Abstract
Background
ALK fusion genes occur in a subset of non-small-cell lung cancers (NSCLCs). We assessed the tolerability and activity of crizotinib in patients with NSCLC who were prospectively identified to have an ALK fusion within the first-in-man phase 1 crizotinib study.Methods
In this phase 1 study, patients with ALK-positive stage III or IV NSCLC received oral crizotinib 250 mg twice daily in 28-day cycles. Endpoints included tumour responses, duration of response, time to tumour response, progression-free survival (PFS), overall survival at 6 and 12 months, and determination of the safety and tolerability and characterisation of the plasma pharmacokinetic profile of crizotinib after oral administration. Responses were analysed in evaluable patients and PFS and safety were analysed in all patients. This study is registered with ClinicalTrials.gov, number NCT00585195.Findings
Between Aug 27, 2008, and June 1, 2011, 149 ALK-positive patients were enrolled, 143 of whom were included in the response-evaluable population. 87 of 143 patients had an objective response (60·8%, 95% CI 52·3-68·9), including three complete responses and 84 partial responses. Median time to first documented objective response was 7·9 weeks (range 2·1-39·6) and median duration of response was 49·1 weeks (95% CI 39·3-75·4). The response rate seemed to be largely independent of age, sex, performance status, or line of treatment. Median PFS was 9·7 months (95% CI 7·7-12·8). Median overall survival data are not yet mature, but estimated overall survival at 6 and 12 months was 87·9% (95% CI 81·3-92·3) and 74·8% (66·4-81·5), respectively. 39 patients continued to receive crizotinib for more than 2 weeks after progression because of perceived ongoing clinical benefit from the drug (12 for at least 6 months from the time of their initial investigator-defined disease progression). Overall, 144 (97%) of 149 patients experienced treatment-related adverse events, which were mostly grade 1 or 2. The most common adverse events were visual effects, nausea, diarrhoea, constipation, vomiting, and peripheral oedema. The most common treatment-related grade 3 or 4 adverse events were neutropenia (n=9), raised alanine aminotransferase (n=6), hypophosphataemia (n=6), and lymphopenia (n=6).Interpretation
Crizotinib is well tolerated with rapid, durable responses in patients with ALK-positive NSCLC. There seems to be potential for ongoing benefit after initial disease progression in this population, but a more formal definition of ongoing benefit in this context is needed.Free full text

Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study
Associated Data
Summary
Background
ALK fusion genes occur in a subset of non-small-cell lung cancers (NSCLCs). We assessed the tolerability and activity of crizotinib in patients with NSCLC who were prospectively identified to have an ALK fusion within the first-in-man phase 1 crizotinib study.
Methods
In this phase 1 study, patients with ALK-positive stage III or IV NSCLC received oral crizotinib 250 mg twice daily in 28-day cycles. Endpoints included tumour responses, duration of response, time to tumour response, progression-free survival (PFS), overall survival at 6 and 12 months, and determination of the safety and tolerability and characterisation of the plasma pharmacokinetic profile of crizotinib after oral administration. Responses were analysed in evaluable patients and PFS and safety were analysed in all patients. This study is registered with ClinicalTrials.gov, number NCT00585195.
Findings
Between Aug 27, 2008, and June 1, 2011, 149 ALK-positive patients were enrolled, 143 of whom were included in the response-evaluable population. 87 of 143 patients had an objective response (60·8%, 95% CI 52·3–68·9), including three complete responses and 84 partial responses. Median time to first documented objective response was 7·9 weeks (range 2·1–39·6) and median duration of response was 49·1 weeks (95% CI 39·3–75·4). The response rate seemed to be largely independent of age, sex, performance status, or line of treatment. Median PFS was 9·7 months (95% CI 7·7–12·8). Median overall survival data are not yet mature, but estimated overall survival at 6 and 12 months was 87·9% (95% CI 81·3–92·3) and 74·8% (66·4–81·5), respectively. 39 patients continued to receive crizotinib for more than 2 weeks after progression because of perceived ongoing clinical benefit from the drug (12 for at least 6 months from the time of their initial investigator-defined disease progression). Overall, 144 (97%) of 149 patients experienced treatment-related adverse events, which were mostly grade 1 or 2. The most common adverse events were visual effects, nausea, diarrhoea, constipation, vomiting, and peripheral oedema. The most common treatment-related grade 3 or 4 adverse events were neutropenia (n=9), raised alanine aminotransferase (n=6), hypophosphataemia (n=6), and lymphopenia (n=6).
Interpretation
Crizotinib is well tolerated with rapid, durable responses in patients with ALK-positive NSCLC. There seems to be potential for ongoing benefit after initial disease progression in this population, but a more formal definition of ongoing benefit in this context is needed.
Funding
Pfizer.
Introduction
Activation of the ALK gene has been described in several human cancers, including non-small-cell lung cancer (NSCLC), inflammatory myofibroblastic tumours, neuroblastomas, and diffuse large B-cell lymphomas, suggesting that ALK-mediated signalling might play a part in the development or progression of these tumours.1–3 Activation of the ALK gene is usually through chromosomal rearrangement resulting in the placement of one of several different 5′ fusion partners and their associated promoter region upstream of the kinase domain of ALK.
