCR (TCRMOG)/B cell–deficient (JHT; TCRMOG/JHT) mice. In comparison with OSE in IgHMOG-ki × TCRMOG mice, the onset of OSE was delayed and severity was less in IgHMOG-mem/JHT × TCRMOG/JHT mice. Our results demonstrate that in models that require participation of B cells, their cellular function is necessary and sufficient for induction of CNS autoimmunity.
RESULTS
B cell IL-6 production promotes proinflammatory T cell polarization in rhMOG-induced EAE
Immunization with rhMOG induces EAE in C57BL/6J WT mice (Fig. 1 A and Table 1), but not in B cell–deficient (JHT) mice (Fig. 1 B and Table 1) or in B cell–depleted mice (not depicted). In contrast, MOG p35–55 and recombinant mouse MOG (rmMOG) can induce EAE in either WT mice (Fig. 1 A) or B cell–deficient mice (Fig. 1 B; Hjelmström et al., 1998; Fillatreau et al., 2002; Oliver et al., 2003). Thus, only rhMOG-induced EAE is considered B cell dependent (Lyons et al., 1999; Oliver et al., 2003; Marta et al., 2005).
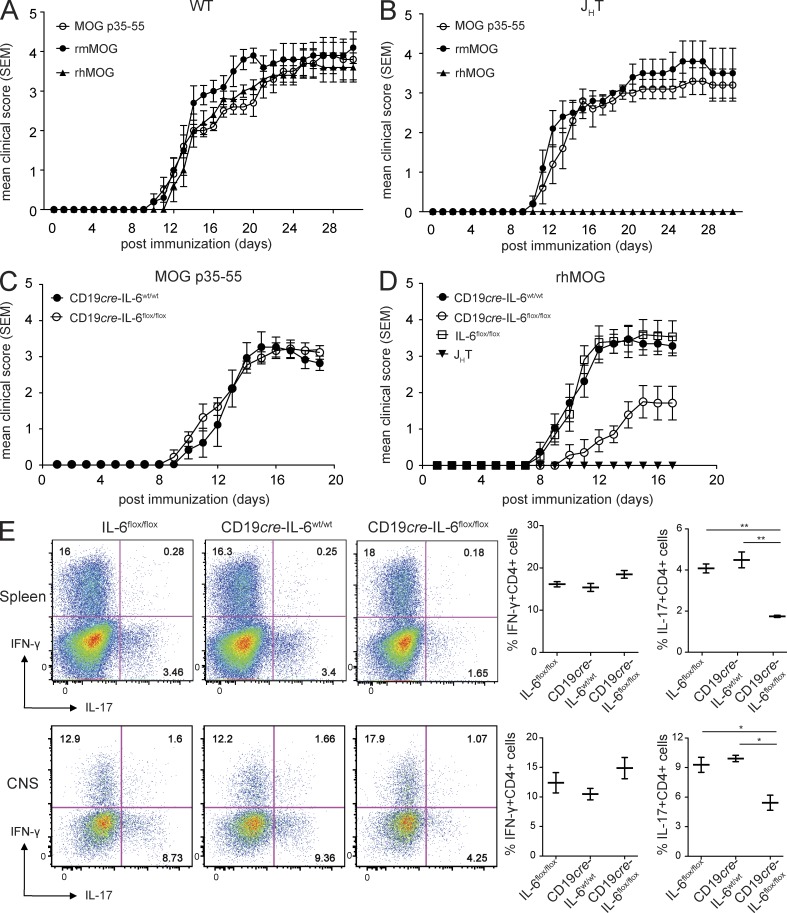
B cell IL-6 production was required for induction of EAE by rhMOG. (A and B) EAE was induced in C57BL/6J WT (A) or B cell–deficient JHT mice (B) by injecting MOG p35–55 (50 µg s.c.), rmMOG (100 µg s.c.), or rhMOG (100 µg s.c.) emulsified in CFA along with heat-killed Mtb (200 µg) and B. pertussis toxin (200 ng i.v.) on days 0 and 2, and survival was monitored. Results shown are representative of five independent experiments of n = 5 mice/group. (C and D) EAE was induced in mice harboring IL-6–deficient B cells (CD19cre-IL-6flox/flox) or WT B cells (CD19cre–IL-6wt/wt or IL-6flox/flox) or mice lacking B cells (JHT) with MOG p35–55 or rhMOG as in A, and survival was monitored. (E) Lymphocytes were isolated from the spleen and CNS of the indicated mouse strains after disease onset and stimulated with PMA + ionomycin for 6 h, and IL-17 and IFN-γ production was assessed by flow cytometry. Flow cytometry plots from a representative mouse are shown (left), and graphs (right) show quantitative data from five to six mice/group. Results are representative of two independent experiments (n = 5 mice/group [C]; n = 7–8 mice/group [D]). For all EAE experiments, mean disease score ± SEM is shown. For all experiments: *, P < 0.05; **, P < 0.01 by Mann–Whitney U test.
Table 1.
Evaluation of clinical signs and histological EAE in WT, B cell–deficient (JHT), B–MHC II+/+, and B–MHC II−/− mice
| Mice and Ag | Incidence | Onset | Mean maximal clinical score | Cumulative score | Mean number of foci (±SEM)a | ||
| Meninges | Parenchyma | Total | |||||
| WT | |||||||
| MOG p35–55 | 5/5 | 11.8 (±0.6) | 3.5 (±0.5) | 295.0 | 126 (±69) | 137 (±69) | 263 (±138) |
| rmMOG | 5/5 | 12.0 (±0.6) | 4.0 (±0.3) | 336.5 | 122 (±42) | 149 (±45) | 271 (±87) |
| rhMOG | 5/5 | 12.8 (±0.5) | 3.5 (±0.4) | 291.0 | 154 (±47) | 150 (±10) | 304 (±57) |
| JHT | |||||||
| MOG p35–55 | 5/5 | 11.8 (±0.7) | 3.5 (±0.3) | 288.0 | 98 (±25) | 128 (±55) | 226 (±80) |
| rmMOG | 5/5 | 11.2 (±0.4) | 3.9 (±0.5) | 325.0 | 103 (±18) | 125 (±24) | 228 (±42) |
| rhMOG | 0/5 | - | 0.0 (±0.0) | 0.0 | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) |
| B–MHC II+/+ | |||||||
| MOG p35–55 | 5/5 | 8.8 (±1.3) | 3.2 (±0.3) | 113.5 | 178 (±13) | 147 (±8) | 325 (±21) |
| rmMOG | 5/5 | 8.4 (±0.5) | 3.4 (±0.2) | 124.0 | 151 (±43) | 131 (±19) | 282 (±62) |
| rhMOG | 5/5 | 8.2 (±0.8) | 3.7 (±0.2) | 136.5 | 109 (±17) | 148 (±27) | 257 (±34) |
| B–MHC II−/− | |||||||
| MOG p35–55 | 5/5 | 10.2 (±0.8) | 2.9 (±0.2) | 95.0 | 142 (±22) | 177 (±43) | 319 (±65) |
| rmMOG | 5/5 | 8.8 (±0.4) | 2.8 (±0.1) | 95.5 | 107 (±33) | 87 (±7) | 194 (±40) |
| rhMOG | 0/5 | - | 0.0 (±0.0) | 0.0 | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) |
Onset indicates mean day of disease onset (paralytic EAE) ± SEM. Clinical score shows mean maximal score of paralytic EAE of diseased mice ± SEM. Cumulative score shows sum of the daily scores of all animals from day 0 until the end of the experiment. Results are representative of five separate experiments (five mice/group/experiment).
When serving as APCs, B cells can produce proinflammatory cytokines that promote development and activation of encephalitogenic T cells (Weber et al., 2010). As IL-6 has a key role in Th17 differentiation (Okuda et al., 1999; Bettelli et al., 2006b), we first addressed whether selective IL-6 production by B cells is required in rhMOG-induced EAE. No difference in disease course was observed between mice containing selective B cell IL-6 deficiency (CD19cre–IL-6flox/flox) and control (CD19cre–IL-6wt/wt) mice that were immunized with MOG p35–55 (Fig. 1 C). In contrast, when immunized with rhMOG, mice containing IL-6–deficient B cells developed less severe EAE in comparison with control (CD19cre–IL-6wt/wt and IL-6flox/flox) mice (Fig. 1 D) and exhibited reduced IL-17 production in both the periphery and the CNS (Fig. 1 E). These results confirmed the importance of B cell IL-6 production in a model of B cell–dependent CNS autoimmunity.
B cell MHC II expression is required for rhMOG-induced EAE
Although cytokine production by accessory cells can promote proinflammatory T cell polarization, expression of MHC II on APCs is considered an obligate requirement for presentation and activation of Ag-specific CD4+ T cells (Schwartz, 1985). Thus, to determine the role of Ag presentation by B cells in EAE, we constructed mice containing MHC II–deficient B cells. Irradiated WT mice were reconstituted with mixed BM cells in a ratio of 80% JHT and 20% MHC II−/− (B–MHC II−/−) or WT (B–MHC II+/+) BM cells. Thus, in B–MHC II−/− mice, MHC II deficiency was restricted to B cells, whereas other APCs, including DCs and monocytes, expressed MHC II (Fig. 2 A). The frequencies of B cells and CD4+ and CD8+ T cells were equivalent in BM chimeras containing either MHC II−/− or MHC II+/+ B cells (Fig. 2 A). As shown in Fig. 2 B, BM chimera mice containing MHC II+/+ B cells developed EAE in response to immunization with rhMOG. In contrast, BM chimera mice containing MHC II–deficient B cells were resistant to clinical or histological EAE induction by rhMOG (Fig. 2 B and Table 1), although, like B cell–deficient JHT or WT mice, BM chimera mice selectively deficient of MHC II on B cells did develop EAE in response to MOG p35–55 or rmMOG. Thus, EAE induction by rhMOG requires B cell MHC II expression.
