Abstract
Free full text

Influenza A Virus Exacerbates Staphylococcus aureus Pneumonia in Mice by Attenuating Antimicrobial Peptide Production
Associated Data
Abstract
Influenza A represents a significant cause of morbidity and mortality worldwide. Bacterial complications of influenza A confer the greatest risk to patients. TH17 pathway inhibition has been implicated as a mechanism by which influenza A alters bacterial host defense. Here we show that preceding influenza causes persistent Staphylococcus aureus infection and suppression of TH17 pathway activation in mice. Influenza does not inhibit S. aureus binding and uptake by phagocytic cells but instead attenuates S. aureus induced TH17 related antimicrobial peptides necessary for bacterial clearance in the lung. Importantly, exogenous lipocalin 2 rescued viral exacerbation of S. aureus infection and decreased free iron levels in the bronchoalveolar lavage from mice coinfected with S. aureus and influenza. These findings indicate a novel mechanism by which influenza A inhibits TH17 immunity and increases susceptibility to secondary bacterial pneumonia. Identification of new mechanisms in the pathogenesis of bacterial pneumonia could lead to future therapeutic targets.
Pneumonia is a leading cause of death worldwide, accounting for the highest mortality in children younger than 5 years of age, resulting in approximately 1.4 million deaths in 2010 [1]. In addition, pneumonia ranks 9th overall as a cause of death annually in the United States [2]. Numerous bacterial organisms cause pneumonia, including Staphylococcus aureus. The prevalence of S. aureus has increased in recent years with the emergence of methicillin-resistant S. aureus. MRSA currently accounts for 20%–40% of hospital-acquired and ventilator-acquired pneumonias [3] and 9% of community-acquired pneumonias [4]. A primary cofactor involved in mortality due to community acquired MRSA infection is preceding influenza-like illness [5].
Influenza is a common respiratory illness that affects 5%–20% of the US population yearly and results in approximately 30 000 deaths annually. Although most cases of influenza are not fatal, complications such as bacterial pneumonia can have serious consequences. Increased intensive care admission, mechanical ventilation, and mortality have been described in children and young adults with influenza A and concomitant S. aureus infection compared to those with either influenza or S. aureus infection alone [6]. Tissue histologic specimens and bacteriologic samples recovered from autopsies performed during the 1918 pandemic reveal that most deaths were likely caused by secondary bacterial pneumonias [7]. Given the significant morbidity and mortality associated with influenza and secondary bacterial pneumonia, the identification of therapeutic immune targets to alleviate this copathogenesis will have substantial clinical benefits.
The role of T cells in host defense against bacterial pneumonia is recently emerging. We have previously demonstrated that mice sequentially infected with influenza A and S. aureus resulted in influenza A-induced attenuation of S. aureus driven TH17 pathway activation and increased susceptibility to bacterial pneumonia [8]. However, the breadth and kinetics of influenza A-induced inhibition of the TH17 pathway and the explanation for the lung's increased susceptibility to bacterial infection remains unknown. In addition, the specific mechanism(s) by which the TH17 pathway promotes S. aureus clearance has not been previously examined.
METHODS
Mice
Six to eight week-old male C57BL/6 mice were purchased from Taconic Farms (Germantown, NY). Mice were maintained under pathogen-free conditions and studies performed on age- and sex-matched mice.
S. aureus Infection
Methicillin-sensitive S. aureus (MSSA; ATCC 49775) and methicillin-resistant S. aureus (MRSA; USA 300) were used to inoculate mice with either 1 × 108 colony-forming units (CFU) of MSSA or 5 × 107 of MRSA (in 50 µL sterile phosphate-buffered saline [PBS]) by oropharyngeal aspiration.
Influenza A Infection
Influenza A PR/8/34 H1N1 and Influenza A CA/07/2009 H1N1 [9, 10] were used to inoculate mice with 100 plaque-forming units (PFU) of influenza (in 40 μL sterile PBS) by oropharyngeal aspiration. Viral burden was determined by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) on lung RNA for viral matrix protein as described [8].
Analysis of Lung Inflammation
Mouse lungs were lavaged with 1 mL sterile PBS for inflammatory cell counts. Lung homogenate was used for bacterial colony counting and cytokine analysis by Lincoplex (Millipore, Billerica, MA) or by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). RNA extraction was performed (Agilent Technologies, Santa Clara, CA), and RNA analysis was performed by RT-PCR using Assay on Demand Taqman probes and primers (Applied Biosystems, Foster City, CA).
Depletion Antibodies
BioXcell (West Lebanon, NH) antibodies clones RB6-8C5 (anti-Ly6G/C) and 1A8 (anti-Ly6G) and anti-IL-17 were used. Mice received 250 µg (300 µg anti-IL-17) of antibody 24 hours prior to S. aureus challenge.
Lipocalin 2 Protein
Lipocalin 2 (Lcn2) recombinant protein purification was performed as described elsewhere [11, 12] with modifications.
S. aureus Macrophage Binding and Uptake
S. aureus was labeled with fluorescein isothiocyanate (FITC; Life Technologies). FITC-labeled S. aureus were instilled into mice, and bronchoalveolar lavage (BAL) was performed 24 hours after challenge. BAL cells were stained with fluorescent conjugated antibodies against F4/80, Ly6G, Ly6G/C (GR-1), and Cd11c for flow cytometry. For in vitro studies, alveolar macrophages were isolated from the BAL of naive, PBS control, and influenza infected mice. Flow cytometry was performed to determine total number of FITC-positive cells and the total number of FITC-positive cells in the presence and absence of interferon γ and/or interferon β.