ALK rearrangements in NSCLC were first described in 20074,5 and have an estimated prevalence of 3–5% in series mostly dominated by adenocarcinoma on histology.6,7 EML4–ALK is the most common ALK fusion gene in NSCLC and occurs as several variants with different breakpoints in the EML4 gene.8,9 Other, more rare non-EML4 fusions, including KIF5B–ALK and TFG-ALK, have also been described in lung cancer.5,9 Their exact frequency and clinical significance remain under investigation but, by analogy with EML4 and other oncogenic ALK fusions,10 they also probably represent targets for therapeutic ALK inhibition in NSCLC. ALK fusions typically occur independently of EGFR and KRAS gene mutations,11–15 although these aberrations are not mutually exclusive.11,15,16 In the recent Lung Cancer Mutation Consortium series,17 8% of ALK-positive adenocarcinomas were also positive for either an EGFR or KRAS mutation.
Crizotinib (PF-02341066) is a potent, orally available, ATP-competitive, small-molecule inhibitor of ALK and c-Met receptor tyrosine kinase, with half maximum inhibitory concentration values of 5–25 nmol/L.18,19 Preclinical testing against over 120 kinases showed crizotinib to be highly (>20 times) selective for these targets.18
The first-in-man crizotinib study began in 2006 with a dose-escalation phase undertaken in patients with solid tumours, which was followed by protocol-defined patient prescreening for evidence of ALK or MET activation in specific tumour types. Patients with ALK-positive or MET-positive tumours were enrolled into a series of molecularly defined expansion cohorts at the proposed recommended phase 2 dose (250 mg twice daily in 28-day cycles).
After the discovery of ALK gene rearrangements in NSCLC and promising results in two patients with ALK-positive NSCLC enrolled during the dose-escalation phase of the study,20,21 the protocol was amended and an additional ALK-positive NSCLC expanded cohort was instigated in 2008. Response data from the first 19 evaluable patients with ALK-positive NSCLC within the cohort revealed a high proportion of objective responses (53%).20 Subsequent data from the first 82 patients confirmed these findings (57%).21
Here, we present an updated analysis of patients with ALK-positive NSCLC who were treated with crizotinib in the first-in-man single-arm crizotinib study before the data cutoff of June 1, 2011.
Methods
Patients
The design, methods, and objectives of this phase 1 single-arm study have been described previously21 and are briefly summarised here. Patients aged 18 years or older with measurable ALK-positive stage III or IV NSCLC (defined by a break-apart fluorescence in-situ hybridisation assay), adequate organ function, and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1 were eligible for enrolment. Patients with an ECOG PS score of 2 were eligible on investigator and sponsor agreement. With the exception of alopecia, resolution of all previous acute treatment-related toxic effects to grade 1 or less was required and patients were excluded if they had received systemic anticancer treatment, radiation treatment, or major surgery within 2 weeks before starting study treatment. Additional key exclusion criteria included previous ALK-directed treatment; previous high-dose chemotherapy needing haemopoietic-stem-cell rescue; brain metastases, spinal cord compression, carcinomatous meningitis, or lepto-meningeal disease unless appropriately treated and neurologically stable for at least 2 weeks; myocardial infarction, severe or unstable angina, coronary or peripheral artery bypass graft, congestive heart failure, or cerebrovascular accident including transient ischaemic attack within 12 months or pulmonary embolus within 6 months before starting study treatment; ongoing cardiac dysrhythmias of National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 grade 2 or higher, uncontrolled atrial fibrillation of any grade, or QT interval, corrected over 470 ms; uncontrolled hypertension; and use of drugs that are known potent cytochrome P450 3A4 inducers within 12 days before the first dose of crizotinib. Patients participating in the study were treated at sites in the USA, Australia, and South Korea. The protocol was approved by the investigational review board at each study site, and all patients provided written informed consent before enrolment.
Procedures
Patients received oral crizotinib 250 mg twice daily in 28-day cycles. Tumour response was assessed every 8 weeks (two cycles) using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0,22 with confirmation of complete response or partial response a minimum of 4 weeks after initial response. The response-evaluable population was defined as patients who received at least one dose of crizotinib and had an adequate baseline disease assessment (ie, had a scan done no more than 35 days before the first dose of the study drug and had a scan showing disease that was evaluable per RECIST) plus had either at least one post-baseline disease assessment at least 6 weeks after the first dose or had withdrawn from the study, or those patients who had withdrawn from the study or progressed or died without receiving a second scan at least 6 weeks after the first dose. Patients who had withdrawn, progressed, or died in these latter groups were classified as non-responders. Patients with investigator-defined tumour progression were allowed to continue study treatment if, in the opinion of the investigator, there was reasonable evidence of ongoing clinical benefit. In such patients, local ablative treatments such as surgery or radiation to sites of progression could be used, but the use of additional systemic anticancer drugs, other than the continued use of crizotinib after disease progression, was not allowed.
Safety was assessed at least every 2 weeks for the first 8 weeks of treatment and at least every 4 weeks thereafter until cycle 10, when visits every 8 weeks were permissible. Safety assessments included physical examination, documentation of adverse events, and routine laboratory tests including haematology (eg, haemoglobin, platelet, and white blood cell counts), chemistry (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and lactate dehydrogenase concentrations), coagulation (prothrombin time and activated partial thromboplastin time), and urinalysis. Adverse events were graded according to NCI CTCAE version 3.0. After initial reports of visual disturbances,21 the clustered term visual effects—including diplopia, photopsia, blurred vision, visual impairment, and vitreous floaters—was introduced to record such reports effectively.
