Abstract
Free full text

Can Levofloxacin Be a Useful Alternative to Trimethoprim-Sulfamethoxazole for Treating Stenotrophomonas maltophilia Bacteremia?
Abstract
A retrospective study was conducted to evaluate the efficacy of levofloxacin in the treatment of Stenotrophomonas maltophilia bacteremia. The 30-day mortality rates were similar between the trimerthoprim-sulfamethoxazole (TMP-SMX) and levofloxacin treatment groups. Adverse events related to antibiotics occurred more frequently in patients receiving TMP-SMX, and recurrent bacteremia due to levofloxacin-resistant S. maltophilia strains developed in patients treated with levofloxacin. Our data suggest that levofloxacin can be a useful alternative option for treating S. maltophilia infections.
TEXT
Stenotrophomonas maltophilia is an emerging nosocomial pathogen, mainly in immunocompromised patients, and is associated with high mortality (1,–4). Trimethoprim-sulfamethoxazole (TMP-SMX) is recommended as the treatment of choice for S. maltophilia infections (5,–7). However, because of concern regarding adverse events related to TMP-SMX, levofloxacin has also been used for treating these infections. The purpose of this study was to compare clinical outcomes between TMP-SMX and levofloxacin for the treatment of S. maltophilia bacteremia.
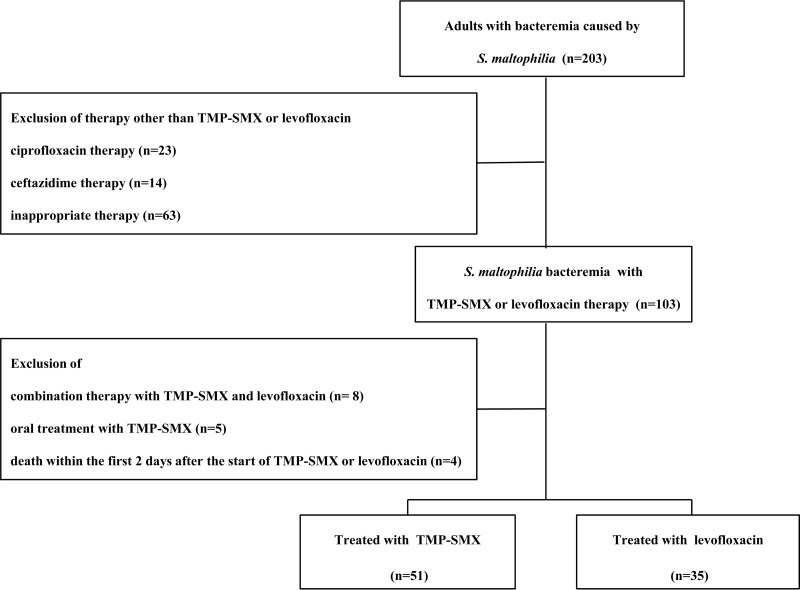
A retrospective cohort study that involved adult patients with S. maltophilia bacteremia at Samsung Medical Center, a 1,945-bed tertiary care facility in Seoul, South Korea, was undertaken from 2000 to 2012. Antimicrobial susceptibility testing was performed using a Vitek 2 system (bioMérieux, Inc., Hazelwood, MO, USA), and breakpoints were those defined by the Clinical and Laboratory Standards Institute (8). Patients were included in the study if they received either intravenous TMP-SMX or levofloxacin for more than 48 h and if the causative isolate was found to be susceptible to the prescribed drug. TMP-SMX was administered in a dose of 15 to 20 mg/kg of body weight/day (based on the trimethoprim component), and levofloxacin was administered in a dose of 750 mg once daily in patients with normal renal function. Dosage adjustments were made according to creatinine clearance in patients with renal impairment. Demographic characteristics, underlying diseases or conditions, severity of the underlying disease, severity of bacteremia, site of infection, and antibiogram results were recorded. Definite catheter-related infection (CRI) was defined according to the Infectious Diseases Society of America guidelines (9). Possible CRI was indicated by the finding of a positive blood culture with no apparent source of bacteremia other than the catheter. Recurrent bacteremia was defined as the reappearance of S. maltophilia bacteremia within the first 3 months. The main outcome measure used was the overall 30-day mortality. We also assessed length of hospital stay, adverse events, and recurrent bacteremia. Continuous variables were compared using Student's t test, and categorical variables were compared using χ2 or Fisher's exact test. Multivariate logistic regression analysis was used to evaluate the effects of levofloxacin treatment. A P value of less than 0.05 was considered statistically significant. SPSS statistical software for Windows, PASW version 18.0, was used for the statistical analyses.