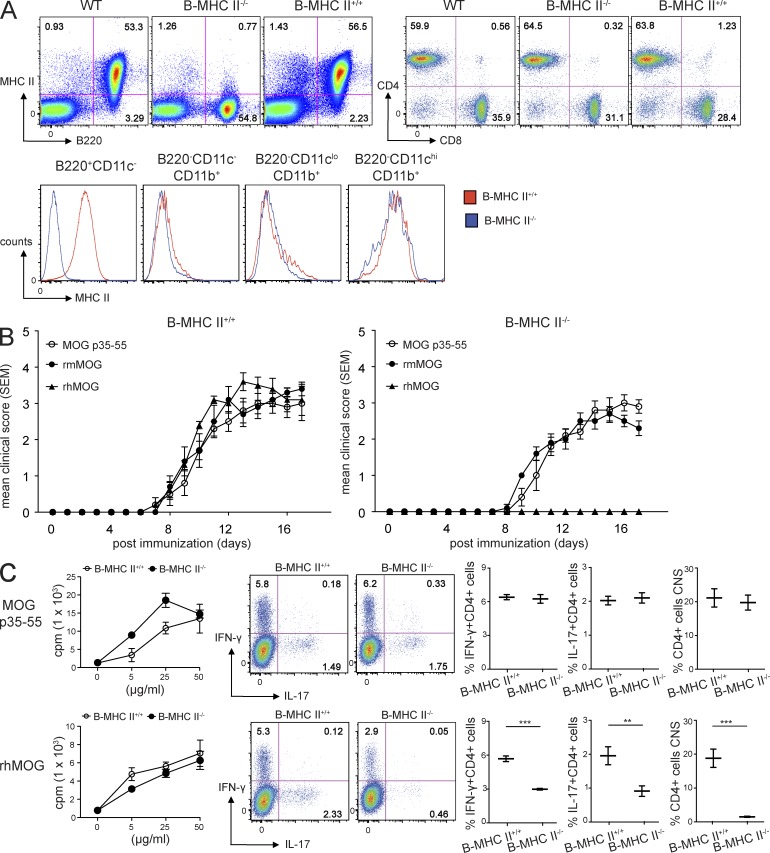
B cell MHC II expression promoted proinflammatory T cell cytokine production in rhMOG-induced EAE. Mixed BM chimera mice containing MHC II–deficient B cells were generated by reconstituting C57BL/6J (WT) mice with mixed BM from JHT + MHC II–deficient mice (MHC II−/−) mice. (A) B and T cell reconstitution of secondary lymphoid organs was evaluated by FACS analysis of spleen cells 6–8 wk after BM transplantation. Panels show frequencies of MHC II–expressing cells (left) and CD4+ and CD8+ cells (right) from B–MHC II−/− and B–MHC II+/+ chimeras. FACS plots and histograms are representative of five independent experiments (n = 3 mice/group). (B) EAE was induced in B–MHC II+/+ or B–MHC II−/− chimeras by injecting MOG p35–55 (50 µg s.c.), rmMOG (100 µg s.c.), or rhMOG (100 µg s.c.) emulsified in CFA along with heat-killed Mtb (200 µg) and B. pertussis toxin (200 ng i.v.) on days 0 and 2. Survival was monitored daily. Results shown are representative of five independent experiments of n = 5 mice/group. Mean disease score ± SEM is shown. (C) Analyses were performed 14 d after immunization. Spleen cells of B–MHC II+/+ and B–MHC II−/− mice were cultured with the indicated concentrations of MOG p35–55 or rhMOG for 48 h, and T cell proliferation was measured 18 h later by incorporation of [3H]thymidine (cpm; left). Data are presented as means of triplicate values ± SD and are representative of at least three independent experiments. Single-cell suspensions from spleens and CNS of the indicated mice were stimulated with PMA + ionomycin for 6 h, and frequencies of IL-17+ CD4+ Th17 and IFN-γ+ CD4+ Th1 cells were assessed by flow cytometry. Representative FACS plots and quantification (mean ± SD) of n = 5 mice/group are shown (middle and right). For all experiments: **, P < 0.01; ***, P < 0.001 by Mann–Whitney U test.
Next, we examined how deficiency of MHC II on B cells influenced T cell activation and proinflammatory polarization in EAE induced by MOG p35–55 or rhMOG. Selective B cell MHC II deficiency did not influence proliferative responses to either Ag (Fig. 2 C), presumably because MHC II–competent myeloid cells also can process and present Ags. The contribution of MHC II expression by B cells in the development of pathogenic cells in vivo was also evaluated. No significant differences were observed in peripheral Th1 or Th17 responses in MOG p35–55–immunized BM chimera mice containing either MHC II+/+ or MHC II−/− B cells. Also, there were no differences in the frequency of CNS-infiltrating T cells or the number of CNS infiltrates in these mice (Fig. 2 C and Table 1). In contrast, in response to immunization with rhMOG, the frequency of splenic CD4+ T cells expressing IFN-γ and IL-17A was significantly decreased in B–MHC II−/− chimera mice. Furthermore, CNS inflammation was not detected in mice completely lacking MHC II only on B cells. Therefore, MHC II expression on B cells is critical for pathogenic T cell responses in rhMOG-induced EAE.
MOG-reactive antibodies partially restore EAE susceptibility to rhMOG in B–MHC II−/− mice
Antibodies specific for rhMOG are known to be pathogenic (Marta et al., 2005). Although MHC II expression is required for B cell APC function, data indicate that MHC II is also necessary for germinal center formation and subsequent formation of long-lived plasma cells in T cell–dependent responses (Shimoda et al., 2006). Thus, we analyzed production of MOG-specific antibodies in our B–MHC II−/− mice. Consistent with previous findings, MOG-specific antibodies were produced in control (B–MHC II+/+) mice in response to rhMOG and rmMOG, but not to MOG p35–55 (Weber et al., 2010). In contrast, anti-MOG IgG was not detected in BM chimera mice containing MHC II–deficient B cells (Fig. 3 A). Interestingly, these data are consistent with the initial work that demonstrated the importance of MHC II in humoral immunity (McDevitt et al., 1972). Absence of MHC II–expressing B cells was also associated with reduction of T follicular helper cells (Tfh cells; Deenick et al., 2010), a specialized T cell subset which provides help for B cell maturation and isotype switching (Fig. 3 B; Vinuesa et al., 2005; Deenick et al., 2010). As B–MHC II−/− mice did not produce anti-MOG IgG, we tested whether antibodies could restore EAE susceptibility after immunization with rhMOG. Whereas transfer of isotype control OVA-specific mAb had no effect on EAE in B–MHC II−/− mice immunized with rhMOG (Fig. 3 C and Table 2), administration of pathogenic anti-MOG mAb, 8-18C5, led to development of mild clinical disease, but did not restore EAE severity to the level observed in B–MHC II+/+ mice. Together, these results emphasize that the humoral contribution of B cells is insufficient to compensate for the lack of MHC II–dependent cellular function in MOG protein–induced EAE.
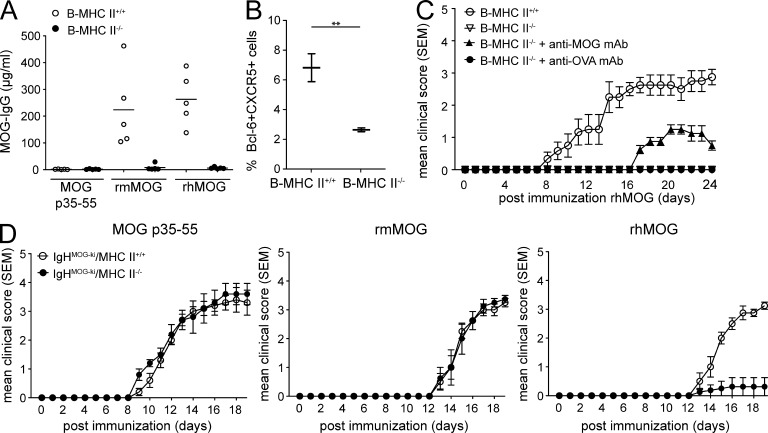
MOG-specific antibodies partially restored susceptibility to rhMOG-induced EAE in B–MHC II−/− mice. (A) Sera were obtained from B–MHC II−/− and B–MHC II+/+ mice on day 14 after immunization with MOG p35–55, rmMOG, or rhMOG. Total anti-MOG IgG levels (µg/ml) determined by ELISA (1:1,000 dilution) are shown (n = 5 mice/group). Horizontal bars indicate mean. (B) Numbers indicate the percentages of splenic Bcl-6+CXCR5+ Tfh cells (pregated on CD4+CD44+ICOS+PD-1+ T cells) evaluated by intracellular FACS staining 14 d after EAE induction with rhMOG. (C) B–MHC II−/− and B–MHC II+/+ mice were immunized with rhMOG (n = 4–6/mice/group), and clinical scores were monitored at the indicated days after immunization. When B–MHC II+/+ mice showed EAE signs (score ~2.0), B–MHC II−/− mice received i.p. injections of 150 µg of either anti-MOG 8-18C5 mAb (closed triangles) or an equal amount of anti-OVA 1B7.11 mAb (closed circles) three times at 48-h intervals. Results are representative of two independent experiments. In one experiment, sera from IgHMOG-ki mice and non-Tg littermate mice were used in lieu of 8-18C5 and 1B7.11 mAbs. (D) BM chimera mice with MHC II deficiency restricted to MOG-specific B cells from IgHMOG-ki mice were generated by reconstituting C57BL/6J mice with mixed BM from JHT + IgHMOG-ki mice or JHT + IgHMOG-ki mice backcrossed onto the MHC II−/− background. Chimera mice with MOG-specific MHC II–deficient B cells (IgHMOG-ki/MHC II−/−) or MOG-specific MHC II–competent B cells (IgHMOG-ki/MHC II+/+) were immunized with MOG p35–55, rmMOG, or rhMOG (n = 5 mice/group), and clinical scores were monitored at the indicated days after immunization. Data shown are representative of four independent experiments. Mean disease score ± SEM is shown for all experiments displayed. For all experiments: **, P < 0.01 by Mann–Whitney U test.
Table 2.
Passive transfer of MOG-specific antibodies partially restored EAE susceptibility in B–MHC II−/− mice
| Ag | Incidence | Onset | Mean maximal clinical score | Mean clinical score day 12 | Mean clinical score day 18 | Mean clinical score day 24 | Cumulative score |
| B–MHC II+/+ | |||||||
| rhMOG | 6/6 | 15.0 (±1.2) | 2.5 (±0.4) | 1.3 (±0.5) | 2.6 (±0.3) | 2.9 (±0.2) | 150.0 |
| B–MHC II−/− | |||||||
| rhMOG | 0/4 | - | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 |
| +anti-MOG mAb | 4/4 | 3.0 (±0.0) p.i. | 1.0 (±0.1) | 0.0 (±0.0) | 0.9 (±0.1) | 0.9 (±0.1) | 32.0 |
| +anti-OVA mAb | 0/4 | - | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 |
p.i., post injection. Onset indicates mean day of disease onset (paralytic EAE) ± SEM. Clinical score shows mean maximal score of paralytic EAE of diseased mice ± SEM. Mean clinical score shows mean score of paralytic EAE of disease mice ± SEM. Cumulative score shows sum of the daily scores of all animals from day 0 until the end of the experiment.
Besides serving as APCs or a source of autoantibodies, data indicate that through their cell surface–specific BCRs, B cells can transfer their target Ag to other professional APCs, a process which may be MHC II independent (Harvey et al., 2007). Thus, we addressed whether MOG-specific B cells contribute to MOG protein–induced EAE independent of their expression of MHC II molecules. BM chimera mice containing only IgHMOG-ki B cells that expressed MHC II (IgHMOG-ki/MHC II+/+) were susceptible to EAE induced by MOG p35–55, rmMOG, or rhMOG (Fig. 3 D). In contrast, BM chimera mice containing MHC II–deficient IgHMOG-ki B cells (IgHMOG-ki/MHC II−/−) were susceptible to EAE induced by MOG p35–55 or rmMOG but not to EAE induced by rhMOG (Fig. 3 D). Therefore, these results do not support BCR-mediated Ag exchange in rhMOG-induced EAE.