In Vitro Epithelial Cell Experiments
Mouse C10 cells were treated with interleukin 17A (IL-17A; 10 ng/mL), interleukin 22 (IL-22; 20 ng/mL), and/or tumor necrosis factor α (TNF-α; 1 ng/mL) for 24 hours. Cell RNA was isolated by RNeasy kit (Qiagen, Valencia, CA) for RT-PCR analysis of gene expression.
Free Iron Quantification
At indicated time points, mouse lungs were lavaged with 1 mL sterile PBS and Ferrous (Fe2+) ions were measured using an iron assay kit (Abcam, Cambridge MA). Lavage samples were analyzed without assay buffer or iron reducing reagents in order to detect free iron.
Statistical Analysis
The data are presented as the mean ± standard error of the mean (SEM). Significance was tested by unpaired t test (for 2 means) or one-way analysis of variance (ANOVA; for multiple data groups) followed by Tukey post hoc test. Data were analyzed using GraphPad Prism and/or Microsoft Excel software.
RESULTS
Preceding Influenza A Infection Prolongs S. aureus Pneumonia and Attenuates Acute and Chronic TH17 Pathway Activation by S. aureus
We have previously demonstrated that preceding influenza A infection results in increased susceptibility to bacterial pneumonia at 24 and 48 hours following bacterial challenge [8], but the persistence of bacterial colonization remains unknown. We challenged C57BL/6 mice with influenza A PR/8/34 H1N1 for 6 days followed by S. aureus (ATTC 49775) and after 6, 24, 48, 72, 96, and 120 hours, bacterial and viral clearance and lung inflammation were assessed. Preceding influenza infection resulted in decreased clearance of S. aureus in the lung up to 120 hours following bacterial challenge (Figure (Figure11A). There was also significant exacerbation of lung inflammation for up to 120 hours (Supplementary Figure 1A). Histology of lungs coinfected with influenza A and S. aureus at 120 hours postbacterial challenge revealed increased inflammation and lung damage compared to S. aureus alone (Figure (Figure11C). These data show that preceding influenza A infection causes increased inflammation and S. aureus burden, as well as bacterial persistence in the lung. We proposed that there would be rapid and persistent attenuation of the TH17 pathway in coinfected mice following bacterial challenge. S. aureus induced a more robust TH17 effector cytokine response up to 120 hours following bacterial challenge in mice infected with S. aureus alone compared to coinfected mice (Figure (Figure11B, Supplementary Figure 1B and C). Importantly, we examined early TH17 cytokine responses and found that S. aureus failed to induce IL-17 (Figure (Figure11D) or interleukin 23 (IL-23; Supplementary Figure 1D) at early time points following challenge in mice previously infected with influenza. Finally, we examined whether 2009 pandemic influenza followed by S. aureus challenge would also result in decreased bacterial clearance compared to mice infected with S. aureus alone. We challenged C57BL/6 mice with influenza CA/07/2009/A H1N1 for 6 days followed by S. aureus (ATTC 49775) and after 24 hours, assessed bacterial clearance. Preceding 2009 H1N1 influenza infection resulted in decreased clearance of S. aureus in the lung following bacterial challenge (Figure (Figure11E).
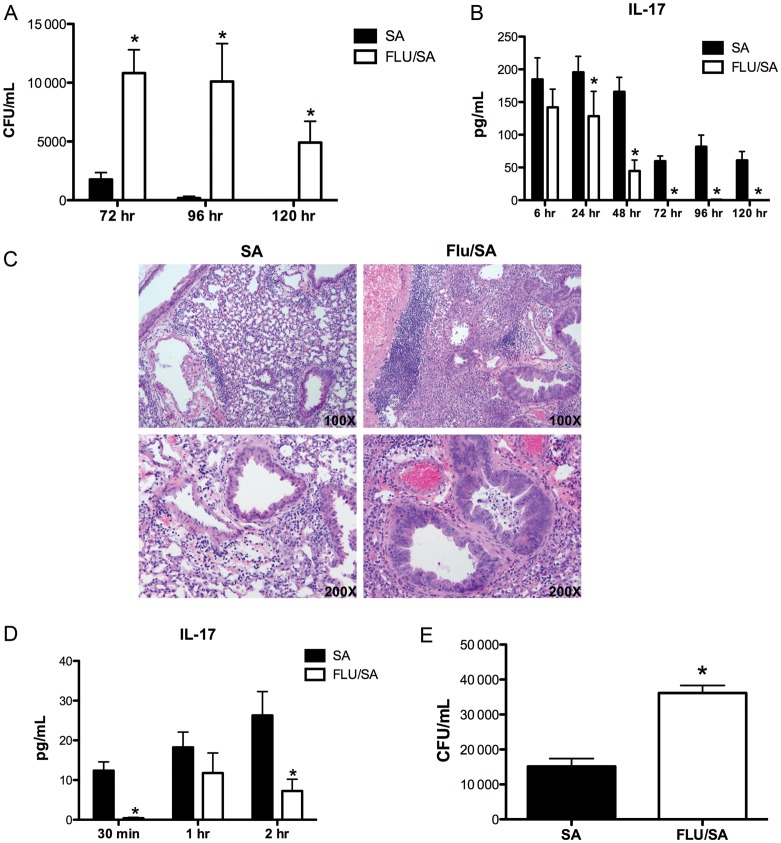
Influenza A increases the susceptibility and prolongs Staphylococcus aureus pneumonia and inhibits TH17 pathway induction by S. aureus following bacterial challenge. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 days, mice were then challenged with 108 CFU of S. aureus for 6–120 hours. A, Bacterial colony counts in the lung (n = 6–7). B, IL-17 protein production in lung homogenate as measured by ELISA (n = 6–7). C, Representative histological sections of lung at 120 hours postinfection. D, IL-17 protein production in lung homogenate as measure by Lincoplex (n = 3–4). C57BL/6 mice were infected with 100 PFU of influenza A CA/07/2009 or vehicle for 6 days, mice were then challenged with 108 CFU of S. aureus for 6–120 hours. E, Bacterial colony counts in the lung (n = 8). *P < .05 vs S. aureus alone. Significance was tested by unpaired t test. Abbreviations: CFU, colony-forming units; ELISA, enzyme-linked immunosorbent assay; PFU, plaque-forming units.