Endpoints included assessment of antitumour activity as measured by tumour response by RECIST, duration of response, time to tumour response, progression-free survival (PFS), overall survival at 6 and 12 months, and determination of the safety and tolerability and characterisation of the plasma pharmacokinetic profile of crizotinib after oral administration.
Statistical analyses
Primary tumour response analyses were based on investigator assessment of tumour data, as per RECIST version 1.0.22 Time-to-event data were estimated using the Kaplan–Meier method to generate median event times with two-sided 95% CIs (by the Brookmeyer–Crowley method) and 6-month and 12-month overall survival probabilities. Median duration of follow-up for PFS and overall survival including quartiles were estimated using the reverse Kaplan–Meier method. All analyses were done with SAS statistical software, version 9.2.
This study is registered with ClinicalTrials.gov, number NCT00585195.
Role of the funding source
The sponsor of the study participated in the study design, data collection, data analysis, and data interpretation. The report was written by the corresponding author with contributions and review by all coauthors, including those employed by the sponsor. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The first patient in the ALK-positive NSCLC cohort was enrolled on Aug 27, 2008, and received their first dose on Aug 28, 2008, and the last patient was enrolled on May 29, 2011, and received their first dose on June 1, 2011. The data cutoff for this study was June 1, 2011. Table 1 lists the baseline clinicopathological characteristics for the 149 patients enrolled before the data cutoff. At the data cutoff, the median duration of treatment was 43·1 weeks (range 0·1–138·6) and treatment was ongoing in 82 patients (55%), 52 of whom had yet to experience disease progression per RECIST.
Table 1
Patient demographics and baseline disease characteristics
| Patients (n=149) | |
|---|---|
| Age (years) | 52 (21–86) |
|
| |
| Men | 73 (49%) |
|
| |
| Women | 76 (51%) |
|
| |
| Ethnic origin | |
 White White | 95 (64%) |
 Asian Asian | 41 (28%) |
 Other Other | 13 (9%) |
|
| |
| Smoking status | |
 Never Never | 106 (71%) |
 Former Former | 42 (28%) |
 Present Present | 1 (<1%) |
|
| |
| Histological findings | |
 Adenocarcinoma Adenocarcinoma | 144 (97%) |
 Large-cell carcinoma Large-cell carcinoma | 1 (<1%) |
 Squamous-cell carcinoma Squamous-cell carcinoma | 2 (1%) |
 Other Other | 2 (1%) |
|
| |
| ECOG PS score | |
 0 0 | 56 (38%) |
 1 1 | 75 (50%) |
 ≥2 ≥2 | 18 (12%) |
|
| |
| Number of previous advanced or metastatic treatment regimens | |
 0 0 | 24 (16%) |
 1 1 | 47 (32%) |
 2 2 | 31 (21%) |
 3 3 | 19 (13%) |
 ≥4 ≥4 | 28 (19%) |
Data are median (range) or number (%). Some % do not sum to 100 because of rounding. ECOG PS=Eastern Cooperative Oncology Group performance status.
The overall response-evaluable population consisted of 143 patients. The remaining six patients did not have adequate baseline scans. Within this population, 87 achieved an objective response (60·8%, 95% CI 52·3–68·9): three patients had a complete response and 84 had a partial response. Disease control (ie, complete response, partial response, or stable disease) was achieved by 118 patients (82·5%, 95% CI 75·3–88·4) at week 8 and 101 patients (70·6%, 62·4–77·9) at week 16. Figure 1 shows the best percent change from baseline in size of target lesions for patients with measurable disease (n=133), excluding those with early death before repeat imaging, those without an interpretable response assessment scan, or those who had only non-target lesions. 125 patients (94%) experienced some degree of tumour shrinkage during the study (figure 1).
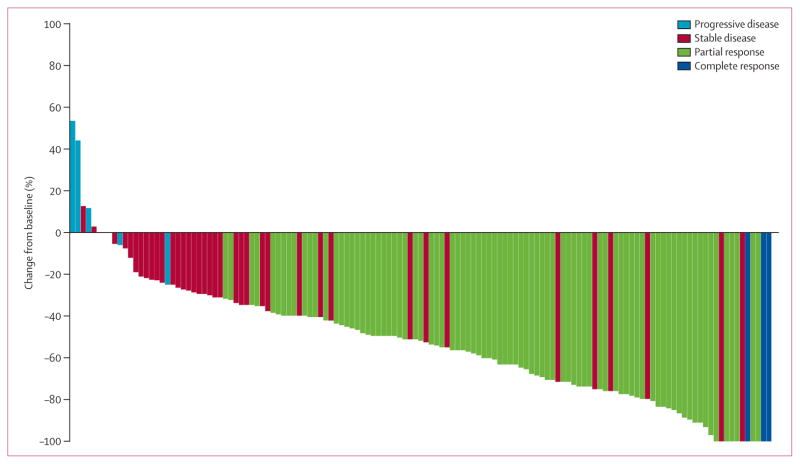
*Excludes patients with early death before re-imaging, non-measurable non-target disease, or indeterminate responses: five patients with a best overall response of indeterminate either had available on-study scans that could not be assessed or discontinued the study before to obtaining adequate scans to assess response; three patients died within 42 days from first dose; and two patients had non-target lesions only.