Generation of IgHMOG-mem Tg mice
In the experimental system we used thus far, disruption of cellular function (i.e., MHC II deficiency) of B cells was accompanied by a reduction in humoral autoimmunity. It was therefore important to generate a model that would permit examination of the role of MHC II–competent MOG-specific B cells without possible contribution of antibodies. Tg mice containing B cells that express membrane IgH only have been useful for studying APC function in systemic and organ-specific autoimmune diseases (Chan et al., 1999; Wong et al., 2004). Thus, we created Tg mice containing B cells that express membrane MOG-specific Ig (IgHMOG-mem) but are incapable of secreting Ig. We subcloned the rearranged heavy chain Ig gene from 8-18C5, the same gene used to create the IgHMOG-ki mice, which are capable of secreting all Ig isotypes, into a construct containing the IgMa constant region from which the exon encoding for secretion was excised (Fig. 4 A; see Materials and methods; Chan et al., 1999). Mice bearing the IgHMOG-mem Tg were backcrossed onto the B cell–deficient JHT strain (IgHMOG-mem/JHT; Chen et al., 1993) to prevent the development of B cells expressing endogenous Ig. More than 50% of Tg B cells in the founder line #3088, which was chosen for most of our subsequent experiments, bound MOG protein similarly to B cells in other founder lines and in IgHMOG-ki mice (Fig. 4 B and not depicted). B cells in those Tg mice also expressed MHC II comparably with IgHMOG-ki and WT mice (Fig. 4 C). B cells in IgHMOG-mem/JHT Tg mice expressed cell surface IgM only (not depicted). Comparable frequencies of CD4+, CD8+, naive, and effector/memory T cells were detected in IgHMOG-mem/JHT, WT, and IgHMOG-ki mice (not depicted).
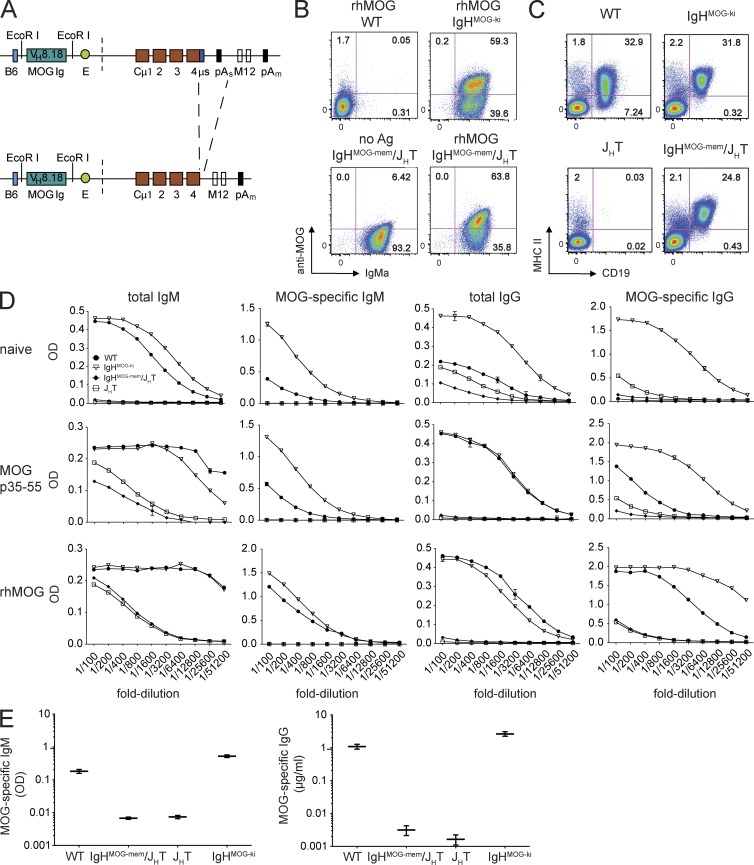
Generation and characterization of IgHMOG-mem Tg mice. (A) Schematic representation of the construct used to generate IgHMOG-mem Tg mice. The DNA directing secretion (µs) and the transcription termination site (pAs) have been deleted (Chan et al., 1999). VH8.18 is the rearranged heavy chain V(D)J gene segment from the hybridoma 8-18C5 (Linnington et al., 1984), which was used to generate IgHMOG-ki mice (Litzenburger et al., 1998). 8.18-C5 is known to recognize rhMOG1–125 (Menge et al., 2007). E indicates the heavy chain intronic enhancer. Much of the switch region was deleted; a small segment of the residual switch region is indicated. (B) Spleen cells of WT, IgHMOG-ki, JHT, and IgHMOG-mem/JHT mice were analyzed for surface expression of IgMa and binding of rhMOG by flow cytometry (gated on B220+ cells). Percentages of rhMOG-binding or -nonbinding IgMa-positive B cells are indicated. (C) Spleen cells from WT, JHT, IgHMOG-ki, and IgHMOG-mem/JHT mice were analyzed by FACS for CD19 and MHC II expression. Data shown in B and C are representative of five independent experiments. (D) Sera from individual naive and immunized (MOG p35–55, rhMOG) WT, JHT, IgHMOG-ki, and IgHMOG-mem/JHT mice were obtained on day 14 after immunization, and serum Ig levels were calculated. Total and MOG-specific IgM and IgG titers are expressed as mean OD values ± SD from one of three representative experiments (performed in triplicate) from 1:2 serial dilutions. (E) Quantification of MOG-specific IgM and IgG (n = 6 mice/group) determined by ELISA are expressed as means of OD values or µg/ml ± SD of one of three representative experiments (performed in triplicate), respectively, and are shown on the logarithmic y axis.
The absence of secreted antibody in IgHMOG-mem/JHT mice was confirmed in separate analyses of total and MOG-specific IgM, as well as total and MOG-specific IgG in naive, MOG p35–55–immunized, and MOG protein–immunized mice (Fig. 4, D and E). These antibodies were undetectable or observed only in trace amounts in IgHMOG-mem/JHT mice and were never significantly more than in B cell–deficient mice. In general, antibodies were 102–103 times lower than WT controls. In contrast, naive and immunized IgHMOG-ki mice secreted much higher concentrations of MOG-specific IgM and IgG.
MOG-specific B cells, but not antibodies, are required for induction of B cell–dependent EAE
IgHMOG-mem/JHT mice were tested for susceptibility to EAE by immunization with MOG p35–55, rmMOG, and rhMOG. Onset, severity, and disease course in IgHMOG-mem/JHT, WT, and IgHMOG-ki mice immunized with either MOG p35–55 or rmMOG were similar (Fig. 5 A and Table 3). Onset and initial clinical severity were also comparable when these mice were immunized with rhMOG. However, the IgHMOG-mem/JHT mice appeared to have a slightly milder disease course after the acute stage, although this difference was not statistically significant. A similar EAE course was observed in another IgHMOG-mem/JHT Tg founder line after immunization with rhMOG (not depicted). There were no obvious differences in the extent of meningeal or parenchymal CNS infiltration among any of these mice with EAE (Fig. 5 B and Table 3).
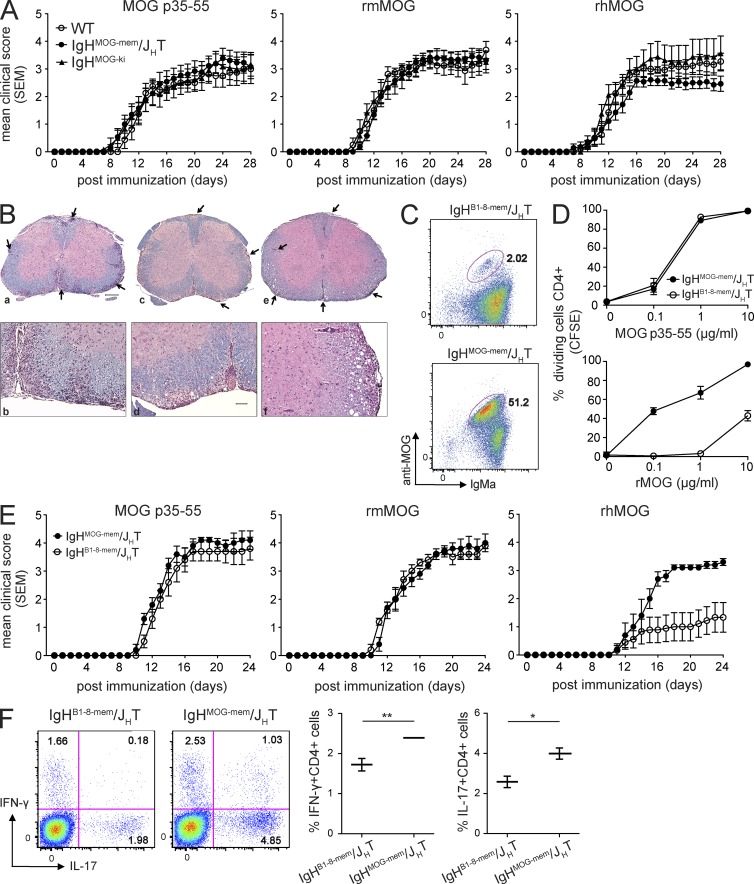
Myelin-specific BCR contributed to B cell APC function and susceptibility to CNS autoimmune disease independent of antibodies. (A) EAE was induced in WT, IgHMOG-mem/JHT, or IgHMOG-ki mice by injecting MOG p35–55 (50 µg s.c.), rmMOG (100 µg s.c.), or rhMOG (100 µg s.c.) emulsified in CFA along with 200 µg heat-killed Mtb and B. pertussis toxin (200 ng i.v.) on days 0 and 2, and survival was monitored. Composite disease course of three experiments is shown (n = 3–6 mice per group/experiment). Mean disease score ± SEM is shown. (B) Spinal cord tissues from mice immunized with rhMOG were collected on day 28, fixed in neutral-buffered formalin, processed routinely, and embedded in paraffin (a, b: WT; c, d: IgHMOG-ki; e, f: IgHMOG-mem/JHT). 5-µm sections were processed and stained with H&E (for assessment of inflammation) and LFB (for demyelination). Meningeal and parenchymal inflammatory/demyelinating foci are indicated by arrows. Bars: (a, c, and e) 200 µm; (b, d, and f) 50 µm. (C) Splenic B cells (B220+) from IgHMOG-mem/JHT and IgHB1-8-mem/JHT mice were stained for cell surface IgMa, and capability to bind MOG protein and then analyzed by flow cytometry. (D) Purified CD4+ T cells from TCRMOG mice were stained with CFSE and cultured with purified B cells from IgHMOG-mem/JHT or IgHB1-8-mem/JHT mice in the presence of various concentrations of MOG p35–55 or rhMOG. T cell proliferation (CFSE dilution) was analyzed by FACS. Data are presented as means of triplicate values ± SD and are representative of two independent experiments. (E) EAE was induced in IgHMOG-mem/JHT or IgHB1-8-mem/JHT mice (n = 5 mice/group) as in A. Survival was monitored daily. Clinical EAE scores are shown as mean ± SEM. EAE data shown are representative of five independent experiments. (F) Phenotype of splenic T cells was evaluated by intracellular FACS staining for IL-17A and IFN-γ (gated on CD4+ T cells) 14 d after disease induction. Representative FACS plots (left) and quantification (mean ± SD; right; n = 5 mice/group) are shown. For all experiments: *, P < 0.05; **, P < 0.01 by Mann–Whitney U test.
Table 3.