S. aureus induced production of the TH17 cytokine-associated chemokines G-CSF, and KC was inhibited by influenza infection (Supplementary Figure 2). There was no change in IFN-γ production between the groups (Figure (Figure22A), whereas interleukin 10 (IL-10) was increased coinfected mice compared to those that received S. aureus alone (Figure (Figure22B). Acute TNF-α production was also reduced in co-infected mice (Figure (Figure22C). Interleukin 6 (IL-6) production was significantly elevated at 6 hours in the mice that received S. aureus only compared to the coinfected mice but remained similar in the coinfected mice at all time points, suggesting that preceding influenza infection does not result in suppression of all cytokines below S. aureus induced levels (Figure (Figure22D). These data confirm that S. aureus induces a TH17 immune response in the lung during bacterial challenge. Further, preceding influenza A infection significantly attenuates the S. aureus-driven TH17 pathway as evidence by decreased levels of TH17 effector cytokines, TH17 promoting cytokine, and TH17 cytokine-induced chemokines as early as 30 minutes and up to 120 hours following bacterial challenge.
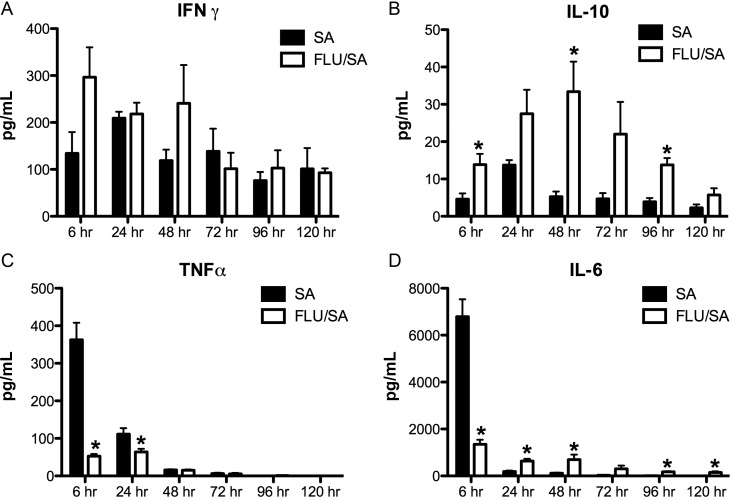
Influenza A alters inflammatory cytokine production following bacterial challenge with Staphylococcus aureus. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 days, mice were then challenged with 108 CFU of S. aureus for 6–120 hours. A–D, cytokine concentrations in lung homogenate measured by Lincoplex (n = 6–7). *P < .05 vs S. aureus alone. Significance was tested by unpaired t test. Abbreviations: CFU, colony-forming units; PFU, plaque-forming units.
Neutrophils Are Not the Primary Mechanism by Which the TH17 Pathway Promotes Bacterial Host Defense
A known function of IL-17A is to enhance inflammation through induction of neutrophil and macrophage chemokines and growth factors [13]. We have published data that suggested the decreased clearance of S. aureus in TH17 pathway knockout mice was not due to a lack of neutrophil recruitment [8]. In order to further investigate the contribution of neutrophils to S. aureus clearance in the lungs, C57BL/6 mice received neutrophil depletion antibodies anti-Ly6G (1A8) or anti-Ly6G/C (RB6-8C5 (RB6)) followed by administration of S. aureus (ATTC 49775) 24 hours later. Bacterial clearance and lung inflammation were assessed 24 hours following bacterial challenge. Mice that received RB6 antibody had severely attenuated clearance of S. aureus, whereas mice that received 1A8 antibody cleared S. aureus similar to control (Figure (Figure33A). Both RB6 antibody and 1A8 antibody resulted in neutrophil depletion in the BAL, whereas only RB6 antibody resulted in macrophage depletion (Figure (Figure33B). Mice that received RB6 or 1A8 antibody prior to bacterial challenge had increased proinflammatory and neutrophil recruitment cytokine production (Figure (Figure33C–F). Histological sections of lungs confirmed suppression of inflammation in the mice that received 1A8 or RB6 (Figure (Figure33G). Interestingly, although the RB6 exacerbated S. aureus infection, there was typical bacterial clearance in the mice that received the 1A8 antibody (neutrophil specific), suggesting that neutrophils are not the mechanism by which the TH17 pathway promotes bacterial host defense.