During the study period, we identified 203 adult patients with S. maltophilia bacteremia. Among all S. maltophilia isolates, the rates of susceptibility to TMP-SMX and levofloxacin were 96.1% and 87.1%, respectively. After the exclusion of patients according to our study criteria, 53 patients received TMP-SMX and 35 patients received levofloxacin. Of the 53 patients who received TMP-SMX, the causative isolates from 2 patients were resistant in vitro to TMP-SMX, and these patients were excluded from the study. However, in the levofloxacin treatment group, there were no patients excluded because of resistance to levofloxacin (Fig. 1).
The baseline characteristics between the groups were similar (Table 1). However, compared to patients in the TMP-SMX treatment group, patients in the levofloxacin group were predominantly male. In the levofloxacin treatment group, no resistance to TMP-SMX was detected, whereas 7 patients were nonsusceptible to levofloxacin in the TMP-SMX treatment group. Outcomes are presented in Table 2. The overall 30-day mortality rates did not show statistically significant differences between the TMP-SMX treatment group and the levofloxacin treatment group (14 of 51 patients [27.5%] versus 7 of 35 patients [20.0%], P = 0.429). After exclusion of patients who had combination therapy or polymicrobial bacteremia from the analysis, there was no difference in the 30-day mortality rates between the groups (9 of 31 patients [29.0%] versus 5 of 27 patients [18.5%], P = 0.351). In a univariate analysis, old age, septic shock, and pneumonia were significantly associated with increased mortality of S. maltophilia bacteremia, while definite CRI was found to be a protective factor for 30-day mortality (Table 3). In multivariate analyses, pneumonia and septic shock were significantly associated with 30-day mortality. However, levofloxacin treatment was not associated with 30-day mortality. In 10 patients treated with TMP-SMX, TMP-SMX was withdrawn due to adverse events. The most common adverse events related to TMP-SMX were cytopenia and hepatotoxicity. Treatment with levofloxacin was switched to TMP-SMX in eight patients. However, among these patients, treatment failure related to levofloxacin was not observed. Of these eight patients, three developed rash or cytopenia after changing to TMP-SMX. Additionally, in two patients, levofloxacin was changed to ceftazidime or tigecycline for broader coverage. Among eight patients with recurrent bacteremia, levofloxacin-resistant S. maltophilia was isolated from four patients (two patients from the levofloxacin group and two patients in whom TMP-SMX was switched to levofloxacin).
TABLE 1
Demographic and clinical characteristics of patients in the TMP-SMX and levofloxacin groups
| Characteristica | No. of patients (%) with characteristicb | P value | ||
|---|---|---|---|---|
| Total (n = 86) | TMP-SMX group (n = 51) | Levofloxacin group (n = 35) | ||
| Age, median (IQR) | 58 (45–67) | 56 (40–67) | 61 (49–68) | 0.153 |
| Older age (≥65 yr) | 30 (34.9) | 16 (31.4) | 14 (40.0) | 0.410 |
| Gender, male | 56 (65.1) | 28 (54.9) | 28 (80.0) | 0.016 |
| Hospital-acquired infection | 79 (91.9) | 45 (88.2) | 34 (97.1) | 0.233 |
| ICU-acquired infection | 22 (25.6) | 13 (25.5) | 10 (28.6) | 0.751 |
| Underlying disease | ||||
