Abstract
Free full text

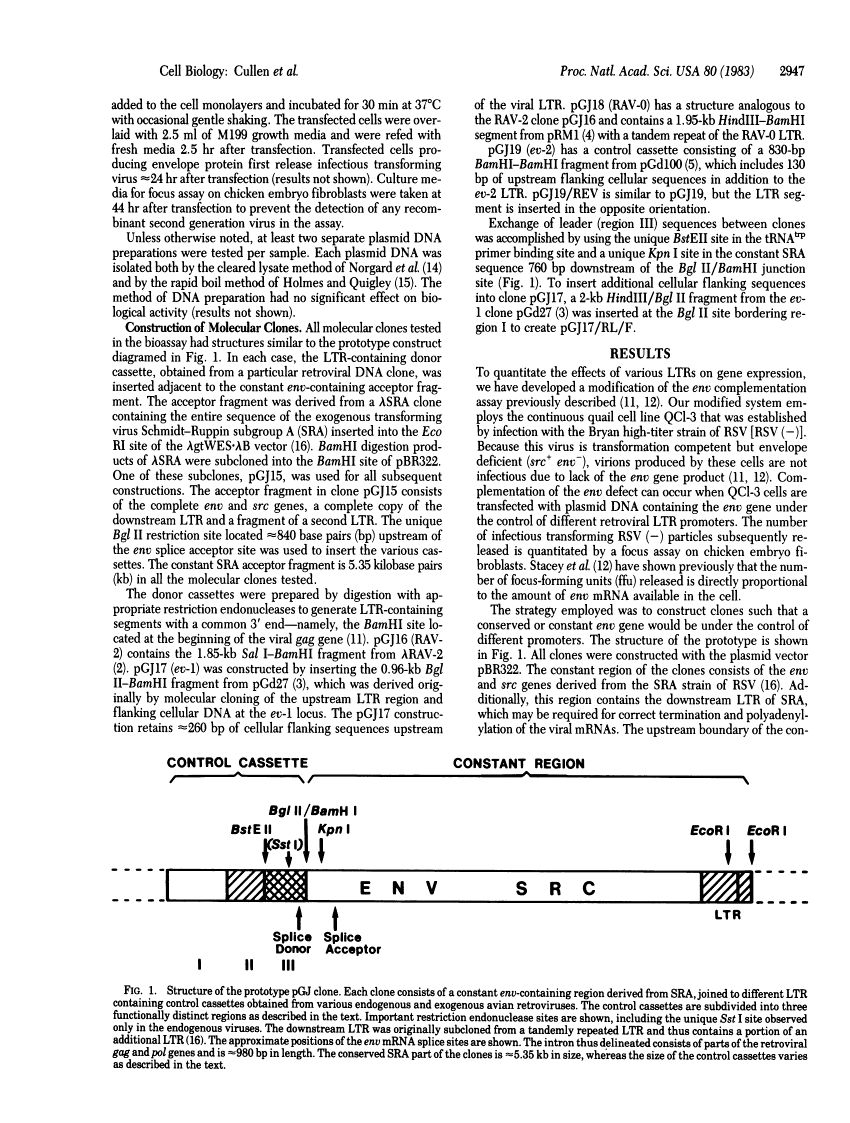
Endogenous avian retroviruses contain deficient promoter and leader sequences.