The median time to first documented objective response was 7·9 weeks (range 2·1–39·6)—ie, at the first protocol-specified assessment. However, some patients had responded within days of treatment with crizotinib, as shown on non-protocol-mandated scans done at individual investigators’ discretion.23,24 Responses seemed durable, with an estimated median response duration of 49·1 weeks (95% CI 39·3–75·4; based on Kaplan–Meier estimates). At the time of this analysis (June 1, 2011) 46 (53%) of 87 responders had disease progression or had died.
In an analysis of response according to patient characteristics, the proportion of patients with an objective response was similar regardless of age (<65 years, ≥65 years) or sex (table 2). The proportion of patients with an objective response was high in patients with a poor ECOG PS score and among those who had received multiple lines of previous treatment for advanced or metastatic disease. The proportion of patients who had an objective response seemed to be higher in Asian than in non-Asian patients (table 2).
Table 2
Objective response rate according to patient characteristics
| n/N | Proportion with objective response (95% CI)* | |
|---|---|---|
| Age | ||
|
| ||
| <65 years | 74/123 | 60·2% (50·9–68·9) |
| ≥65 years | 13/20 | 65·0% (40·8–84·6) |
|
| ||
| Sex | ||
|
| ||
| Men | 46/71 | 64·8% (52·5–75·8) |
| Women | 41/72 | 56·9% (44·7–68·6) |
|
| ||
| ECOG PS score | ||
|
| ||
| 0 | 29/53 | 54·7% (40·4–68·4) |
| 1 | 46/72 | 63·9% (51·7–74·9) |
| 2 | 12/17 | 66·7% (44·0–89·7) |
| 3 | 0/1 | 0·0% (0·0–97·5) |
|
| ||
| Number of previous advanced or metastatic systemic treatments | ||
|
| ||
| 0 | 14/22 | 63·6% (40·7–82·8) |
| 1 | 26/44 | 59·1% (43·2–73·7) |
| 2 | 20/31 | 64·5% (45·4–80·8) |
| ≥3 | 27/46 | 58·7% (43·2–73·0) |
|
| ||
| Ethnic origin | ||
|
| ||
| Asian | 30/39 | 76·9% (60·7–88·9) |
| Non-Asian | 57/104 | 54·8% (44·7–64·6) |
143 patients were evaluable for response. ECOG PS=Eastern Cooperative Oncology Group performance status.
Median follow-up for PFS was 16·3 months (95% CI 13·8–18·4; quartiles: 25% 10·4, 75% 20·9), and the estimated median PFS was 9·7 months (95% CI 7·7–12·8) for all patients who received at least one dose of crizotinib (figure 2). In patients receiving first-line crizotinib (n=24), median PFS was 18·3 months (95% CI 8·3 to not reached; appendix), and in patients receiving crizotinib as second-line or later treatment (n=125), median PFS was 9·2 months (95% CI 7·3–12·7). At the time of data cutoff, there had been 85 PFS events (69 disease progressions and 16 deaths without documented disease progression) and 64 patients were censored. 52 (81%) of the 64 censored patients remained in follow-up for PFS, with the others censored because of absence of adequate baseline assessments (n=2), no on-study disease assessments (n=4), starting of a new anticancer treatment before tumour progression (n=2), and unacceptably long gap between disease progression or death and the most recent disease assessment (n=4). Among the 67 patients who had stopped treatment, the reasons for doing so were RECIST-defined progressive disease (n=41), death (n=15), adverse events (n=6; three were crizotinib-related: two pneumonitis and one increased ALT), and clinical or non-RECIST-defined disease progression (n=5). Dosing was interrupted in 63 patients, although in 21 of these patients the dose interruption lasted less than 1 week.
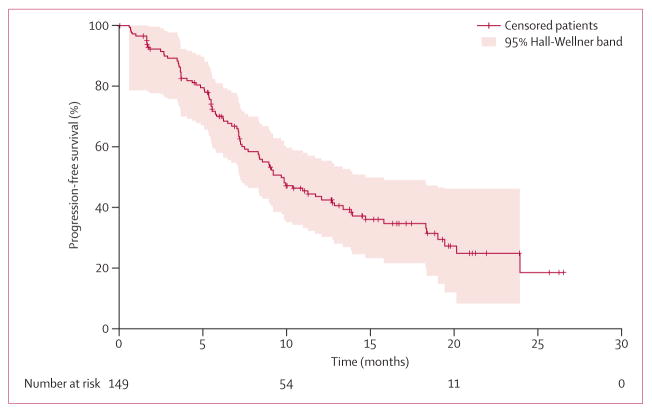
64 patients were censored, of whom 52 remained in follow-up for progression-free survival.
At the time of data cutoff, median overall survival had not been reached; 101 (68%) of 149 of the patients were still in follow-up for survival, 46 (31%) had experienced events, and the remaining two (1%) were censored and no longer assessed for survival. The median duration of follow-up for overall survival was 16·6 months (95% CI 15·0–18·6; quartiles: 25% 11·6, 75% 21·5). Preliminary estimates of the 6-month and 12-month overall survival were 87·9% (95% CI 81·3–92·3) and 74·8% (66·4–81·5), respectively.
Of the 69 patients with investigator-documented disease progression, 39 continued to receive crizotinib for more than 2 weeks after disease progression because, in the opinion of the investigators, they were deriving ongoing clinical benefit from the drug (table 3; figure 3). 12 of these patients received crizotinib for at least 6 months from the time of their initial investigator-defined disease progression. Excluding predefined target lesions, the most common sites of investigator-defined disease progression in these 39 patients were brain (n=10), lung (n=5), and liver (n=3).