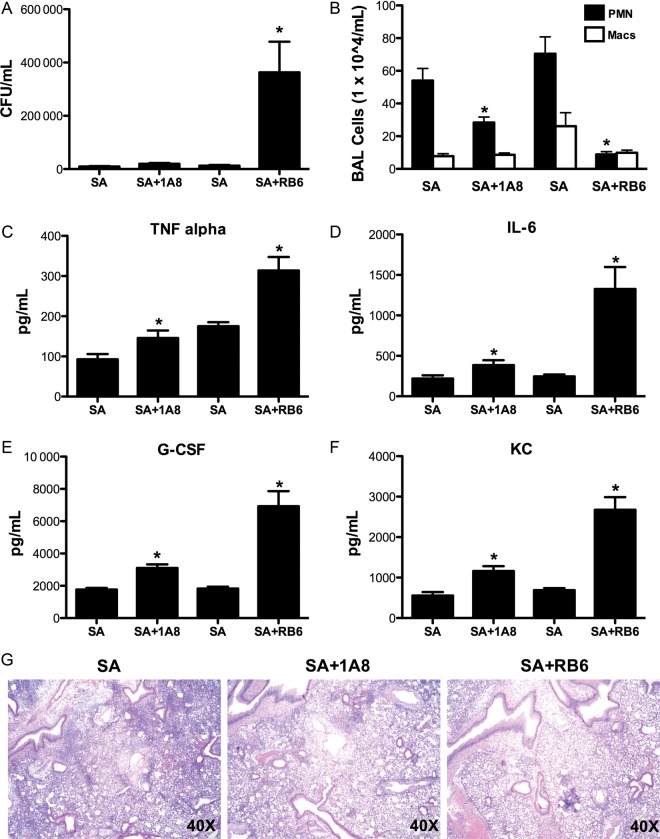
Specific neutrophil depletion does not significantly attenuate Staphylococcus aureus clearance. C57BL/6 mice received 250 µg of RB6–8C5 (anti-Ly6G/C) or 1A8 (anti-Ly6G) antibody 24 hours prior to challenge with 108 CFU of S. aureus for 24 hours. A, Bacterial colony counts in the lung (n = 10). B, Bronchoalveolar lavage cell differential counts (n = 10). C–F, TH17 pathway cytokine concentrations in lung homogenate measured by Lincoplex (n = 10). G, Representative histological sections of lung. *P < .05 vs S. aureus alone. Significance was tested by unpaired t test. Abbreviation: CFU, colony-forming units.
Alveolar Macrophage and Neutrophil Binding and Uptake of S. aureus Are Not Impaired by Preceding Influenza Infection
It is possible that preceding influenza infection directly attenuates innate immune cell killing of S. aureus in the lung (despite increased cell numbers), increasing susceptibility to secondary bacterial pneumonia. To test this, we infected mice with FITC-labeled S. aureus for 24 hours and performed flow cytometry. Examination of the S. aureus positive macrophages revealed no difference in the total number of FITC-positive cells during coinfection (Figure (Figure44A and and44B). There was a decrease in the percentage of S. aureus positive neutrophils in influenza coinfected lungs, however, coinfected mice had elevated neutrophil numbers compared to S. aureus only infected animals. The total number of S. aureus positive neutrophils was not different between groups (data not shown). Further, we isolated alveolar macrophages from influenza infected or control lungs and performed flow cytometry. Macrophages from influenza-infected lungs were able to bind and take up significantly more S. aureus than naive macrophages as determined by percent positivity and mean fluorescence (Figure (Figure44C and Supplementary Figure 3A). Finally, interferons have been suggested to inhibit macrophage uptake of bacteria during influenza co-infection [14]. We treated naive alveolar macrophages with IFN-β and/or IFN-γ prior to in vitro flow cytometry. Interferon treatment did not alter macrophage binding and uptake of S. aureus (Figure (Figure44E and Supplementary Figure 3B). These data suggest that there is no innate cellular defect in influenza-infected lungs that results in aberrant S. aureus clearance.
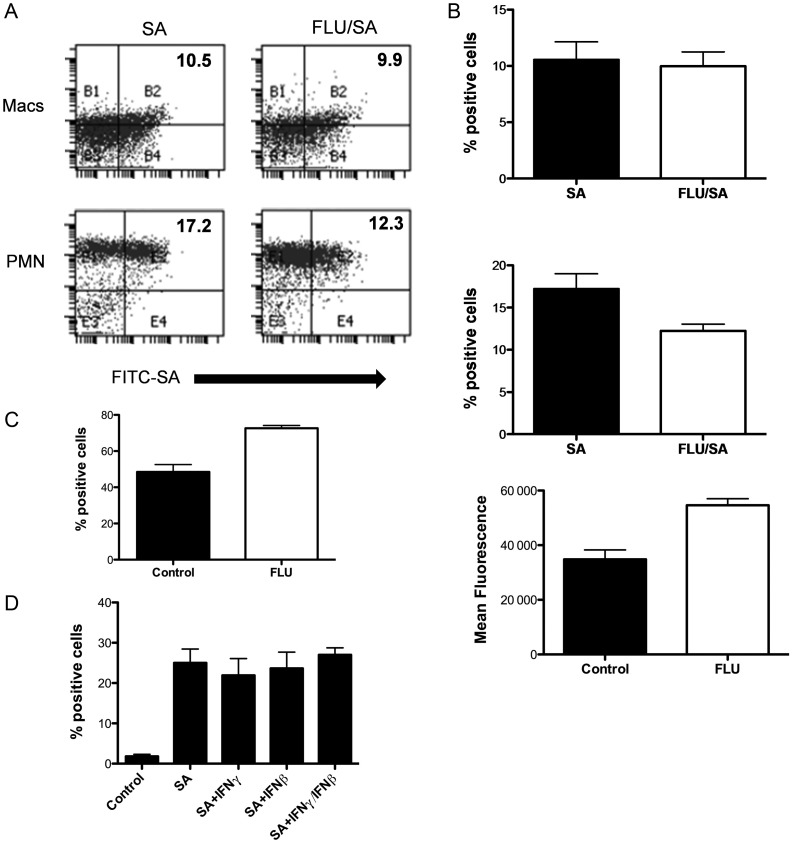
Influenza A infection does not impair alveolar macrophage or neutrophil binding and uptake of Staphylococcus aureus. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 days, mice were then challenged with 1 × 108 CFU FITC-labeled S. aureus or vehicle for 24 hours. A and B, Total FITC-positive macrophages and neutrophils from S. aureus and co-infected lungs by flow cytometry (n = 6–8). C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 days, macrophages were then collected and suspended with 1 × 107 CFU FITC-labeled S. aureus to allow for phagocytosis. C, Total FITC-positive macrophages from control and influenza-infected lungs as determined by percent positivity and mean fluorescence by flow cytometry (n = 8). Naive alveolar macrophages were stimulated with IFN-β(100 U/mL) and/or IFN-γ (5 ng/mL) prior to stimulation with FITC-labeled S. aureus. D, Total FITC-positive cells in lung by flow cytometry (n = 8–17). *P < .05 vs S. aureus alone, **P < .05 vs control. Significance was tested by unpaired t test (for 2 means) or one-way ANOVA (for multiple data groups) followed by Tukey post hoc test. Abbreviations: ANOVA, analysis of variance; CFU, colony-forming units; FITC, fluorescein isothiocyanate; IFN, interferon; PFU, plaque-forming units.