Evaluation of clinical signs and histological EAE in WT, IgHMOG-mem/JHT, and IgHMOG-ki mice
| Ag and mice | Incidence | Onset | Mean maximal clinical score | Mean number of foci (±SEM)a | ||
| Meninges | Parenchyma | Total | ||||
| MOG p35–55 | ||||||
| WT | 15/15 | 11.5 (±0.4) | 3.6 (±0.3) | 238 (±53) | 203 (±39) | 441 (±92) |
| IgHMOG-mem/JHT | 18/18 | 11.4 (±1.1) | 3.7 (±0.3) | 117 (±23) | 117 (±24) | 234 (±47) |
| IgHMOG-ki | 15/15 | 11.6 (±1.2) | 3.8 (±0.2) | 118 (±28) | 138 (±23) | 256 (±51) |
| rmMOG | ||||||
| WT | 15/15 | 11.3 (±0.6) | 3.9 (±0.2) | 160 (±33) | 199 (±41) | 339 (±74) |
| IgHMOG-mem/JHT | 18/18 | 11.4 (±0.2) | 3.9 (±0.2) | 189 (±18) | 199 (±12) | 388 (±30) |
| IgHMOG-ki | 15/15 | 11.8 (±0.8) | 3.8 (±0.3) | 137 (±16) | 145 (±24) | 282 (±30) |
| rhMOG | ||||||
| WT | 18/18 | 11.8 (±0.5) | 3.7 (±0.2) | 154 (±47) | 150 (±10) | 304 (±57) |
| IgHMOG-mem/JHT | 18/18 | 11.8 (±0.7) | 3.0 (±0.2) | 118 (±28) | 139 (±23) | 257 (±51) |
| IgHMOG-ki | 17/18 | 10.4 (±0.4) | 4.2 (±0.3) | 146 (±25) | 139 (±18) | 285 (±43) |
Onset indicates mean day of disease onset (paralytic EAE) ± SEM. Clinical score shows mean maximal score of paralytic EAE of diseased mice ± SEM. Composite data from three independent experiments with five to six mice per group are indicated.
Data from our IgHMOG-mem/JHT mice demonstrated that it was the cellular and not the humoral contribution that was required for development of acute disease in B cell–dependent EAE. We therefore examined the role of BCR specificity by comparing EAE induction in our IgHMOG-mem/JHT mice with Tg mice containing B cells that express a fixed cell surface IgH specific for nitrophenyl, an irrelevant Ag. Membrane-only nitrophenyl-specific IgH (B1-8; IgHB1-8-mem/JHT) mice differ from IgHMOG-mem/JHT mice only in the specificity of their rearranged heavy chain gene (Chan et al., 1999). In comparison with B cells from IgHMOG-mem/JHT, a much lower frequency of B cells from IgHB1-8-mem/JHT were capable of binding rhMOG (Fig. 5 C). As anticipated, B cells from IgHB1-8-mem/JHT mice could present MOG p35–55 to MOG-specific 2D2 cells but were inefficient in presentation of MOG protein (Fig. 5 D), which requires processing (Slavin et al., 2001; Bettelli et al., 2003). Like IgHMOG-mem/JHT mice, IgHB1-8-mem/JHT mice were susceptible to EAE induced by MOG p35–55 or rmMOG, independent of BCR specificity. However, IgHB1-8-mem/JHT mice developed a much milder EAE course than IgHMOG-mem/JHT mice after immunization with rhMOG (Fig. 5 E), which corresponded to a reduction in MOG-specific proinflammatory Th1 and Th17 cytokine responses in rhMOG-induced EAE (Fig. 5 F). These results clearly support an important role for CNS auto-Ag BCR specificity in B cell APC function in vivo.
MOG-specific T and B cells cooperate in development of spontaneous OSE in the absence of MOG-specific antibodies
It was previously observed when TCRMOG mice were crossed with IgHMOG-ki mice, the progeny developed spontaneous paralysis, which was associated with meningeal and parenchymal inflammatory lesions within the optic nerves and spinal cord (OSE; Bettelli et al., 2006a; Krishnamoorthy et al., 2006), a lesion topography reminiscent of neuromyelitis optica. OSE was also associated with elevation in MOG-specific IgG1, and as IgHMOG-ki B cells can also serve as APCs, it was not clear whether it was their potential role as APCs or production of MOG-specific autoantibodies that promoted development of OSE. Thus, to evaluate the participation of MOG-specific B cells, independent of MOG-specific antibodies, we crossed IgHMOG-mem/JHT with TCRMOG/JHT mice and evaluated their progeny for spontaneous development of OSE. 55% of IgHMOG-mem/JHT × TCRMOG/JHT mice and 78% of IgHMOG-ki × TCRMOG developed paralysis spontaneously (Table 4). In contrast, spontaneous paralysis was not observed in IgHB1-8-mem/JHT × TCRMOG/JHT, TCRMOG/JHT, or IgHMOG-ki mice. Onset of paralysis occurred earlier and the clinical severity was greater in IgHMOG-ki × TCRMOG mice than in IgHMOG-mem/JHT × TCRMOG/JHT mice, possibly reflecting participation of MOG-specific antibodies in the manifestation of OSE. Interestingly, the numbers of inflammatory lesions were similar in both IgHMOG-mem/JHT × TCRMOG/JHT and IgHMOG-ki × TCRMOG mice. Prominent meningeal and parenchymal inflammatory foci with demyelination and formation of meningeal lymphoid follicle-like aggregates (Fig. 6, A–E) were observed in IgHMOG-mem/JHT × TCRMOG/JHT mice, as was described previously in IgHMOG-ki × TCRMOG mice with spontaneous OSE (Bettelli et al., 2006a), and resembled similar structures observed in SPMS (Magliozzi et al., 2007). Meningeal foci in IgHMOG-mem/JHT × TCRMOG/JHT mice were composed predominantly of B cells that infiltrate the parenchyma (Fig. 6 C). Relatively fewer meningeal and parenchymal T cells were detected in these areas (Fig. 6 D). Follicle-like organization was evident using a reticulin stain (Fig. 6 E). Meningeal inflammation and follicle-like structures were not detected in clinically normal IgHMOG-mem/JHT × TCRMOG/JHT mice (Fig. 6, F–J). Our observations indicate that the APC function of MOG-specific B cells is sufficient to cooperate with MOG-specific T cells in development of OSE and further suggest that in the context of CNS inflammation, anti-MOG antibodies augment phenotypic expression of this spontaneous disease.
Table 4.
IgHMOG-mem/JHT × TCRMOG/JHT mice developed spontaneous EAE
| Mice | Incidence | Onset | Mean maximal clinical score | Mean number of foci (±SEM)a | ||
| Meninges | Parenchyma | Total | ||||
| % | ||||||
| IgHMOG-mem/JHT × TCRMOG/JHT | 55 (21/38) | 60 (±4) | 2.9 (±0.2) | 43 (±25) | 55 (±18) | 98 (±43) |
| IgHB1-8-mem/JHT × TCRMOG/JHT | 0 (0/22) | n/a | 0.0 | 0.0 | 0.0 | 0.0 |
| TCRMOG/JHT | 0 (0/52) | n/a | 0.0 | 0.0 | 0.0 | 0.0 |
| IgHMOG-mem/JHT | 0 (0/56) | n/a | 0.0 | 0.0 | 0.0 | 0.0 |
| IgHMOG-ki | 0 (0/45) | n/a | 0.0 | 0.0 | 0.0 | 0.0 |
| IgHMOG-ki × TCRMOG | 78 (38/49) | 38 (±2) | 3.8 (±0.1) | 60 (±14) | 51 (±14) | 111 (±24) |
n/a, not applicable. Onset indicates mean day of disease onset (paralytic EAE) ± SEM. Clinical score shows mean maximal score of paralytic EAE of diseased mice ± SEM.
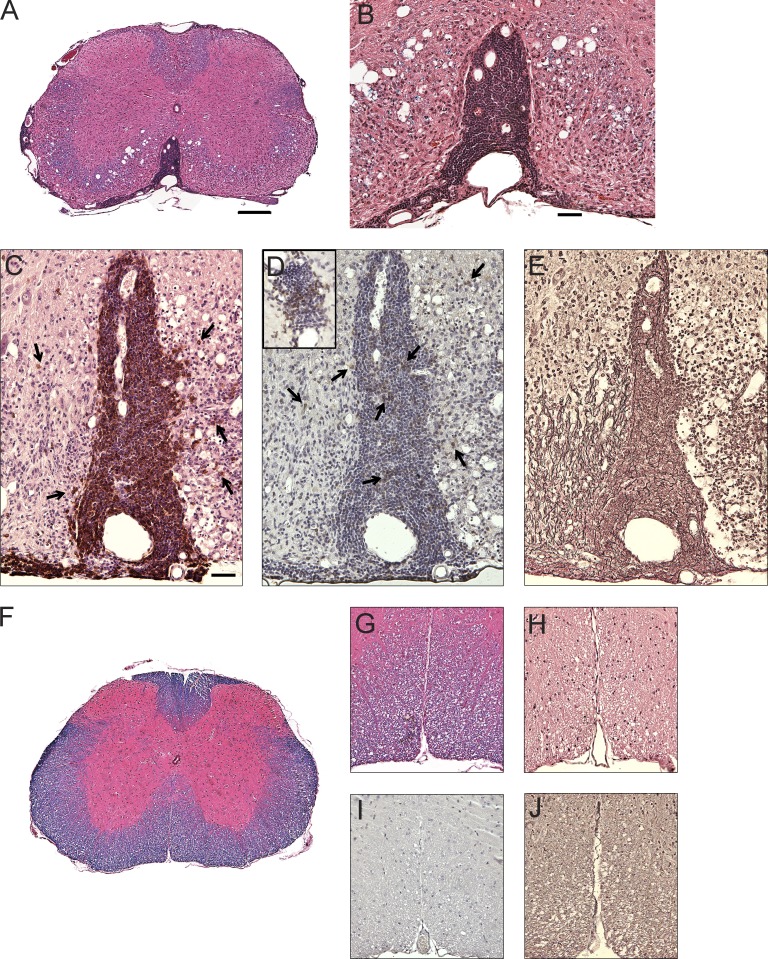
Meningeal B cell follicle–like aggregates were observed in IgHMOG-mem/JHT × TCRMOG/JHT mice that developed spontaneous OSE. (A–E) Spinal cords of IgHMOG-mem/JHT × TCRMOG/JHT mice that developed spontaneous OSE (A and B). (F–J) Spinal cord of a clinically normal IgHMOG-mem/JHT × TCRMOG/JHT mouse. (C and H) Immunostaining for B cells (CD45R). (C) Arrows indicate meningeal foci that contained B cells that infiltrated the parenchyma. (D and I) Immunostaining for T cells (CD3). (D) Arrows and inset show meningeal and parenchymal T cells. (E and J) Reticulin stain. (A, B, F, G) LFB/H&E; (C and H) H&E counterstain; (D and I) hematoxylin counterstain; (E and J) reticulin stain. Histological analysis of IgHMOG-mem × 2D2/JHT mice with and without spontaneous OSE is representative of three independent experiments with at least three mice per group. The entire CNS was sampled in a single slide for each mouse. Bars: (A and F) 200 µm; (B and G–J) 50 µm; (C–E) 70 µm; (D, inset [same bar as in C]) 35 µm.