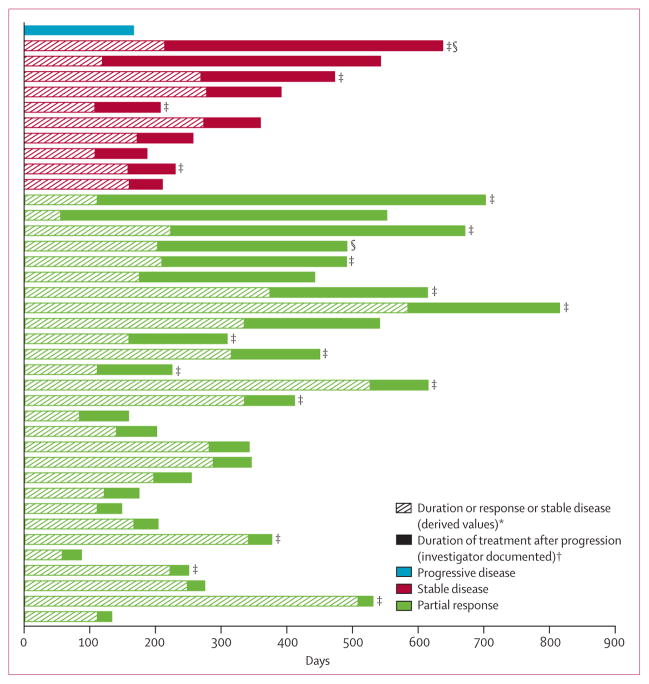
Patients are ordered by initial best response before progression and duration of crizotinib treatment after progression (n=39). *Defined as the time (in weeks) from the first documentation of objective tumour response (complete response or partial response) that was subsequently confirmed, to the first documentation of progressive disease or death. Stable disease duration was calculated from the date of the first dose to the date of first documented disease progression. †Defined as time from investigator-documented progressive disease to the last date of crizotinib dose or censor at the time of analysis. Disease progression and best objective response were derived according to Response Evaluation Criteria in Solid Tumors. ‡Treatment ongoing at the time of analysis. §Received crizotinib as first-line treatment.
Table 3
Patients who received crizotinib for more than 2 weeks after progression
| Best objective response | Site of initial progression | Duration of treatment after progression (days)* | |
|---|---|---|---|
| 1 | Progressive disease | Brain | 169 |
| 2 | Stable disease | Target lesions | 49 |
| 3 | Stable disease | Target lesions | >70 |
| 4 | Stable disease | Diaphragm | 79 |
| 5 | Stable disease | Brain | 82 |
| 6 | Stable disease | Target lesions | 85 |
| 7 | Stable disease | Lung, pleural effusion | >98 |
| 8 | Stable disease | Target lesions | 114 |
| 9 | Stable disease | Brain | >203 |
| 10 | Stable disease | Target lesions | >422 |
| 11 | Stable disease | Brain | 422 |
| 12 | Partial response | Target lesions | >22 |
| 13 | Partial response | Target lesions, lung, liver | 22 |
| 14 | Partial response | Target lesions, pleural effusion | 26 |
| 15 | Partial response | Clinical progression | >28 |
| 16 | Partial response | Liver | 29 |
| 17 | Partial response | Target lesions | >34 |
| 18 | Partial response | Target lesions | 35 |
| 19 | Partial response | Target lesions | 38 |
| 20 | Partial response | Target lesions, lymph nodes | 53 |
| 21 | Partial response | Target lesions, gluteal soft tissue | 57 |
| 22 | Partial response | Target lesions | 57 |
| 23 | Partial response | Target lesions | 60 |
| 24 | Partial response | Liver | 62 |
| 25 | Partial response | Target lesions | 75 |
| 26 | Partial response | Target lesions, lung nodules | >77 |
| 27 | Partial response | Brain | >88 |
| 28 | Partial response | Brain | >114 |
| 29 | Partial response | Brain | >134 |
| 30 | Partial response | Target lesions | >150 |
| 31 | Partial response | Lung nodules | 205 |
| 32 | Partial response | Clinical progression | >233 |
| 33 | Partial response | Brain | >241 |
| 34 | Partial response | Target lesions | 266 |
| 35 | Partial response | Brain | >282 |
| 36 | Partial response | Adrenal glands | 288 |
| 37 | Partial response | Target lesions | >447 |
| 38 | Partial response | Target lesions | 496 |
| 39 | Partial response | Brain | >591 |
CNS imaging before progression and body or CNS surveillance, decision-making, and capture of additional progression events after initial progression were not standardised within this study.
144 (97%) of 149 patients experienced treatment-related adverse events (table 4), 108 of whom reported adverse events of grade 1 or 2 severity. The most frequently occurring treatment-related adverse events were visual effects, gastrointestinal events (nausea, diarrhoea, vomiting, and constipation), and peripheral oedema. Nausea, vomiting, and diarrhoea occurred early in treatment (range of median times to first onset 2–5 days; range 1–518), with visual effects occurring slightly later (median time to onset 14·5 days; range 1–173). By contrast, the median time to onset of oedema was 85 days (range 1–617). During treatment, the prevalence of common treatment-related grade 1 gastrointestinal adverse events and visual effects decreased over time, whereas that of oedema increased with continuing treatment (figure 4). The prevalence of treatment-related grade 2 events remained stable throughout treatment cycles.