S. aureus Induces TH17 Pathway Associated Antimicrobial Peptides
Because innate immune cell uptake of S. aureus was similar in S. aureus and coinfected mice, we examined alternative pathways by which TH17 activation may promote bacterial clearance. To test if the TH17 pathway stimulates antimicrobial peptides (AMPs), C10 airway epithelial cells were stimulated with combinations of IL-17A, IL-22, with or without TNF-α. It has been shown previously that TH17 cytokines and TNF-α have synergistic effects on epithelial cells [15]. We found that TNF-α, IL-17A + TNF-α, and IL-17A + IL-22 + TNF-α treatment synergistically induced Lcn2 expression in C10 cells (Figure (Figure55A). TH17 cytokines induced expression of RegIIIβ (Figure (Figure55B) and CAMP in all groups stimulated with TNF-α (Figure (Figure55C). To determine whether S. aureus induces TH17 pathway associated AMPs in vivo, C57BL/6 mice were challenged with influenza A followed by S. aureus. S. aureus enhanced production of IL-17 and IL-22 associated AMPs: Lcn2, RegIIIαβγ, CAMP, and S100A8-9 (Figure (Figure55D–H), particularly at acute time points (6, 24 hours). Preceding influenza infection resulted in marked suppression of AMP production. To test whether IL-17 is required for S. aureus induction of AMPs in vivo, C57BL/6 mice were challenged with MRSA following the administration of anti-IL-17 antibody. At 24 hours following bacterial challenge, there was overall decreased expression of AMPs (Figure (Figure55I). These data support that S. aureus induces TH17 pathway associated AMPs and illustrate a potential mechanism by which the TH17 pathway may promote bacterial immunity in the lung. Further, these findings demonstrate suppression of AMP production by influenza A, a novel mechanism for exacerbation of secondary bacterial pneumonia.
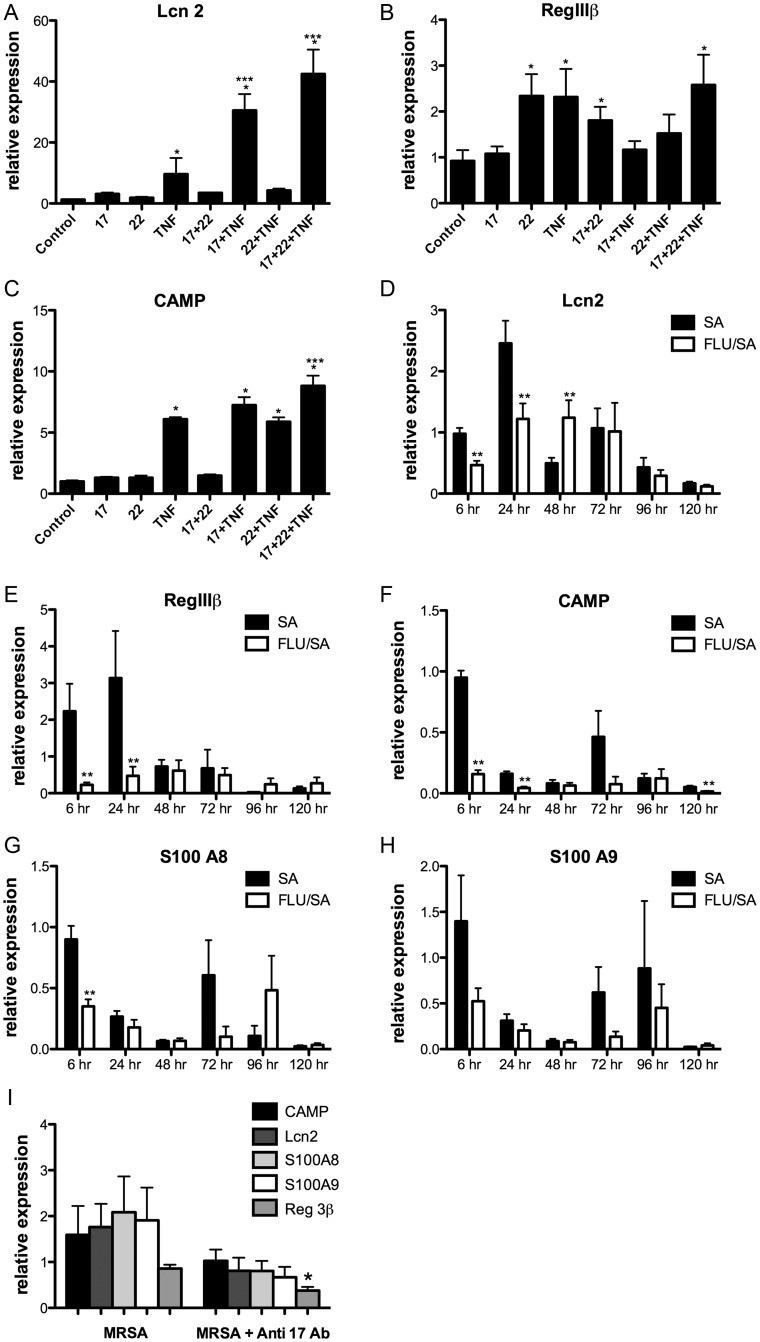
TH17 cytokines induce antimicrobial peptides in vitro, Influenza A inhibits Staphylococcus aureus induced antimicrobial peptides in vivo. C10 airway epithelial cells were stimulated with combinations of IL-17A, IL-22, and TNF-α (n = 6). A–C, TH17 pathway associated antimicrobial peptide gene expression in cell RNA. *P < .05 vs control, ***P < .05 vs TNF-α. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 days, mice were then challenged with 108 CFU of S. aureus for 6–120 hours. D–H, TH17 pathway associated antimicrobial peptide gene expression in lung RNA (n = 6–7). C57BL/6 mice received 300 µg of anti-IL-17 antibody 24 hours prior to challenge with 5 × 107 CFU of S. aureus for 24 hours. I, TH17 pathway associated antimicrobial peptide gene expression in lung RNA (n = 8). *P < .05 vs control, **P < .05 vs S. aureus alone. Significance was tested by unpaired t test (for 2 means) or 1-way ANOVA (for multiple data groups) followed by Tukey post hoc test. Abbreviations: ANOVA, analysis of variance; CFU, colony-forming units; IL-17A, interleukin 17A; IL-22, interleukin 22; PFU, plaque-forming units; TNF-α, tumor necrosis factor α.
Exogenous Lcn2 Improves S. aureus Clearance in the Lung and Rescues Influenza Exacerbation During Co-infection
We proposed that Lcn2 might aid in lung clearance of S. aureus. To test this, C57BL/6 mice received Lcn2 or a control protein and 4 hours later were challenged with S. aureus (USA 300); 24 hours after bacterial challenge, bacterial burden and lung inflammation were assessed. S. aureus clearance was increased in mice that received exogenous Lcn2 compared to control (Figure (Figure66A). There was also decreased lung inflammation (Figure (Figure66B). Next, mice were coinfected with influenza A and MRSA in the presence of Lcn2 or control protein. There was increased bacterial clearance in the mice that received exogenous Lcn2 compared to control (Figure (Figure66C–D). Thus, exogenous Lcn2 rescued S. aureus clearance in both mice infected with S. aureus alone and coinfected with influenza A. Finally, there were lower levels of ferrous (Fe2+) ions in the S. aureus challenged and coinfected mice that received exogenous Lcn2 compared to control (Figure (Figure66E). These data provide evidence that TH17 pathway induction of AMPs is a likely mechanism used to aid bacterial killing. In addition, these data suggest that Lcn2 depletes iron in the lung microenvironment, inhibiting the growth of S. aureus.
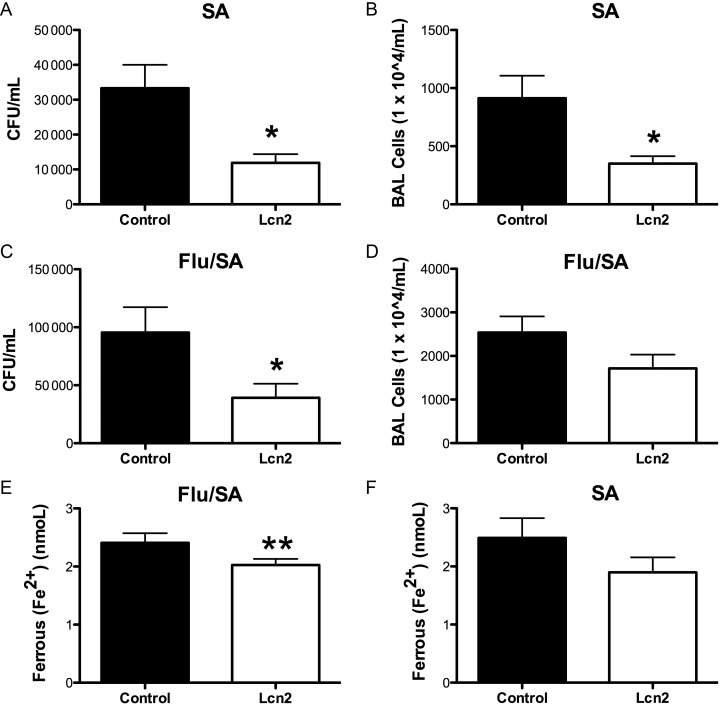
Exogenous Lcn2 rescues Staphylococcus aureus clearance. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 days, then received 100 µg of lipocalin 2 or BSA control and after 6 hours, mice were then challenged with 5 × 107 CFU of S. aureus for 24 hours. A, Bacterial colony counts in the lung of mice infected with S. aureus alone (n = 8). B, Bronchoalveolar lavage cell total counts (n = 8). C, Bacterial colony counts in the lung of mice co-infected influenza A and S. aureus (n = 8). D, Bronchoalveolar lavage cell total counts (n = 8). E, Ferrous (Fe2+) levels in the bronchoalveolar lavage (n = 7–8). *P < .05 vs control, **P < .10 vs control. Significance was tested by unpaired t test. Abbreviation: CFU, colony-forming units.