DISCUSSION
Recent MS trials indicate that B cell–depleting agents have a profound beneficial effect, underscoring the importance of B cells in MS pathogenesis (Bar-Or et al., 2008; Hauser et al., 2008; Marriott and O’Connor, 2010; Kappos et al., 2011). Although it was the appreciation of the potential humoral contribution of B cells that inspired testing B cell depletion in MS, the clinical response was not associated with reduction in antibody levels (Bar-Or et al., 2008; Hauser et al., 2008; Marriott and O’Connor, 2010; Kappos et al., 2011). B cells afford multiple functions. Besides serving as a source of antibody-producing plasma cells, B cells can participate as APCs (Lanzavecchia, 1985; Constant et al., 1995a,b; Chan et al., 1999) in Ag transport (Phan et al., 2007) and as regulatory cells (Matsushita et al., 2008). Although MHC II is expressed constitutively on B cells, it is unknown whether B cell Ag presentation may be required for activation of pathogenic CNS auto-Ag–specific T cells in vivo in EAE pathogenesis. In this investigation, we have created models that permitted us to evaluate and distinguish the role of B cells as APCs in vivo from their contribution to humoral CNS autoimmunity. Our results clearly demonstrate how B cell MHC II expression can be pivotal for activation of proinflammatory Th1 and Th17 cells in vivo and susceptibility to CNS autoimmunity. By generation of Tg mice that expressed cell surface myelin-specific BCR, we provided unequivocal evidence that B cell APC function, in the absence of humoral autoimmunity, is sufficient to drive proinflammatory T cell activation in vivo and EAE pathogenesis. Although BCR specificity for MOG protein promoted activation of encephalitogenic proinflammatory T cells, expression of this myelin-specific BCR was insufficient to drive proinflammatory T cell differentiation in the absence of B cell MHC II molecules (Fig. 3 D), providing further evidence for the MHC II-dependent APC participation of B cells. Elimination of B cell APC function can paralyze T cell activation and histological and clinical manifestations of CNS autoimmunity, findings which provide insight regarding B–T communication in MS pathogenesis and the influence of B cell depletion in MS therapy.
With greater appreciation for the role of B cells in MS, it is important to study MS models that require B cells for development of CNS autoimmunity. There are several different murine EAE models, and each one has merits that permit investigation of specific aspects involved in pathogenesis of CNS autoimmunity. With identification of encephalitogenic T cell epitopes within individual myelin Ags, most studies use those corresponding encephalitogenic peptides for EAE induction. In this regard, MOG p35–55 is frequently used in EAE studies. In general, B cells recognize conformational determinants created by longer contiguous or noncontiguous amino acid sequences within native proteins (Roelants, 1972). Whereas MOG p35–55 activates encephalitogenic T cells (Kuchroo et al., 2002), it does not efficiently activate B cells or promote MOG-specific antibody production (Weber et al., 2010). In contrast, MOG protein efficiently leads to both B cell activation and production of anti-MOG antibodies (Weber et al., 2010), and it is recognized that rhMOG, but not rodent MOG isoforms, induces EAE in a B cell–dependent manner (Marta et al., 2005). Interestingly, this model is not DC dependent, as mice depleted of this myeloid APC subset were susceptible to EAE induced by MOG protein (Isaksson et al., 2012). The B cell dependence of rhMOG-induced EAE is attributed to the presence of proline 42 (P42) in its sequence (Oliver et al., 2003; Marta et al., 2005), as substitution of serine, the corresponding residue in rodent MOG, with P42 in rodent MOG protein confers B cell–dependent induction of EAE (Oliver et al., 2003; Marta et al., 2005). Use of rhMOG-induced EAE also permitted us to evaluate the role of B cell cytokine production in expansion of proinflammatory T cells in vivo. Selective deficiency in MHC II–dependent B cell APC function was associated with significant reductions in both Th1 and Th17 cells. As it is recognized that B cells are a source of IL-6, a key Th17-polarizing cytokine (Bettelli et al., 2006b), we investigated how B cell production of IL-6 itself influenced EAE susceptibility. Although selective B cell IL-6 deficiency did not significantly alter the course of MOG p35–55–induced EAE, onset of rhMOG-induced EAE was delayed, severity was attenuated and the frequency of Th17 cells only was significantly reduced in B cell IL-6–deficient mice. Similar results were obtained in mixed BM chimera mice containing IL-6–deficient B cells (unpublished data). These results underscore the importance of IL-6 production by activated B cells in Th17 differentiation in vivo in rhMOG-induced EAE. Interestingly, a recent study evaluated B cell IL-6 production in MOG p35–55–induced EAE and observed that B cell IL-6 production was not required for initiation of EAE, but that the disease course was reduced in mice containing IL-6–deficient B cells (Barr et al., 2012). Thus, B cell IL-6 deficiency may have a variable effect when EAE is induced by MOG p35–55, but it is clear that the impact of B cell IL-6 production is greater in rhMOG-induced EAE. As rhMOG is currently the only known B cell–dependent encephalitogen, it may be advantageous to examine this myelin protein when evaluating the pathogenic role of activated myelin-specific B cells in EAE.
In this study, selective MHC II deficiency in B cells was evaluated using mixed BM chimera mice reconstituted with B cells from either WT mice or from MHC II–deficient mice, ensuring in the latter a complete absence of B cell MHC II expression and MHC II–restricted Ag presentation. As an alternative, we also examined selective B cell MHC II deficiency in CD19-targeted MHC II–deficient (CD19cre–MHC IIflox/flox) mice (Shimoda et al., 2006). It is known that ~95% of B cells in CD19cre–MHC IIflox/flox mice are MHC II deficient (Shimoda et al., 2006). Although a minority of B cells expressed MHC II molecules, CD19cre–MHC IIflox/flox mice were also resistant to EAE induced with rhMOG (unpublished data). Thus, with both approaches, the results were consistent in demonstrating the in vivo requirement for B cell MHC II expression for EAE induced by this myelin protein. Although selective MHC II deficiency eliminated B cell APC function, it was also associated with a reduction in MOG-specific antibodies. One could contend that the absence of B cell MHC II expression simply eliminated the humoral response, which itself was primarily responsible for EAE pathogenesis. In our study, B cell MHC II deficiency resulted in a reduction in Tfh cells, a subset of CD4+ T cells that participate in germinal center formation, generation of memory B cells, and long-lived plasma cells (Crotty, 2011). In this regard, B cell APC function is a prerequisite for humoral immunity as Ag presentation by responding B cells is required for development and maintenance of Tfh, especially when Ag is limiting (Deenick et al., 2010). Nevertheless, it should be recognized that transfer of MOG-specific antibodies alone, or Tg-driven secretion of MOG-specific antibodies alone, does not lead to CNS inflammation and EAE (Linington et al., 1988; Genain et al., 1995; Litzenburger et al., 1998), and, as we have observed, transfer of pathogenic anti-MOG antibodies into B–MHC II−/− mice that were immunized with rhMOG led to a partial restoration of EAE. Furthermore, despite MHC II expression on myeloid APCs, including DCs, there was a reduction in development of MOG-reactive Th17 cells in mice selectively deficient in B cell MHC II expression, implying direct B–T interaction in vivo.
IgH “membrane-only” BCR Tg mice can distinguish B cell Ag presentation in vivo from humoral immunity (Chan et al., 1999). Although not targeted to a defined self-Ag, IgHB1-8-mem Tg mice have served to distinguish the contribution of B cells in Ag presentation from their role in the generation of antibodies in murine lupus and arthritis (Chan et al., 1999; O’Neill et al., 2005). To completely dissociate B lymphocyte cellular function from the potential contribution of humoral autoimmunity in EAE pathogenesis, we generated IgHMOG-mem/JHT Tg mice. B cells from our IgHMOG-mem/JHT mice efficiently presented MOG protein to MOG-specific 2D2 T cells and, despite the inability to secrete MOG-specific IgG or IgM, these mice were completely susceptible to acute clinical and histological EAE induced by rhMOG. Consistent with observations that B cells are more efficient APCs when they recognize the same target Ag as the responding T cells (Lanzavecchia, 1985; Constant et al., 1995a,b), we observed that B cells from IgHMOG-mem/JHT mice presented rhMOG to 2D2 T cells and promoted proinflammatory Th1 and Th17 differentiation more efficiently than B cells from IgHB1-8-mem/JHT mice, which bound rhMOG less effectively. Furthermore, in contrast to IgHMOG-mem/JHT mice, IgHB1-8-mem/JHT mice were less susceptible to EAE induction by rhMOG. Collectively, these in vitro and in vivo findings demonstrate how myelin-specific B cells can have a critical role in presentation to myelin-specific T cells in CNS autoimmunity. Although onset of acute EAE was similar in IgHMOG-mem/JHT, IgHMOG-ki, and WT mice, it was of particular interest that the chronic phase appeared less severe in IgHMOG-mem/JHT mice than either IgHMOG-ki or WT mice. Indeed, greater severity could reflect contribution of pathogenic MOG-specific antibodies elicited by immunization of IgHMOG-ki or WT mice with rhMOG. However, given minor differences observed in the disease course, it may be experimentally challenging to dissect the contribution of anti-MOG antibodies in this model.
With the recent introduction of new agents for relapsing-remitting MS, the unmet need for successful therapy of progressive MS has become more evident (Fox et al., 2012; Brück et al., 2013). Understanding of the genetic and pathogenic mechanisms involved in relapsing-remitting MS may also be advancing more rapidly (Sawcer et al., 2011). Furthermore, it is recognized that there is a paucity of models for progressive MS (Amor et al., 1994; Bettelli et al., 2006a; Krishnamoorthy et al., 2006; Basso et al., 2008). Although models do not entirely reflect spontaneous human disease, they can provide insight regarding specific pathogenic mechanisms. Meningeal B cell follicle–like structures have been observed in SPMS and in IgHMOG-ki × TCRMOG mice with OSE. IgHMOG-mem/JHT and IgHMOG-ki mice are complementary; whereas IgHMOG-mem/JHT mice cannot produce any antibodies, IgHMOG-ki mice can secrete all Ig isotypes. Here, by analysis of IgHMOG-mem/JHT × TCRMOG/JHT mice, we observed it is the cellular participation of MOG-reactive B lymphocytes that is necessary and sufficient for the development of spontaneous OSE and formation of meningeal follicle–like structures. Clinical signs of OSE appeared less severe in IgHMOG-mem/JHT × TCRMOG/JHT mice than in IgHMOG-ki × TCRMOG mice and the onset occurred later, suggesting that MOG-specific antibodies may augment the phenotypic expression. Thus, IgHMOG-mem/JHT × TCRMOG/JHT mice should provide a foundation to evaluate which individual isotypes of MOG-specific antibodies cooperate with MOG-specific B and T cells in OSE. IgHMOG-mem mice provide a powerful reagent to directly assess the contribution of select genes that may participate in B–T communication or development of OSE and formation of meningeal follicle–like structures without influence of soluble antibodies or potential antibody–Ag complexes. In summary, our observations in this study underscore the central importance of MHC II–dependent B cell Ag presentation in CNS autoimmunity. Use of IgHMOG-mem/JHT mice to study development of spontaneous OSE should provide further insight regarding peripheral and CNS involvement of myelin-specific B cells in MS progression.
MATERIALS AND METHODS
Mice.