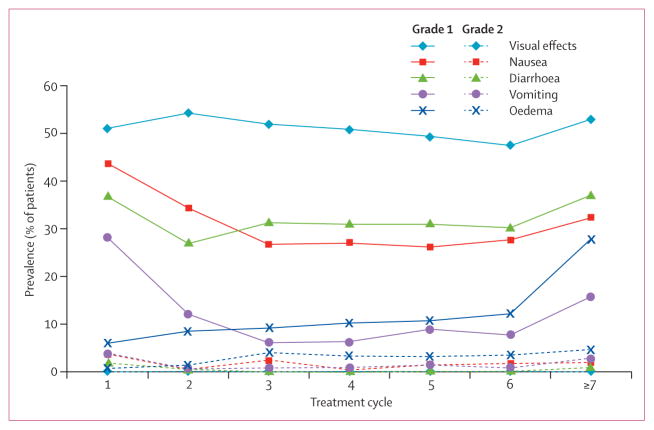
Prevalence of adverse events for cycle 7 and beyond is presented as the proportion of patients at risk who experienced a particular event.
Table 4
Treatment-related adverse events reported in at least 10% of patients in the safety population (N=149), by descending frequency (all grades)
| All grades | Grade 3 or 4 | |
|---|---|---|
| Any adverse event | 144 (97%) | 36 (24%) |
| Visual effects* | 96 (64%) | 0 (0%) |
| Nausea | 84 (56%) | 1 (<1%) |
| Diarrhoea | 74 (50%) | 0 (0%) |
| Vomiting | 58 (39%) | 1 (<1%) |
| Peripheral oedema | 44 (30%) | 0 (0%) |
| Constipation | 41 (28%) | 1 (<1%) |
| Dizziness | 31 (21%) | 0 (0%) |
| Decreased appetite | 24 (16%) | 0 (0%) |
| Fatigue | 24 (16%) | 2 (1%) |
| Increased alanine aminotransferase | 18 (12%) | 6 (4%) |
| Rash | 17 (11%) | 0 (0%) |
| Dysgeusia | 16 (11%) | 0 (0%) |
| Increased aspartate aminotransferase | 15 (10%) | 5 (3%) |
Data are number (%). See text for further details of grade 3 or 4 adverse events.
Visual effects were of grade 1 severity and were described as light trails, flashes, or brief image persistence (post-flashbulb effect). The flipped dark-light registration of high-contrast images, such as stripes, was also reported. Investigators reported that these visual effects usually occurred at the edges of the visual field and were most pronounced on changing from low to bright light conditions. No patient needed dosing interruption, dose reduction, or permanent discontinuation of crizotinib treatment because of visual effects.
36 patients experienced treatment-related grade 3 or 4 events (grade 3 unless otherwise stated) comprising neutropenia (n=9, including one grade 4), raised ALT (n=6, including one grade 4), hypophosphataemia (n=6), lymphopenia (n=6), raised AST (n=5), pneumonitis (n=3, including one grade 4), fatigue (n=2), and nausea, vomiting, constipation, dysphagia, anaemia, peripheral neuropathy, dyspnoea, hyponatraemia, subcutaneous emphysema, increased aminotransferases, urinary tract infection, spontaneous abortion (experienced by the patient’s partner), increased blood glucose, increased blood triglycerides, leucocytosis, abnormal liver function test, pneumomediastinum, pneumonia, respiratory distress, respiratory failure, and traumatic lung injury (all n=1). Ten patients (7%) needed a dose reduction because of treatment-related adverse events (increased ALT or AST, or both [n=6]; neutropenia [n=2]; nausea [n=1]; and fatigue [n=1]).
Three patients permanently discontinued as a result of treatment-related adverse events (one with grade 4 and one grade 2 pneumonitis, and one patient with grade 3 raised ALT). 46 deaths had occurred at the time of data cutoff, none of which was judged to be treatment related.
Discussion
In this updated analysis, crizotinib was well tolerated and resulted in rapid and durable responses in patients with ALK-positive advanced NSCLC (panel), with more than 60% of patients having an objective response and median PFS of almost 10 months.
Clinical and demographic details from the 149 patients revealed many of the features now thought to be characteristic of patients with ALK-positive lung cancer.11,12 The median age was young (52 years), although the age range was wide. Although some patterns of metastatic spread at diagnosis, notably pleural, pericardial, and liver disease, have been associated with ALK-positivity,27 we were unable to expand on this issue from the baseline scans used within this study because the time since diagnosis was not standardised in the patients and there was no relevant comparator group. There was a preponderance of never smokers and tumours with adenocarcinoma on histology. Histology was not centrally reviewed, and patients were not randomly screened. Potential bias in the screened population because of increasing knowledge of clinical and pathological features associated with ALK-positivity in lung cancer therefore cannot be excluded.11 Because of limitations of available tissue, extension of reverse transcriptase PCR analysis to either confirm EML4 as the 5′ fusion partner or to identify the exact EML4-ALK breakpoints in patients beyond that already reported was not possible.21
In the ALK-positive lung cancer population within this study, crizotinib showed marked efficacy, with tumour shrinkage in over 90% of patients and with 61% achieving an objective response. The efficacy results within this study have remained consistent as the number of patients assessed has increased over time, showing the potential of robust efficacy data to be generated from even small numbers of patients when the population is molecularly predefined.20,21 Responses seemed to be rapid and durable. The proportion of patients with an objective response seemed to be largely independent of age, sex, performance status, or line of therapy, which is consistent with the presence of the ALK rearrangement being the primary driver of benefit from crizotinib. The greatest proportions of objective responses were noted in treatment-naive patients, those with the lowest performance status score, and Asian patients. However, because of the small numbers of patients involved, no univariate or multivariate statistical comparisons were undertaken to formally compare the response rate of different categories within or between subgroups. Differences in crizotinib pharmacokinetics between Asian and non-Asian patients have been reported28 and suggest that Asian patients might be subject to greater crizotinib exposure than non-Asians. Further analysis with a more robust sample size from multiple trial centres is needed to confirm these data.