DISCUSSION
Our findings demonstrate a novel mechanism by which preceding influenza infection impairs immunity against bacterial pneumonia. The data substantiate a crucial role for the TH17 pathway in host defense against S. aureus pneumonia. Here we show that preceding influenza in mice causes persistent S. aureus infection and suppression of the TH17 pathway. Our study shows that influenza does not inhibit inflammation or S. aureus binding and uptake by phagocytic cells. Rather, influenza inhibits S. aureus induced TH17 pathway associated AMPs and attenuates bacterial clearance in the lung. Importantly, exogenous Lcn2 improved S. aureus clearance and rescued viral exacerbation of infection. In addition, exogenous Lcn2 decreased levels of free iron in BAL fluid. These data indicate a key mechanism by which TH17 pathway activation results in S. aureus clearance in the lung. Exogenous AMPs may be useful as a therapeutic target in co-infection.
Multiple mechanisms for increased susceptibility to bacterial infections following viral infection have been investigated [16]. Our current findings demonstrate that the impairment of S. aureus clearance and attenuation of the TH17 pathway by preceding influenza A is both an acute and chronic response of the immune system. The early inhibition of IL-17 production likely reflects γδT cell suppression as well as TH17. Mice challenged with S. aureus alone induced high levels of IL-17 in the lung that remained elevated following bacterial clearance. Influenza coinfection reduced this TH17 pathway activation throughout the study. Numerous influenza A viruses cause disease in mice and humans. We observed impaired S. aureus clearance during coinfection with 2 different strains of murine H1N1 influenza A infections. Different types of influenza viruses may elicit differing responses of the innate immune response and future studies will have to be completed in order to address these differences. Although we observed influenza A attenuation of the S. aureus-driven TH17 pathway, there was increased IL-10 in the coinfected mice compared to those that received S. aureus alone. Previous studies have shown that influenza infection induces IL-10 [17] and that IL-10 expression suppresses the development of TH17 cytokines during influenza infection [18]. Influenza-induced IL-10 has also been reported to enhance susceptibility to secondary pneumococcal infection [19], although a recent study showed that IFN-γ attenuation of S. pneumoniae clearance in mice was IL-10 independent [14]. In that study, IFN-γ levels were elevated in coinfected mice resulting in suppression of macrophage uptake of bacteria. We found no difference in IFN-γ levels in coinfected mice vs S. aureus alone, suggesting a minimal role for IFN-γ in the S. aureus model. In addition, we observed decreased TNF-α levels induced by S. aureus in the lungs of coinfected mice. TNF-α production by natural killer (NK) cells has been suggested to be required for S. aureus killing in the influenza coinfection model [20]. It is possible that this suppression of TNF-α in coinfected mice plays a role in impaired host defense independent of the TH17 pathway, although TNF-α is known to synergize with IL-17 in promoting immunity.
IL-17 is known to induce neutrophilia in response to infection. We have previously observed decreased S. aureus clearance in TH17 pathway knockout mice was not solely due to a lack of neutrophil recruitment to the lung [8]. Other groups have suggested influenza-induced defects in innate immune cell uptake of bacteria as a mechanism for viral exacerbation of secondary infection [14]. We investigated the role of neutrophils through antibody depletion. RB6 antibody depletes neutrophils, macrophages, and dendritic cells, whereas 1A8 antibody specifically depletes neutrophils. We observed standard clearance of S. aureus in mice that received the 1A8 antibody, confirming that there are alternate mechanisms by which influenza A inhibits S. aureus immunity. When monocytes were depleted with RB6 antibody, we observed a large increase in bacterial burden, suggesting a role for antigen presenting cells in S. aureus host defense. Decreased phagocytosis of bacteria by neutrophils in the context of influenza and S. pneumonia infection has been reported [21]. We performed in vitro and in vivo studies to determine if inflammatory cells in the lung were capable of phagocytosis of S. aureus in the presence and absence of preceding influenza infection and found that there was no change in the binding and uptake of S. aureus by macrophages or neutrophils. Further, interferon treatment did not alter macrophage binding and uptake of S. aureus in vitro. This mechanism has been suggested in the context of influenza, streptococcal infection [14]. Of note, the assay we used to determine phagocytosis measures both extracellular bound and ingested bacteria. Our data suggest that no innate cellular defect in influenza-infected lungs is present during S. aureus coinfection. Future studies will have to be performed in order to differentiate between extracellular bound and ingested S. aureus by macrophages.