WT C57BL/6J CD45.1 mice as well as CD45.2 MHC II−/− (B6.129S2-H2dlAb1-Ea/J), IL-6−/− (B6.129S2-Il6tm1Kopf/J), and CD19cre/cre (homozygous for cre cassette, B6.129P2(C)-Cd19tm1(cre)Cgn/J) mice were purchased from the Jackson Laboratory. C57BL/6J B cell–deficient JHT mice (B6.129P2-Igh-Jtm1Cgn/J; Gu et al., 1993) were provided by K. Rajewsky (Harvard University, Boston, MA), and C57BL/6J MOG p35–55–specific TCR Tg (2D2, TCRMOG) mice (Bettelli et al., 2003) were provided by V.K. Kuchroo (Harvard University). C57BL/6J MOG-BCR knockin (IgHMOG-ki, also referred to as Th) mice (Litzenburger et al., 1998) were provided by H. Wekerle. C57BL/6 IL-6flox/flox mice generated by gene targeting technology (Quintana et al., 2013) were provided by J. Hidalgo. For selective deletion of floxed IL-6 gene in B lymphocytes, CD19cre/cre mice were crossed with IL-6flox/flox mice. Litters heterozygous for the cre cassette (CD19cre–IL-6flox/flox, CD19cre–IL-6wt/wt) were used for experiments. IgHB1-8 (IgHB1-8-mem) mice (Chan et al., 1999) were backcrossed onto the C57BL/6J background and provided by J.-A. Lyons (University of Wisconsin, Milwaukee, WI) and A.H. Cross (Washington University School of Medicine in St. Louis, St. Louis, MO). IgHB1-8-mem mice were crossed onto the JHT background, and IgHMOG-ki mice were crossed onto the MHC II−/− background. Animals were housed in a specific pathogen–free barrier facility at the Parnassus Research Center of the University of California, San Francisco (UCSF). All breeding and experiments were reviewed and approved by the UCSF Institutional Animal Care and Use Committee.
Generation of IgHMOG-mem and IgHMOG-mem × TCRMOG Tg mice.
To generate IgHMOG-mem mice, the genomic DNA fragment containing the rearranged heavy chain VDJ of the pathogenic MOG-specific Ig 8-18C5 mAb (Litzenburger et al., 1998) was inserted into the IgMa constant region of a vector from which the secreted exon and polyadenylation site were excised (IgM-mem), which permits expression of membrane-bound Ig only (Chan et al., 1999). Specifically, we excised the 3.6-kb fragment containing the rearranged heavy chain enhancer and rearranged VDJ gene segment of the pathogenic MOG-specific Ig by digestion with EcoRI. This fragment was then subcloned into the EcoRI sites in the IgM-mem only construct. Correct orientation was determined first by PCR and restriction analysis and then confirmed by DNA sequencing of the regions containing the integration sites. The IgHMOG-mem construct was microinjected into C57BL/6J oocytes, which provided separate founders that were identified by PCR and confirmed by Southern blot. All founders contained B cells that expressed transgene-directed cell surface IgMa and bound MOG. IgHMOG-mem mice were crossed to C57BL/6J JHT mice to generate IgHMOG-mem/JHT mice.
TCRMOG-mem mice were crossed onto the JHT background to generate TCRMOG/JHT mice. IgHMOG-mem/JHT and IgHB1-8-mem/JHT mice were intercrossed to TCRMOG/JHT mice to generate IgHMOG-mem/JHT × TCRMOG/JHT and IgHB1-8-mem/JHT × TCRMOG/JHT mice. For comparison of IgHMOG-mem/JHT × TCRMOG/JHT mice, IgHMOG-ki mice were crossed with TCRMOG mice to generate IgHMOG-ki × TCRMOG mice (Bettelli et al., 2006a; Krishnamoorthy et al., 2006).
Generation of mixed BM chimera mice.
1,200 cGy γ-irradiation (Cs source) was administered to 6-wk-old recipient C57BL/6J mice. On the same day, recipients received 5 × 106 donor BM cells. All BM preparations were depleted of T cells by labeling with a biotinylated anti–Thy-1 (clone T24) before incubation with streptavidin-microbeads (Miltenyi Biotec) for negative selection via magnetic-activated cell sorting (MACS) column (Miltenyi Biotec). To restrict genetic MHC II deficiency to B cells, the BM inocula consisted of 80% BM from JHT mice supplemented with 20% BM from MHC II–deficient mice. The control group received BM inocula consisting of 80% BM from JHT mice supplemented with 20% BM from WT mice. In certain experiments, chimeras were generated with 80% BM from JHT mice and 20% BM from either IgHMOG-ki/MHC II−/− or IgHMOG-ki mice. BM chimeras were housed for at least 8 wk to reconstitute their peripheral lymphoid system before use in EAE experiments. FACS analysis confirmed hematopoietic reconstitution of sublethally irradiated C57BL/6J CD45.1 mice with CD45.2+ donor BM for expression of CD45.2 on peripheral blood mononuclear cells. Engraftment of donor CD45.2+ cells was ≥ 95%. The BM chimera containing MHC II–deficient B cells showed numbers of CD19+ splenocytes equivalent to numbers found in normal WT mice.
Ags.
Mouse MOG p35–55 (MEVGWYRSPFSRVVHLYRNGK) was synthesized by Auspep. rmMOG was provided C.C.A. Bernard. rhMOG was provided by C.C.A. Bernard and D.E. Jenne. MOG proteins were synthesized, purified, and refolded as previously reported (Clements et al., 2003; Breithaupt et al., 2008).
Induction and assessment of EAE.
C57BL/6J mice were injected s.c. with 50 µg MOG p35–55 or 100 µg rmMOG or 100 µg rhMOG emulsified in CFA (DIFCO Laboratories) containing 200 µg heat-killed Mycobacterium tuberculosis (Mtb) H37RA (DIFCO Laboratories) on day 0. Additionally, mice received i.v. 200 ng Bordetella pertussis toxin (List Biological Laboratories) in 0.2 ml PBS on days 0 and 2. Individual animals were observed daily, and clinical scores were assessed with a 0- to 5-point scoring system, as follows: 0 = no clinical disease, 1 = loss of tail tone only, 2 = mild monoparesis or paraparesis, 3 = severe paraparesis, 4 = paraplegia and/or quadriparesis, and 5 = moribund or death. Moribund mice were given disease severity scores of 5 and euthanized.
Generation of Ag-specific antibodies.
IgHMOG-ki mice were immunized with rmMOG (100 µg/mouse). 14–16 d later, mice were bled via cardiac puncture. Blood was allowed to clot, and the serum was removed after centrifugation and frozen at −80°C until use. The presence of high-titer serum antibodies was confirmed by ELISA (described below). The 8-18C5 hybridoma cell line was provided by C. Linington. An IgG1 mouse anti-OVA hybridoma cell line (1B7.11) was purchased from UCSF hybridoma core. Hybridoma cells were cultured in a BD Cell mAb Medium Serum-Free (BD) in large-scale flasks (Corning). mAbs were purified from hybridoma culture supernatants by the UCSF hybridoma core. The concentrations of the proteins were determined by means of the BCA protein assay (Thermo Fisher Scientific), according to the manufacturer’s recommendations.
Antibody administration.
B–MHC II+/+ and B–MHC II−/− mice were immunized with 100 µg rhMOG emulsified in CFA. 200 ng B. pertussis toxin was injected on days 0 and 2. When B–MHC II+/+ mice showed clinical EAE signs (score ~2.0), B–MHC II−/− mice received i.p. injections of 150 µg anti-MOG IgG1 mAb 8-18C5, an equal amount of isotype control OVA-specific mAb 1B7.11, or 250 µl IgHMOG-ki serum, or non-Tg littermate mice serum three times at 48 h-intervals (Schluesener et al., 1987; Pöllinger et al., 2009). Sera were obtained through cardiac puncture from IgHMOG-ki or non-Tg littermate mice.
Detection of anti-MOG antibodies.
Total serum MOG-specific IgG was quantified using a commercially available anti–mouse MOG (1–125) IgG quantitative ELISA kit (SensoLyte; AnaSpec). Wells were precoated with rmMOG (1–125) protein. Results are expressed as IgG concentrations in µg/ml serum (Figs. 3 A and 4 E) or, to facilitate comparison with other Ig measurements, as OD of serial twofold dilutions (Fig. 4 D). Serum MOG-specific IgM antibodies were measured using a noncommercial ELISA. 96-Maxisorb plates (Costar) were precoated with rmMOG (1–117) protein (10 µg/ml in PBS), blocked with BSA (Sigma-Aldrich), and incubated with sera for 2 h at the indicated dilution. After washing, MOG-specific IgM retained by the plate-bound MOG was detected with horseradish-peroxidase–conjugated anti–mouse IgM (IMMUNO-TEK). Total IgM and IgG were measured using quantitative Ig ELISA kits (IMMUNO-TEK). Total serum IgG or IgM was captured by anti–mouse IgG or IgM antibodies immobilized on micro wells. OVA-coated plates were used as negative controls for nonspecific binding. SOFTmax ELISA plate reader (450-nm wavelength) and software (Molecular Devices) were used for data analysis.
Proliferation assays.
Cells were grown in RPMI supplemented with 10% FCS, β-mercaptoethanol, l-glutamine, and penicillin/streptomycin. For thymidine incorporation, either 2.5 × 105 cells/ml of total splenocytes or 104 cells/ml of purified CD4+ T cells plus 2.5 × 105 cells/ml of purified B220+ B cells were cultured in 96-well plates in the presence of different concentrations of the rMOG proteins or MOG p35–55 (range 1–50 µg/ml). After 72 h, cells were pulsed with [3H]thymidine and harvested 16 h later. Thymidine incorporation is expressed as cpm. In certain experiments, T cell proliferation was assessed by CFSE dilution assay after gating on CD4+ T cells.
Cell isolation.
Single-cell leukocyte suspensions from spleens and peripheral lymph nodes (paired axillary and inguinal) were generated by gentle dissection. CNS mononuclear cells were isolated from EAE mice at peak of disease after cardiac perfusion with PBS, as described previously (Zeine and Owens, 1992). In brief, minced CNS (spinal cord) tissues were digested with 2.5 mg/ml collagenase D (Roche) at 37°C for 45 min. Mononuclear cells were isolated by passing the tissue through 70-mm cell strainers (BD), followed by discontinuous Percoll gradient (70/30%; Sigma-Aldrich) centrifugation. Lymphocytes were collected from the 30:70% interface and washed. Total cell numbers were determined by counting on a hemocytometer, and viability was assessed by trypan blue exclusion.
MACS (Miltenyi Biotec) was used to purify lymphocyte populations according to the manufacturer’s instructions. CD4+ T cells were isolated by depletion of non-CD4+ T cells (negative selection, kit #130-090-860). Naive B cells were isolated by depletion from CD43-expressing B cells (activated B cells, plasma cells and CD5+ B-1a cells) and non-B cells (kit #130-090-862) according to the manufacturer’s instructions. When necessary, cells were enriched a second time using a fresh MACS column to obtain >95% cell purities, as assessed by flow cytometry.
Flow cytometric analysis.
For two- to six-color immunofluorescence analysis, single-cell suspensions (106 cells) were incubated with anti–mouse FcRIIB/FcRIIIA mAb (2.4G2; BD) to avoid nonspecific staining and were subsequently stained at 4°C using predetermined optimal concentrations of mAb for 30 min, as described previously(Weber et al., 2010). Blood erythrocytes were lysed before staining using FACS lysing solution (BD). Antibodies to the mouse proteins CD45R (B220), FITC/PE/APC/PE-Cy7/PerCP-Cy5.5/APC-Cy7 (RA3-6B2), CD4 FITC/PE (RM4-4), CD4 APC/PE-Cy7/PerCP-Cy5.5/APC-Cy7 (RM4-5), CD11c-APC (N418), CD11b-PE (ICRF44), CD44-PE (IM7), CD62L-APC (MEL-14), IgM-FITC (eB121-15F9), and IgD-FITC (11-26c) were purchased from eBioscience. MHC II I-Ab-FITC (AF6-120.1) was purchased from BD. Isotype- and concentration-matched control antibodies were used to assess nonspecific staining. In certain experiments, cells were fixed in 0.3% paraformaldehyde and kept at 4°C until analysis. Lymphocytes were examined by forward and side light scatter on a FACSCanto II flow cytometer using FACSDiva software (BD).