Median PFS in the overall population was almost 10 months. The median PFS was longer in the treatment-naive subgroup than in the group in which crizotinib was a second-line or later treatment, which seems out of context with the minor differences in objective responses noted by line of treatment and at odds with what has been reported for EGFR tyrosine kinase inhibitors in EGFR mutant disease,29 suggesting this finding might simply show the high variability associated with data derived from small subgroups. Median overall survival data are not yet mature, but the estimated overall survival at 6 and 12 months from the first dose of crizotinib were 88% and 75%, respectively. When interpreting these single-arm survival data, whether historical NSCLC datasets represent the best comparator group is hard to know. Within this study, many patients had survived to receive several lines of previous treatment, which might preselect for those with a good prognosis. Additionally, because of other inclusion and exclusion criteria, study populations are always likely to include patients who are healthier than those in an otherwise unselected population. Within this study, we did not capture subsequent treatments and patients might have gone on to receive any of several other standard and experimental drugs being explored for their activity in ALK-positive NSCLC, which might or might not have influenced overall survival in this cohort.30–33 Finally, given its recent discovery, the true natural history of advanced ALK-positive NSCLC compared with NSCLC (not otherwise specified) is still being assessed.12,34,35 A series of randomised registration studies are ongoing after the activity of crizotinib was shown within this first-in-man study. Because all of these studies comparing crizotinib in advanced ALK-positive NSCLC with defined chemotherapy standards in the first-line or second-line setting allow patients in the chemotherapy group to cross over to crizotinib after RECIST-defined disease progression, per independent assessment, none of them include an effect on overall survival as their primary endpoint. However, in a separate retrospective study comparing overall survival in ALK-positive patients who received crizotinib with those who had died before crizotinib was available to them, crizotinib use was associated with significantly longer overall survival.36 Within the present study, because less than a third of patients had an overall survival event and because of the small numbers involved, we did not undertake further analyses of overall survival by line of treatment or other subgroups.
Crizotinib seemed to be well tolerated. Most treatment-related adverse events in the ALK-positive lung cancer population were grade 1 or 2. The normal function of ALK in adult human beings is unknown, but it is involved in the development of the gut and visual system in other organisms.2 Therefore, the predominance of gastrointestinal (notably diarrhoea, constipation, nausea, and vomiting) and visual side-effects could represent on-target anti-ALK effects within host tissues. However, crizotinib is also a MET inhibitor, and whether anti-MET effects, other off-target effects, or factors specific to the structure of crizotinib separate from its direct pharmacological targets could also be contributing to these side-effects is unclear. Peripheral oedema has recently been reported as a side-effect from MET-Mab, a monoclonal antibody directed against the extracellular MET domain.37 Almost all of the common adverse events in the present study occurred early and seemed to improve over time, with the exception of the treatment-emergent oedema that seemed to be a late-onset cumulative adverse event. With protracted follow-up, shown by the proportion of total patients at risk who had specific adverse events for cycle 7 and beyond, no other changes in tolerability or prominent new adverse events relating to prolonged exposure were noted. In the initial study design, crizotinib was taken on an empty stomach in the first 1–2 cycles when blood samples were taken to assess pharmacokinetic exposures. Later, after a food-effect study was completed, the protocol was amended to allow dosing with food. Anecdotally, when the tablets were taken with food many patients reported less nausea and vomiting.
Information available on the efficacy and safety of crizotinib within the first-in-man study has increased over time.20,21 As with any anticancer treatment, new clinical challenges emerge when patients start to progress on treatment. In our study, patients experiencing investigator-defined disease progression were allowed to continue on crizotinib if the investigator felt that the patient had ongoing clinical benefit from the drug. This assessment was subjective, and surveillance and decision making after initial disease progression were not standardised. Undoubtedly, guidelines are needed to formalise the definition of ongoing benefit. Nonetheless, several patients received crizotinib treatment for months and in five cases over 1 year after their initial investigator-defined disease progression, suggesting that, at least in the opinion of the investigators, ongoing clinical benefit could be prolonged. In ten of the 39 patients with extended crizotinib treatment, the sole site of initial disease progression was the brain, raising the possibility of primary pharmacokinetic failure within the CNS as a sanctuary site rather than overall biological failure.38 However, CNS imaging was not mandated within this study either at baseline or on study, and so whether this figure represents an accurate estimate of the true CNS failure rate with crizotinib remains uncertain. In contrast to CNS failure, in patients with systemic disease progression, failure is more likely secondary to more classical mechanisms of resistance to targeted treatments.39 Anecdotally, some patients had progression in one lesion only, which might be consistent with clonal evolution at that site. As an example of a possible clonal resistance mechanism, several different ALK mutations, associated with ALK inhibitor resistance in vitro, have been isolated from patients with ALK-positive NSCLC who developed acquired resistance to crizotinib.40–43 By analogy with the acquired resistance to EGFR inhibitors that emerges in EGFR-mutant NSCLC, although resistance develops, sensitive subclones of the disease that are still being suppressed by the inhibitor might continue to exist.42,44 Therefore, if ALK-inhibitor resistance emerges, particularly in isolated sites potentially amenable to local treatment such as radiation therapy, the true potential for deriving ongoing clinical benefit through continuing crizotinib exposure after disease progression deserves further investigation.