Because influenza coinfected mice have elevated innate immune cell recruitment compared to S. aureus alone and there was no evidence of a defect in bacterial binding and uptake by these cells, we examined the ability of S. aureus to induce TH17 pathway associated AMPs. S. aureus drove the expression of several AMPs; however, this was attenuated in influenza coinfected mice. IL-17 and IL-22 can induce production of several AMPs including serum amyloid A, Lcn2, β-defensins, S100 proteins, and RegIIIαβγ [13, 22–26]. Specifically, Lcn2 can inhibit bacterial growth by sequestering enterobactin and depriving bacteria of iron essential for growth [27]. Although previous studies have shown that Lcn2 is important to host defense against gram-negative and gram-positive bacteria, no prior evidence has shown that Lcn2 aids in immunity against MRSA pneumonia [28–31]. Here we show that IL-17, IL-22, with or without TNF-α can induce epithelial production of AMPs. Further, S. aureus induction of these peptides requires IL-17. Our data suggest that preceding influenza A infection attenuates the TH17 pathway and subsequent production of AMPs, allowing for a TH17 dependent mechanism for S. aureus clearance. In support of this finding, exogenous Lcn2 rescued S. aureus clearance in mice infected with S. aureus alone and coinfected with influenza A, confirming the role of AMPs as a mechanism for bacterial killing. Although a prior investigation by Flo et al has shown that Lcn2 deficiency has no effect on bacterial killing of S. aureus [28], these experiments were performed in vitro rather than in vivo. They also found that Lcn2 deficiency had no effect on survival of mice infected with methicillin-sensitive S. aureus intraperitoneally. This conflicting evidence may be a result of different strains of S. aureus or different locations of infection. In our studies, Lcn2 rescued the bacterial clearance of S. aureus independent of rescuing the TH17 pathway (data not shown), suggesting that AMP production occurs downstream of IL-17. Exogenous Lcn2 decreased levels of ferrous ions in the BAL fluid of mice coinfected with S. aureus and influenza A, suggesting that Lcn2 treatment results in iron sequestration and inhibits bacterial growth of gram-positive bacteria. These findings are significant given the morbidity and mortality associated with influenza and secondary bacterial pneumonia. These data expand our current knowledge regarding host defense, and future studies may allow for the identification of exogenous AMPs as therapeutic targets. The role of TH17 immunity is well described in other sites of mucosal host defense, allowing for broader relevance beyond the lung and its epithelium. In addition, influenza A inhibition of TH17 immunity may be important to a spectrum of pathogens in addition to S. aureus. The identification of TH17 effector mechanisms involved in bacterial clearance provides a novel therapeutic target in viral, bacterial coinfection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We would like to thank the Children's Hospital of Pittsburgh of UPMC Flow Cytometry Core and particularly Allison Logar and Joshua Michel for assistance with this study.
We would like to thank the Children's Hospital of Pittsburgh of UPMC Flow Cytometry Core and particularly Allison Logar and Joshua Michel for assistance with this study.
Financial support. This work was supported by the Parker B. Francis Foundation Fellowship (to J. F. A.), the Children's Hospital of Pittsburgh Research Advisory Committee Start-up Grant (to J. F. A.), and the National Institutes of Health
National Heart, Lung, and Blood Institute (R01 HL107380 to J. F. A.).
This work was supported by the Parker B. Francis Foundation Fellowship (to J. F. A.), the Children's Hospital of Pittsburgh Research Advisory Committee Start-up Grant (to J. F. A.), and the National Institutes of Health
National Heart, Lung, and Blood Institute (R01 HL107380 to J. F. A.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Articles from The Journal of Infectious Diseases are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/infdis/jit527
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3935471?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/infdis/jit527
Article citations
Damping excessive viral-induced IFN-γ rescues the impaired anti-Aspergillus host immune response in influenza-associated pulmonary aspergillosis.
EBioMedicine, 108:105347, 30 Sep 2024
Cited by: 0 articles | PMID: 39353282 | PMCID: PMC11472711
RSV enhances <i>Staphylococcus aureus</i> bacterial growth in the lung.
Infect Immun, 92(10):e0030424, 16 Aug 2024
Cited by: 0 articles | PMID: 39150268
Cell-intrinsic regulation of phagocyte function by interferon lambda during pulmonary viral, bacterial super-infection.
PLoS Pathog, 20(8):e1012498, 23 Aug 2024
Cited by: 0 articles | PMID: 39178311 | PMCID: PMC11376568
Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2.
Open Med (Wars), 19(1):20240953, 15 Apr 2024
Cited by: 0 articles | PMID: 38633219 | PMCID: PMC11022039
STAT1 regulates neutrophil gelatinase B-associated lipocalin induction in influenza-induced myocarditis.
Sci Rep, 14(1):11124, 15 May 2024
Cited by: 0 articles | PMID: 38750107 | PMCID: PMC11096373
Go to all (92) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1β production in mice.
J Immunol, 191(10):5153-5159, 02 Oct 2013
Cited by: 93 articles | PMID: 24089191 | PMCID: PMC3827735
Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice.
Am J Physiol Lung Cell Mol Physiol, 309(2):L158-67, 22 May 2015
Cited by: 85 articles | PMID: 26001778 | PMCID: PMC4504975
The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia.
Respir Res, 16:10, 05 Feb 2015
Cited by: 48 articles | PMID: 25651926 | PMCID: PMC4324414
Immunopathogenesis of Staphylococcus aureus pulmonary infection.
Semin Immunopathol, 34(2):281-297, 31 Oct 2011
Cited by: 86 articles | PMID: 22037948 | PMCID: PMC3577067
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (3)
Grant ID: R01HL107380
Grant ID: R37 HL079142
Grant ID: R01 HL107380
NICHD NIH HHS (1)
Grant ID: T32 HD071834