Intracellular cytokine staining.
Lymphocytes were stimulated in vitro with 50 ng/ml PMA (Sigma-Aldrich) and 1 µg/ml ionomycin (Sigma-Aldrich) in the presence of 1 µl/ml brefeldin A (eBioscience) for 6 h before staining. FcRs were blocked before cell surface staining with anti–CD4-PerCP-Cy5.5 (RM4-5; eBioscience). After staining, cells were washed, fixed, and then permeabilized using the Cytofix/Cytoperm Plus Fixation/Permeabilization kit (BD) according to the manufacturer’s instructions. Permeabilized cells were then stained with anti–IL-17–PE-Cy7 (eBio17B7) and anti–IFN-γ–FITC (XMG1.2; all from eBioscience).
Bcl-6 staining.
For detection of Bcl-6, FcRs were blocked followed by cell surface staining with anti–CD4-PE (GK1.5), anti–PD-1 (CD279)–PE-Cy7 (J43), and anti–ICOS (CD278)-FITC (C398.4A; all from eBioscience) and anti CXCR5-PE (2G8; BD). Bcl-6 staining was subsequently performed using the Foxp3/Transcription Factor Staining Buffer set (eBioscience) and anti–Bcl-6-PE (mGI191E; eBioscience).
Histology and immunohistochemistry.
Brains, spinal cords, and optic nerves were removed and fixed in 10% neutral-buffered formalin, paraffin-embedded, and sectioned. Representative sections were stained with Luxol fast blue (LFB)–hematoxylin and eosin (H&E; for inflammation and demyelination) and reticulin preparation (for connective tissue) and examined by light microscopy. Meningeal and parenchymal inflammatory foci (>10 clustered inflammatory cells) were counted by a blinded observer (R.A. Sobel). Standard avidin-biotin immunohistochemical staining was performed on the sections with rabbit anti–mouse CD3 (Abcam) and rat anti–mouse CD45R (B220; BD), using reagents from Vector Laboratories. Normal mouse spleen tissue served as positive staining controls; negative controls included omission of the primary antibody. Neuropathologic analysis was performed by R.A. Sobel.
Statistical analysis.
Data are presented as mean ± SEM or SD. For clinical scores, significance between groups was examined using the Mann–Whitney U test. A value of P < 0.05 was considered significant. All other statistical analysis was performed using a one-way multiple-range ANOVA test for multiple comparisons. A value of P < 0.01 was considered significant.
Acknowledgments
We thank Dr. Patricia Nelson (University of California, San Francisco) for helpful discussion and Kara Pekarek for technical assistance. We thank Drs. Jeri-Anne Lyons and Anne H. Cross for providing us with the IgHB1-8-mem mice.
N. Molnarfi was supported by an advanced researcher fellowship from the Swiss National Science Foundation (SNF, PA00A-119532) and is a recipient of an advanced researcher exchange 2011 fellowship from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Foundation. U. Schulze-Topphoff is a fellow of the National Multiple Sclerosis Society (NMSS) and the Deutsche Forschungsgemeinschaft (DFG; SCHU 2587/1-1). M.S. Weber was supported by the NMSS (RG 4450A1/T). T. Prod’homme received fellowship support from the NMSS and Teva Neuroscience. M. Varrin-Doyer is a fellow of the NMSS. J. Hidalgo was supported by SAF2011-23272. Support for this work was provided to S.S. Zamvil by the National Institutes of Health (NIH; R01 AI-073737 and R01 NS-063008), the NMSS (RG 3913 and RG 4768), the Guthy Jackson Charitable Foundation, the Dana Foundation, and the Maisin Foundation and to M.J. Shlomchik by the NIH (R01 AI-043603).
The authors declare no competing financial interests.
Author contributions: N. Molnarfi, U. Schulze-Topphoff, and S.S. Zamvil designed research, analyzed data, and wrote the paper; M.S. Weber, J.C. Patarroyo, M. Varrin-Doyer, T. Prod’homme, and A.J. Slavin analyzed data, gave conceptual advice, and discussed the results at all stages; N. Molnarfi, U. Schulze-Topphoff, J.C. Patarroyo, and T. Prod’homme performed the experiments; M. Varrin-Doyer and A. Shetty assisted in the performance of experiments; R.A. Sobel performed the neuropathologic analysis; C. Linington, J. Hidalgo, D.E. Jenne, H. Wekerle, C.C.A. Bernard, and M.J. Shlomchik contributed new reagents; and S.S. Zamvil supervised the study. All authors read, commented on, and approved the final manuscript.
Footnotes
Abbreviations used:
- Ag
- antigen
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- LFB
- Luxol fast blue
- MACS
- magnetic-activated cell sorting
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- Mtb
- Mycobacterium tuberculosis
- NP
- nitrophenyl
- OSE
- opticospinal EAE
- rhMOG
- recombinant human MOG
- rmMOG
- recombinant mouse MOG
- SPMS
- secondary progressive MS
- Tfh cell
- T follicular helper cell
- Tg
- transgenic
References
- Amor S., Groome N., Linington C., Morris M.M., Dornmair K., Gardinier M.V., Matthieu J.M., Baker D. 1994. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J. Immunol. 153:4349–4356 [Abstract] [Google Scholar]
- Bar-Or A., Calabresi P.A., Arnold D., Markowitz C., Shafer S., Kasper L.H., Waubant E., Gazda S., Fox R.J., Panzara M., et al. 2008. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann. Neurol. 63:395–400 [published erratum appears in Ann. Neurol. 2008. 63:803] 10.1002/ana.21363 [Abstract] [CrossRef] [Google Scholar]
- Bar-Or A., Fawaz L., Fan B., Darlington P.J., Rieger A., Ghorayeb C., Calabresi P.A., Waubant E., Hauser S.L., Zhang J., Smith C.H. 2010. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann. Neurol. 67:452–461 10.1002/ana.21939 [Abstract] [CrossRef] [Google Scholar]
- Barr T.A., Shen P., Brown S., Lampropoulou V., Roch T., Lawrie S., Fan B., O’Connor R.A., Anderton S.M., Bar-Or A., et al. 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J. Exp. Med. 209:1001–1010 10.1084/jem.20111675 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Basso A.S., Frenkel D., Quintana F.J., Costa-Pinto F.A., Petrovic-Stojkovic S., Puckett L., Monsonego A., Bar-Shir A., Engel Y., Gozin M., et al. 2008. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 118:1532–1543 10.1172/JCI33464 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., Kuchroo V.K. 2003. Myelin oligodendrocyte glycoprotein–specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081 10.1084/jem.20021603 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bettelli E., Baeten D., Jäger A., Sobel R.A., Kuchroo V.K. 2006a. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J. Clin. Invest. 116:2393–2402 10.1172/JCI28334 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006b. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [Abstract] [CrossRef] [Google Scholar]
- Blennow K., Fredman P., Wallin A., Gottfries C.G., Frey H., Pirttilä T., Skoog I., Wikkelsö C., Svennerholm L. 1994. Formulas for the quantitation of intrathecal IgG production. Their validity in the presence of blood-brain barrier damage and their utility in multiple sclerosis. J. Neurol. Sci. 121:90–96 10.1016/0022-510X(94)90161-9 [Abstract] [CrossRef] [Google Scholar]
- Breithaupt C., Schäfer B., Pellkofer H., Huber R., Linington C., Jacob U. 2008. Demyelinating myelin oligodendrocyte glycoprotein-specific autoantibody response is focused on one dominant conformational epitope region in rodents. J. Immunol. 181:1255–1263 [Abstract] [Google Scholar]
- Brück W., Gold R., Lund B.T., Oreja-Guevara C., Prat A., Spencer C.M., Steinman L., Tintoré M., Vollmer T.L., Weber M.S., et al. 2013. Therapeutic decisions in multiple sclerosis: Moving beyond efficacy. JAMA Neurol. 70:1315–1324 10.1001/jamaneurol.2013.3510 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chan O.T., Hannum L.G., Haberman A.M., Madaio M.P., Shlomchik M.J. 1999. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 189:1639–1648 10.1084/jem.189.10.1639 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chen J., Trounstine M., Alt F.W., Young F., Kurahara C., Loring J.F., Huszar D. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5:647–656 10.1093/intimm/5.6.647 [Abstract] [CrossRef] [Google Scholar]
- Clements C.S., Reid H.H., Beddoe T., Tynan F.E., Perugini M.A., Johns T.G., Bernard C.C., Rossjohn J. 2003. The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis. Proc. Natl. Acad. Sci. USA. 100:11059–11064 10.1073/pnas.1833158100 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Constant S., Sant’Angelo D., Pasqualini T., Taylor T., Levin D., Flavell R., Bottomly K. 1995a. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J. Immunol. 154:4915–4923 [Abstract] [Google Scholar]
- Constant S., Schweitzer N., West J., Ranney P., Bottomly K. 1995b. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J. Immunol. 155:3734–3741 [Abstract] [Google Scholar]
- Cross A.H., Stark J.L., Lauber J., Ramsbottom M.J., Lyons J.A. 2006. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 180:63–70 10.1016/j.jneuroim.2006.06.029 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621–663 10.1146/annurev-immunol-031210-101400 [Abstract] [CrossRef] [Google Scholar]
- Deenick E.K., Chan A., Ma C.S., Gatto D., Schwartzberg P.L., Brink R., Tangye S.G. 2010. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 33:241–253 10.1016/j.immuni.2010.07.015 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944–950 10.1038/ni833 [Abstract] [CrossRef] [Google Scholar]
- Fox R.J., Thompson A., Baker D., Baneke P., Brown D., Browne P., Chandraratna D., Ciccarelli O., Coetzee T., Comi G., et al. 2012. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult. Scler. 18:1534–1540 10.1177/1352458512458169 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Genain C.P., Nguyen M.H., Letvin N.L., Pearl R., Davis R.L., Adelman M., Lees M.B., Linington C., Hauser S.L. 1995. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J. Clin. Invest. 96:2966–2974 10.1172/JCI118368 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gu H., Zou Y.R., Rajewsky K. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 73:1155–1164 10.1016/0092-8674(93)90644-6 [Abstract] [CrossRef] [Google Scholar]
- Harvey B.P., Gee R.J., Haberman A.M., Shlomchik M.J., Mamula M.J. 2007. Antigen presentation and transfer between B cells and macrophages. Eur. J. Immunol. 37:1739–1751 10.1002/eji.200636452 [Abstract] [CrossRef] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J., Bar-Or A., Panzara M., Sarkar N., Agarwal S., et al. ; HERMES Trial Group 2008. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358:676–688 10.1056/NEJMoa0706383 [Abstract] [CrossRef] [Google Scholar]
- Hjelmström P., Juedes A.E., Fjell J., Ruddle N.H. 1998. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J. Immunol. 161:4480–4483 [Abstract] [Google Scholar]