On the basis of data from this study and preliminary data from a single-arm phase 2 study,45 accelerated approval for crizotinib in ALK-positive locally advanced or metastatic NSCLC has been granted by the US Food and Drug Administration.
Acknowledgments
We thank all the participating patients, their families, and the network of investigators, research nurses, study coordinators, and operations staff. The phase 1 clinical trial was sponsored by Pfizer. Investigators were supported in part by the National Cancer Institute P50-CA090578 (PAJ, DBC), the American Society of Clinical Oncology Conquer Cancer Foundation (DBC), and by internal funds from the Massachusetts General Hospital Cancer Center and Pathology Department (ATS, AJI). Editorial assistance was provided by Martin Quinn at Acumed (Tytherington, UK) and was funded by Pfizer.
Footnotes
Contributors
The phase 1 clinical trial was designed by Pfizer. DRC, PLR, KR, PS, YT, and KW analysed the data. Y-JB, ELK, AJI, MV-G, SBF, GJR, BS, S-HIO, D-WK, RS, PF, JAE, LG, PAJ, DBC, GIS, JWC, and ATS collected data. DRC wrote the report. All authors read and provided comments on the report.
Conflicts of interest
DRC, Y-JB, ELK, AJI, BS, S-HIO, D-WK, PAJ, DBC, PLR, and ATS received honoraria or consulting fees from Pfizer. ELK, GJR, BS, and JWC received research funding from Pfizer. AJI, MV-G, and GJR received honoraria or consulting fees from Abbott Molecular. GJR, LG, and ATS received honoraria or consulting fees from Chugai. GJR received research funding from Chugai, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Infinity Pharmaceuticals, and Merck; and honoraria or consulting fees from Daiichi and Tragara. GJR and ATS received honoraria or consulting fees from Novartis and Ariad and research funding from Novartis. PF received honoraria or consulting fees from Genentech. DBC received honoraria or consulting fees from AstraZeneca and Roche. KR, YT, and KW are employees and stockholders of Pfizer. ATS received research funding from AstraZeneca. SBF, RS, JAE, GIS, and PS declare that they have no conflicts of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/s1470-2045(12)70344-3
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3936578?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/s1470-2045(12)70344-3
Article citations
Targeted Therapies, Novel Antibodies, and Immunotherapies in Advanced Non-Small Cell Lung Cancer: Clinical Evidence and Drug Approval Patterns.
Clin Cancer Res, 30(21):4822-4833, 01 Nov 2024
Cited by: 0 articles | PMID: 39177967 | PMCID: PMC11528205
Review Free full text in Europe PMC
The Use of Anaplastic Lymphoma Kinase Inhibitors in Non-Small-Cell Lung Cancer Treatment-Literature Review.
Biomedicines, 12(10):2308, 11 Oct 2024
Cited by: 0 articles | PMID: 39457620 | PMCID: PMC11504905
Review Free full text in Europe PMC
Discordant ALK Status in Non-Small Cell Lung Carcinoma: A Detailed Reevaluation Comparing IHC, FISH, and NGS Analyses.
Int J Mol Sci, 25(15):8168, 26 Jul 2024
Cited by: 0 articles | PMID: 39125737 | PMCID: PMC11312000
Understanding the treatment response and resistance to targeted therapies in non-small cell lung cancer: clinical insights and perspectives.
Front Oncol, 14:1387345, 11 Jul 2024
Cited by: 0 articles | PMID: 39055566 | PMCID: PMC11269125
Review Free full text in Europe PMC
Lck Function and Modulation: Immune Cytotoxic Response and Tumor Treatment More Than a Simple Event.
Cancers (Basel), 16(15):2630, 24 Jul 2024
Cited by: 0 articles | PMID: 39123358 | PMCID: PMC11311849
Review Free full text in Europe PMC
Go to all (770) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT00585195
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study.
Lancet Oncol, 15(10):1119-1128, 18 Aug 2014
Cited by: 419 articles | PMID: 25153538
Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial.
Lancet, 390(10089):29-39, 10 May 2017
Cited by: 478 articles | PMID: 28501140
Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer.
N Engl J Med, 377(9):829-838, 06 Jun 2017
Cited by: 1127 articles | PMID: 28586279
Role of anaplastic lymphoma kinase inhibition in the treatment of non-small-cell lung cancer.
Am J Health Syst Pharm, 72(17):1456-1462, 01 Sep 2015
Cited by: 11 articles | PMID: 26294238
Review
Funding
Funders who supported this work.
Conquer Cancer Foundation
Deer Breeders Corporation
Massachusetts General Hospital
NCI NIH HHS (2)
Grant ID: P50 CA090578
Grant ID: P30 CA046934
National Cancer Institute (1)
Grant ID: P50-CA090578