    Hematologic malignancy Hematologic malignancy | 44 (51.8) | 29 (56.9) | 15 (44.1) | 0.249 |
    Solid tumor Solid tumor | 32 (37.2) | 16 (31.4) | 16 (45.7) | 0.176 |
    Liver cirrhosis Liver cirrhosis | 14 (16.3) | 9 (17.6) | 5 (14.3) | 0.678 |
    Diabetes mellitus Diabetes mellitus | 13 (15.1) | 8 (15.7) | 5 (14.3) | 0.859 |
    Cardiovascular disease Cardiovascular disease | 16 (18.6) | 8 (15.7) | 8 (22.9) | 0.401 |
| Comorbid conditions | ||||
    Chemotherapy Chemotherapy | 47 (54.7) | 30 (58.8) | 17 (48.6) | 0.348 |
    Neutropenia Neutropenia | 38 (44.2) | 25 (49) | 13 (37.1) | 0.276 |
    Major surgery Major surgery | 10 (11.6) | 5 (9.8) | 5 (14.3) | 0.734 |
    Invasive procedure Invasive procedure | 18 (20.9) | 10 (9.6) | 8 (22.9) | 0.716 |
    Immunosuppression Immunosuppression | 16 (18.6) | 8 (15.7) | 8 (22.9) | 0.401 |
    CRRT/HD CRRT/HD | 11 (12.8) | 9 (17.6) | 2 (5.7) | 0.187 |
    Mechanical ventilation Mechanical ventilation | 14 (16.3) | 7 (13.7) | 7 (20.0) | 0.439 |
    Tracheostomy Tracheostomy | 9 (10.5) | 4 (7.8) | 5 (14.3) | 0.476 |
    HSCT HSCT | 8 (9.3) | 5 (9.8) | 3 (8.6) | 1.000 |
    SOT SOT | 7 (8.1) | 3 (5.9) | 4 (11.4) | 0.435 |
    Central venous catheter Central venous catheter | 61 (70.9) | 39 (76.5) | 22 (62.9) | 0.172 |
| Catheter removal | 32 (52.5) | 21 (53.8) | 11 (50.0) | 0.773 |
| Time to removal, daysc | 3 (1–5) | 3 (0–4.75) | 3 (1–6.25) | 0.431 |
| CCIc | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.539 |
| CCI of ≥3 | 26 (30.2) | 16 (31.4) | 10 (28.6) | 0.781 |
| Pitt bacteremia scorec | 1 (0–3) | 1 (0–3) | 2 (0–3) | 0.678 |
| Pitt bacteremia score of ≥4 | 16 (18.6) | 10 (19.6) | 6 (17.1) | 0.773 |
| Septic shock | 20 (23.3) | 13 (25.5) | 7 (20.0) | 0.554 |
| Source of bacteremia | ||||
    Definite CRI Definite CRI | 17 (19.8) | 12 (23.5) | 5 (14.3) | 0.290 |
    Possible CRI Possible CRI | 22 (25.6) | 11 (21.6) | 11 (31.4) | 0.303 |
    IAI IAI | 25 (29.1) | 14 (27.5) | 11 (31.4) | 0.690 |
    Pneumonia Pneumonia | 14 (16.3) | 9 (17.6) | 5 (14.3) | 0.678 |
| Polymicrobial bacteremia | 17 (19.8) | 12 (23.5) | 5 (14.3) | 0.290 |
| Combination therapy | 12 (14.0) | 9 (17.6) | 3 (8.6) | 0.345 |
TABLE 2
Comparison of treatment outcomes between TMP-SMX and levofloxacin groups for S. maltophilia bacteremia
| Outcome | No. of patients (%) with outcomea | P value | ||
|---|---|---|---|---|
| Total (n = 86) | TMP-SMX group (n = 51) | Levofloxacin group (n = 35) | ||
| 30-day mortality | 21 (24.4) | 14 (27.5) | 7 (20.0) | 0.429 |
| Length of hospital stay (no. of days)b | 26 (14–49) | 25 (12–51) | 27 (15–52) | 0.824 |
| Antibiotic withdrawal | 20 (23.3) | 10 (19.6) | 10 (28.6) | 0.386 |
| Adverse event | 12 (14.0) | 12 (23.5) | 0 (0) | 0.001 |
| Recurrent bacteremiac | 8 (9.3) | 6 (11.9) | 2 (5.7) | 0.464 |
TABLE 3
Factors associated with 30-day mortality in patients with S. maltophilia bacteremia
| Risk factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Old age (≥65 yr) | 2.66 (0.97–7.31) | 0.053 | ||
| Septic shock | 5.00 (1.68–14.87) | 0.006 | 6.19 (1.81–21.21) | 0.004 |
| Definite CRI | 0.15 (0.02–1.23) | 0.06 | ||
| Pneumonia | 6.01 (1.80–20.44) | 0.004 | 5.33 (1.39–20.37) | 0.015 |
| Levofloxacin treatment | 0.66 (0.24–1.85) | 0.429 | 0.62 (0.19–2.04) | 0.433 |
Abbreviations: CI, confidence interval; OR, odds ratio; CRI, catheter-related infection.