- Isaksson M., Lundgren B.A., Ahlgren K.M., Kämpe O., Lobell A. 2012. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur. J. Immunol. 42:2555–2563 10.1002/eji.201142239 [Abstract] [CrossRef] [Google Scholar]
- Kappos L., Li D., Calabresi P.A., O’Connor P., Bar-Or A., Barkhof F., Yin M., Leppert D., Glanzman R., Tinbergen J., et al. 2011. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 378:1779–1787 10.1016/S0140-6736(11)61649-8 [Abstract] [CrossRef] [Google Scholar]
- Krishnamoorthy G., Lassmann H., Wekerle H., Holz A. 2006. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J. Clin. Invest. 116:2385–2392 10.1172/JCI28330 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kuchroo V.K., Anderson A.C., Waldner H., Munder M., Bettelli E., Nicholson L.B. 2002. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 20:101–123 10.1146/annurev.immunol.20.081701.141316 [Abstract] [CrossRef] [Google Scholar]
- Lanzavecchia A. 1985. Antigen-specific interaction between T and B cells. Nature. 314:537–539 10.1038/314537a0 [Abstract] [CrossRef] [Google Scholar]
- Linington C., Bradl M., Lassmann H., Brunner C., Vass K. 1988. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 130:443–454 [Europe PMC free article] [Abstract] [Google Scholar]
- Linnington C., Webb M., Woodhams P.L. 1984. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J. Neuroimmunol. 6:387–396 10.1016/0165-5728(84)90064-X [Abstract] [CrossRef] [Google Scholar]
- Litzenburger T., Fässler R., Bauer J., Lassmann H., Linington C., Wekerle H., Iglesias A. 1998. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J. Exp. Med. 188:169–180 10.1084/jem.188.1.169 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lyons J.A., San M., Happ M.P., Cross A.H. 1999. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur. J. Immunol. 29:3432–3439 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2 [Abstract] [CrossRef] [Google Scholar]
- Lyons J.A., Ramsbottom M.J., Cross A.H. 2002. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 32:1905–1913 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L [Abstract] [CrossRef] [Google Scholar]
- Magliozzi R., Howell O., Vora A., Serafini B., Nicholas R., Puopolo M., Reynolds R., Aloisi F. 2007. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 130:1089–1104 10.1093/brain/awm038 [Abstract] [CrossRef] [Google Scholar]
- Marriott J.J., O’Connor P.W. 2010. Emerging therapies in relapsing-remitting multiple sclerosis. Rev. Recent Clin. Trials. 5:179–188 10.2174/157488710792007275 [Abstract] [CrossRef] [Google Scholar]
- Marta C.B., Oliver A.R., Sweet R.A., Pfeiffer S.E., Ruddle N.H. 2005. Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology. Proc. Natl. Acad. Sci. USA. 102:13992–13997 10.1073/pnas.0504979102 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Martin Mdel.P., Cravens P.D., Winger R., Kieseier B.C., Cepok S., Eagar T.N., Zamvil S.S., Weber M.S., Frohman E.M., Kleinschmidt-Demasters B.K., et al. 2009. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch. Neurol. 66:1016–1020 10.1001/archneurol.2009.157 [Abstract] [CrossRef] [Google Scholar]
- Matsushita T., Yanaba K., Bouaziz J.D., Fujimoto M., Tedder T.F. 2008. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118:3420–3430 [Europe PMC free article] [Abstract] [Google Scholar]
- McDevitt H.O., Deak B.D., Shreffler D.C., Klein J., Stimpfling J.H., Snell G.D. 1972. Genetic control of the immune response. Mapping of the Ir-1 locus. J. Exp. Med. 135:1259–1278 10.1084/jem.135.6.1259 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Meinl E., Krumbholz M., Hohlfeld R. 2006. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 59:880–892 10.1002/ana.20890 [Abstract] [CrossRef] [Google Scholar]
- Menge T., von Büdingen H.C., Lalive P.H., Genain C.P. 2007. Relevant antibody subsets against MOG recognize conformational epitopes exclusively exposed in solid-phase ELISA. Eur. J. Immunol. 37:3229–3239 10.1002/eji.200737249 [Abstract] [CrossRef] [Google Scholar]
- O’Neill S.K., Shlomchik M.J., Glant T.T., Cao Y., Doodes P.D., Finnegan A. 2005. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J. Immunol. 174:3781–3788 [Abstract] [Google Scholar]
- Okuda Y., Sakoda S., Fujimura H., Saeki Y., Kishimoto T., Yanagihara T. 1999. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35-55 induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 101:188–196 10.1016/S0165-5728(99)00139-3 [Abstract] [CrossRef] [Google Scholar]
- Oliver A.R., Lyon G.M., Ruddle N.H. 2003. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J. Immunol. 171:462–468 [Abstract] [Google Scholar]
- Phan T.G., Grigorova I., Okada T., Cyster J.G. 2007. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8:992–1000 10.1038/ni1494 [Abstract] [CrossRef] [Google Scholar]
- Pöllinger B., Krishnamoorthy G., Berer K., Lassmann H., Bösl M.R., Dunn R., Domingues H.S., Holz A., Kurschus F.C., Wekerle H. 2009. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J. Exp. Med. 206:1303–1316 10.1084/jem.20090299 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Quintana A., Erta M., Ferrer B., Comes G., Giralt M., Hidalgo J. 2013. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav. Immun. 27:162–173 10.1016/j.bbi.2012.10.011 [Abstract] [CrossRef] [Google Scholar]
- Roelants G. 1972. Antigen recognition by B and T lymphocytes. Curr. Top. Microbiol. Immunol. 59:135–165 [Abstract] [Google Scholar]
- Sawcer S., Hellenthal G., Pirinen M., Spencer C.C., Patsopoulos N.A., Moutsianas L., Dilthey A., Su Z., Freeman C., Hunt S.E., et al. ; International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 476:214–219 10.1038/nature10251 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schluesener H.J., Sobel R.A., Linington C., Weiner H.L. 1987. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J. Immunol. 139:4016–4021 [Abstract] [Google Scholar]
- Schwartz R.H. 1985. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu. Rev. Immunol. 3:237–261 10.1146/annurev.iy.03.040185.001321 [Abstract] [CrossRef] [Google Scholar]
- Shimoda M., Li T., Pihkala J.P.S., Koni P.A. 2006. Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response. J. Immunol. 176:2122–2133 [Abstract] [Google Scholar]
- Slavin A.J., Soos J.M., Stuve O., Patarroyo J.C., Weiner H.L., Fontana A., Bikoff E.K., Zamvil S.S. 2001. Requirement for endocytic antigen processing and influence of invariant chain and H-2M deficiencies in CNS autoimmunity. J. Clin. Invest. 108:1133–1139 [Europe PMC free article] [Abstract] [Google Scholar]
- van der Veen R.C., Trotter J.L., Kapp J.A. 1992. Immune processing of proteolipid protein by subsets of antigen-presenting spleen cells. J. Neuroimmunol. 38:139–146 10.1016/0165-5728(92)90098-6 [Abstract] [CrossRef] [Google Scholar]
- Vinuesa C.G., Tangye S.G., Moser B., Mackay C.R. 2005. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5:853–865 10.1038/nri1714 [Abstract] [CrossRef] [Google Scholar]
- von Büdingen H.C., Bar-Or A., Zamvil S.S. 2011. B cells in multiple sclerosis: connecting the dots. Curr. Opin. Immunol. 23:713–720 10.1016/j.coi.2011.09.003 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Weber M.S., Prod’homme T., Patarroyo J.C., Molnarfi N., Karnezis T., Lehmann-Horn K., Danilenko D.M., Eastham-Anderson J., Slavin A.J., Linington C., et al. 2010. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 68:369–383 10.1002/ana.22081 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wong F.S., Wen L., Tang M., Ramanathan M., Visintin I., Daugherty J., Hannum L.G., Janeway C.A., Jr, Shlomchik M.J. 2004. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 53:2581–2587 10.2337/diabetes.53.10.2581 [Abstract] [CrossRef] [Google Scholar]
- Zamvil S.S., Steinman L. 1990. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 8:579–621 10.1146/annurev.iy.08.040190.003051 [Abstract] [CrossRef] [Google Scholar]
- Zeine R., Owens T. 1992. Direct demonstration of the infiltration of murine central nervous system by Pgp-1/CD44high CD45RB(low) CD4+ T cells that induce experimental allergic encephalomyelitis. J. Neuroimmunol. 40:57–69 10.1016/0165-5728(92)90213-5 [Abstract] [CrossRef] [Google Scholar]
Articles from The Journal of Experimental Medicine are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1084/jem.20130699
Read article for free, from open access legal sources, via Unpaywall:
http://jem.rupress.org/content/jem/210/13/2921.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
B Cells Influence Encephalitogenic T Cell Frequency to Myelin Oligodendrocyte Glycoprotein (MOG)38-49 during Full-length MOG Protein-Induced Demyelinating Disease.
Immunohorizons, 8(9):729-739, 01 Sep 2024
Cited by: 0 articles | PMID: 39330967 | PMCID: PMC11447661
HLA-transgenic mouse models to study autoimmune central nervous system diseases.
Autoimmunity, 57(1):2387414, 21 Aug 2024
Cited by: 0 articles | PMID: 39167553
Review
Macrophages and HLA-Class II Alleles in Multiple Sclerosis: Insights in Therapeutic Dynamics.
Int J Mol Sci, 25(13):7354, 04 Jul 2024
Cited by: 0 articles | PMID: 39000461
Review
MOG CNS Autoimmunity and MOGAD.
Neurol Neuroimmunol Neuroinflamm, 11(5):e200275, 12 Jul 2024
Cited by: 3 articles | PMID: 38996203 | PMCID: PMC11256982
Review Free full text in Europe PMC
IL-6 Inhibition as a Therapeutic Target in Aged Experimental Autoimmune Encephalomyelitis.
Int J Mol Sci, 25(12):6732, 19 Jun 2024
Cited by: 0 articles | PMID: 38928437
Go to all (252) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Myelin-reactive B cells exacerbate CD4+ T cell-driven CNS autoimmunity in an IL-23-dependent manner.
Nat Commun, 15(1):5404, 26 Jun 2024
Cited by: 0 articles | PMID: 38926356 | PMCID: PMC11208426
B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity.
Ann Neurol, 68(3):369-383, 01 Sep 2010
Cited by: 201 articles | PMID: 20641064 | PMCID: PMC3375897
MOG extracellular domain (p1-125) triggers elevated frequency of CXCR3+ CD4+ Th1 cells in the CNS of mice and induces greater incidence of severe EAE.
Mult Scler, 20(10):1312-1321, 19 Feb 2014
Cited by: 9 articles | PMID: 24552747
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.
J Neurol Sci, 333(1-2):76-87, 08 Apr 2013
Cited by: 140 articles | PMID: 23578791 | PMCID: PMC3726569
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: R01 AI-043603
Grant ID: R01 AI043603
Grant ID: R01 AI-073737
Grant ID: R01 AI073737
NINDS NIH HHS (2)
Grant ID: R01 NS-063008
Grant ID: R01 NS063008
Swiss National Science Foundation (1)
Grant ID: 119532

 1,2
1,2