Abstract
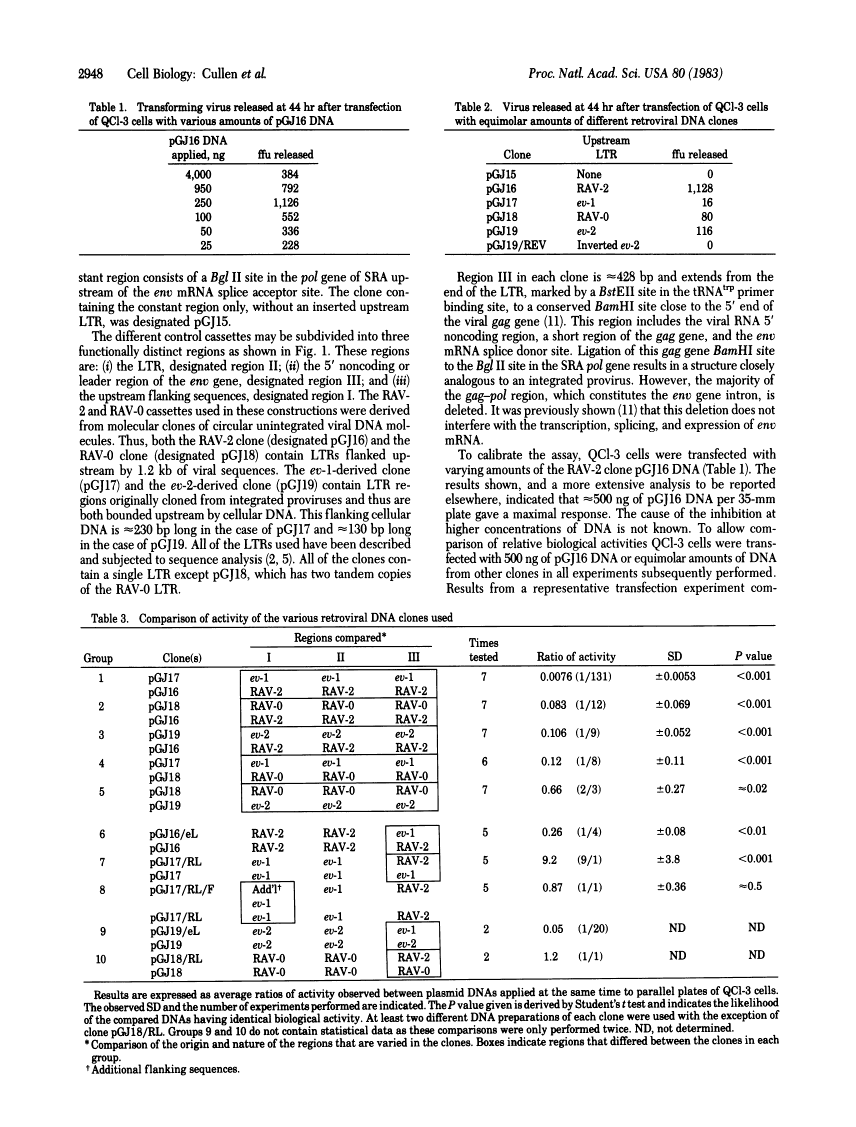
A sensitive and quantitative biological assay has been utilized to measure the ability of the exogenous and endogenous avian retroviral long terminal repeats (LTR) to promote gene expression in avian cells. This assay has revealed that the exogenous virus RAV-2 LTR is approximately equal to 10-fold more active than the LTRs of endogenous viruses RAV-0, ev-1, and ev-2. The endogenous viral LTRs show approximately equal activity. Upstream flanking cellular or viral sequences have no significant modulating effect on gene expression in our assay. Unexpectedly, we have detected and localized an additional defect outside of the LTR in the 5' noncoding leader sequence of ev-1 that further decreases gene expression relative to RAV-0 by approximately equal to 10-fold.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1005K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ju G, Skalka AM. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. [Abstract] [Google Scholar]
- Hishinuma F, DeBona PJ, Astrin S, Skalka AM. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. [Abstract] [Google Scholar]
- Tsichlis PN, Donehower L, Hager G, Zeller N, Malavarca R, Astrin S, Skalka AM. Sequence comparison in the crossover region of an oncogenic avian retrovirus recombinant and its nononcogenic parent: genetic regions that control growth rate and oncogenic potential. Mol Cell Biol. 1982 Nov;2(11):1331–1338. [Europe PMC free article] [Abstract] [Google Scholar]
- Scholl DR, Kahn S, Malavarca R, Astrin S, Skalka AM. Nucleotide sequence of the long terminal repeat and flanking cellular sequences of avian endogenous retrovirus ev-2: variation in Rous-associated virus-0 expression cannot be explained by differences in primary sequence. J Virol. 1983 Feb;45(2):868–871. [Europe PMC free article] [Abstract] [Google Scholar]
- Hayward WS. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. [Europe PMC free article] [Abstract] [Google Scholar]
- Hayward WS, Braverman SB, Astrin SM. Transcriptional products and DNA structure of endogenous avian proviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1111–1121. [Abstract] [Google Scholar]
- Groudine M, Eisenman R, Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. [Abstract] [Google Scholar]
- Neel BG, Hayward WS, Robinson HL, Fang J, Astrin SM. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. [Abstract] [Google Scholar]
- Cullen BR, Kopchick JJ, Stacey DW. Effect of intron size on splicing efficiency in retroviral transcripts. Nucleic Acids Res. 1982 Oct 11;10(19):6177–6190. [Europe PMC free article] [Abstract] [Google Scholar]
- Stacey DW, Allfrey VG, Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. [Europe PMC free article] [Abstract] [Google Scholar]
- Warden D, Thorne HV. The infectivity of polyoma virus DNA for mouse embryo cells in the presence of diethylaminoethyl-dextran. J Gen Virol. 1968 Dec;3(3):371–377. [Abstract] [Google Scholar]
- Norgard MV, Emigholz K, Monahan JJ. Increased amplification of pBR322 plasmid deoxyribonucleic acid in Escherichia coli K-12 strains RR1 and chi1776 grown in the presence of high concentrations of nucleoside. J Bacteriol. 1979 Apr;138(1):270–272. [Europe PMC free article] [Abstract] [Google Scholar]
- Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. [Abstract] [Google Scholar]
- Takeya T, Hanafusa H, Junghans RP, Ju G, Skalka AM. Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Mol Cell Biol. 1981 Nov;1(11):1024–1037. [Europe PMC free article] [Abstract] [Google Scholar]
- Cooper GM, Silverman L. Linkage of the endogenous avian leukosis virus genome of virus-producing chicken cells to inhibitory cellular DNA sequences. Cell. 1978 Oct;15(2):573–577. [Abstract] [Google Scholar]
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. [Europe PMC free article] [Abstract] [Google Scholar]
- Schümperli D, Howard BH, Rosenberg M. Efficient expression of Escherichia coli galactokinase gene in mammalian cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):257–261. [Europe PMC free article] [Abstract] [Google Scholar]
- Harbers K, Schnieke A, Stuhlmann H, Jähner D, Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. [Europe PMC free article] [Abstract] [Google Scholar]
- Conklin KF, Coffin JM, Robinson HL, Groudine M, Eisenman R. Role of methylation in the induced and spontaneous expression of the avian endogenous virus ev-1: DNA structure and gene products. Mol Cell Biol. 1982 Jun;2(6):638–652. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.80.10.2946
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc393950?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.80.10.2946
Article citations
Establishment of a TaqMan-based real-time quantitative PCR method for detection of exogenous fowl adenovirus type Ⅰ, type Ⅲ and avian leukosis virus in human cold adapted live attenuated influenza vaccines.
Heliyon, 9(12):e23033, 29 Nov 2023
Cited by: 2 articles | PMID: 38076100 | PMCID: PMC10703828
Finding, Conducting, and Nurturing Science: A Virologist's Memoir.
Annu Rev Virol, 4(1):1-35, 26 May 2017
Cited by: 1 article | PMID: 28548882
Inhibition of alpharetrovirus replication by a range of human APOBEC3 proteins.
J Virol, 81(24):13694-13699, 03 Oct 2007
Cited by: 33 articles | PMID: 17913830 | PMCID: PMC2168862
Quantitative evaluation of DNA methylation patterns for ALVE and TVB genes in a neoplastic disease susceptible and resistant chicken model.
PLoS One, 3(3):e1731, 05 Mar 2008
Cited by: 15 articles | PMID: 18320050 | PMCID: PMC2254315
Molecular epidemiology of avian leukosis virus subgroup J and evolutionary history of its 3' untranslated region.
Avian Dis, 51(4):942-953, 01 Dec 2007
Cited by: 22 articles | PMID: 18251406
Go to all (42) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nucleotide sequence analysis of avian retroviruses: structural similarities with transposable elements.
Fed Proc, 41(10):2659-2661, 01 Aug 1982
Cited by: 2 articles | PMID: 6286367
Transcriptional activity of avian retroviral long terminal repeats directly correlates with enhancer activity.
J Virol, 53(2):515-521, 01 Feb 1985
Cited by: 45 articles | PMID: 2982034 | PMCID: PMC254665
Structural and functional characterization of the unusually short long terminal repeats and their adjacent regions of a novel endogenous avian retrovirus.
Virology, 173(1):157-166, 01 Nov 1989
Cited by: 23 articles | PMID: 2815581
Transcription termination and polyadenylation in retroviruses.
Microbiol Rev, 57(3):511-521, 01 Sep 1993
Cited by: 23 articles | PMID: 7902524 | PMCID: PMC372924
Review Free full text in Europe PMC