The findings of this study suggest that the severity of bacteremia and the site of infection may be more important for the prognosis of S. maltophilia bacteremia than the choice of either levofloxacin or TMP-SMX for treatment, as long as the causative isolates are susceptible to these antibiotics. In our study, adverse events occurred more frequently in patients receiving TMP-SMX than in those receiving levofloxacin. The new fluoroquinolones, such as moxifloxacin and levofloxacin, show superior in vitro activity against S. maltophilia than earlier quinolones, either alone or in combination (10,–12). However, in the use of levofloxacin for treating S. maltophilia infection, induction of resistance is a matter of concern. Rapid emergence of resistance against quinolones has been observed in vitro and in vivo (13, 14). This finding was observed in our study.
The present study has several limitations. First, treatment groups were assigned retrospectively and were not randomized. Second, our study showed good susceptibility of S. maltophilia to TMP-SMX and levofloxacin. Therefore, our results cannot be generalized in areas with a high prevalence of multidrug-resistant S. maltophilia.
In conclusion, patients receiving levofloxacin showed clinical outcomes similar to those receiving TMP-SMX for the treatment of S. maltophilia bacteremia. The patients receiving TMP-SMX experienced a higher rate of adverse events, while induction of resistance was observed in the levofloxacin group. Our data suggest that levofloxacin can be a useful option for treating S. maltophilia infections, although a clinical trial comparing the two treatment regimens is warranted.
ACKNOWLEDGMENTS
We declare that we have no conflicts of interest.
This study was supported by Samsung Biomedical Research Institute grant SBRI GL1-B2-031-1.
REFERENCES
Articles from Antimicrobial Agents and Chemotherapy are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aac.01682-13
Read article for free, from open access legal sources, via Unpaywall:
https://aac.asm.org/content/aac/58/1/581.full.pdf
Citations & impact
Impact metrics
Article citations
Pathogenic spectrum and drug resistance of bloodstream infection in patients with acute myeloid leukaemia: a single centre retrospective study.
Front Cell Infect Microbiol, 14:1390053, 07 Jun 2024
Cited by: 0 articles | PMID: 38912203 | PMCID: PMC11190328
Clinical Outcomes of Trimethoprim/Sulfamethoxazole in Critically Ill Patients with Stenotrophomonas maltophilia Bacteremia and Pneumonia Utilizing Renal Replacement Therapies.
J Clin Med, 13(8):2275, 14 Apr 2024
Cited by: 0 articles | PMID: 38673547 | PMCID: PMC11051438
Global mapping of antibiotic resistance rates among clinical isolates of Stenotrophomonas maltophilia: a systematic review and meta-analysis.
Ann Clin Microbiol Antimicrob, 23(1):26, 19 Mar 2024
Cited by: 3 articles | PMID: 38504262 | PMCID: PMC10953290
Review Free full text in Europe PMC
Intra-Abdominal Abscess and Bacteremia Due to Stenotrophomonas maltophilia After Total Gastrectomy: A Case Report and Literature Review.
Infect Drug Resist, 16:7197-7204, 10 Nov 2023
Cited by: 0 articles | PMID: 38023400 | PMCID: PMC10644874
Comparative genomics analysis of Stenotrophomonas maltophilia strains from a community.
Front Cell Infect Microbiol, 13:1266295, 28 Nov 2023
Cited by: 0 articles | PMID: 38089814 | PMCID: PMC10715271
Go to all (52) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Trimethoprim-sulfamethoxazole versus levofloxacin for the treatment of Stenotrophomonas maltophilia infections: A multicentre cohort study.
J Glob Antimicrob Resist, 38:42-48, 30 May 2024
Cited by: 0 articles | PMID: 38821443
Emergence of concurrent levofloxacin- and trimethoprim/sulfamethoxazole-resistant Stenotrophomonas maltophilia: Risk factors and antimicrobial sensitivity pattern analysis from a single medical center in Taiwan.
J Microbiol Immunol Infect, 55(1):107-113, 13 Jan 2021
Cited by: 9 articles | PMID: 33500210
Comparisons between patients with trimethoprim-sulfamethoxazole-susceptible and trimethoprim-sulfamethoxazole-resistant Stenotrophomonas maltophilia monomicrobial bacteremia: A 10-year retrospective study.
J Microbiol Immunol Infect, 49(3):378-386, 28 Jul 2014
Cited by: 15 articles | PMID: 25081988
Effective strategies for managing trimethoprim-sulfamethoxazole and levofloxacin-resistant Stenotrophomonas maltophilia infections: bridging the gap between scientific evidence and clinical practice.
Curr Opin Infect Dis, 37(6):554-564, 29 Jul 2024
Cited by: 0 articles | PMID: 39082087
Review
 a
a