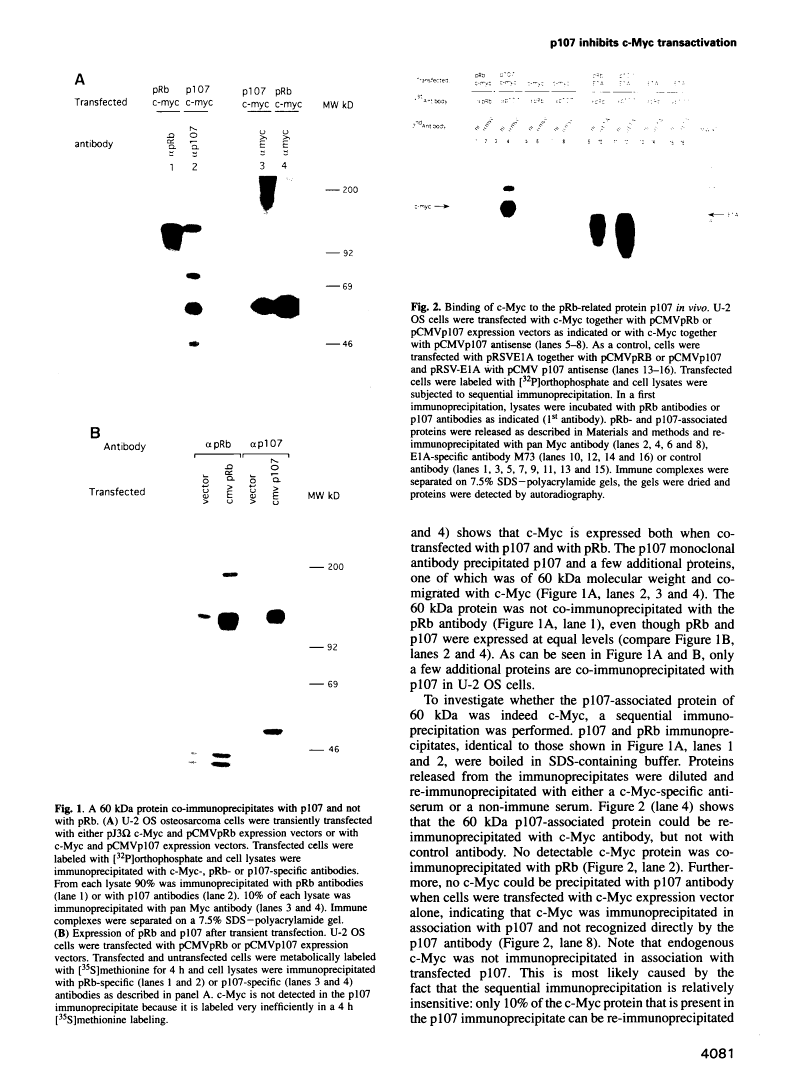
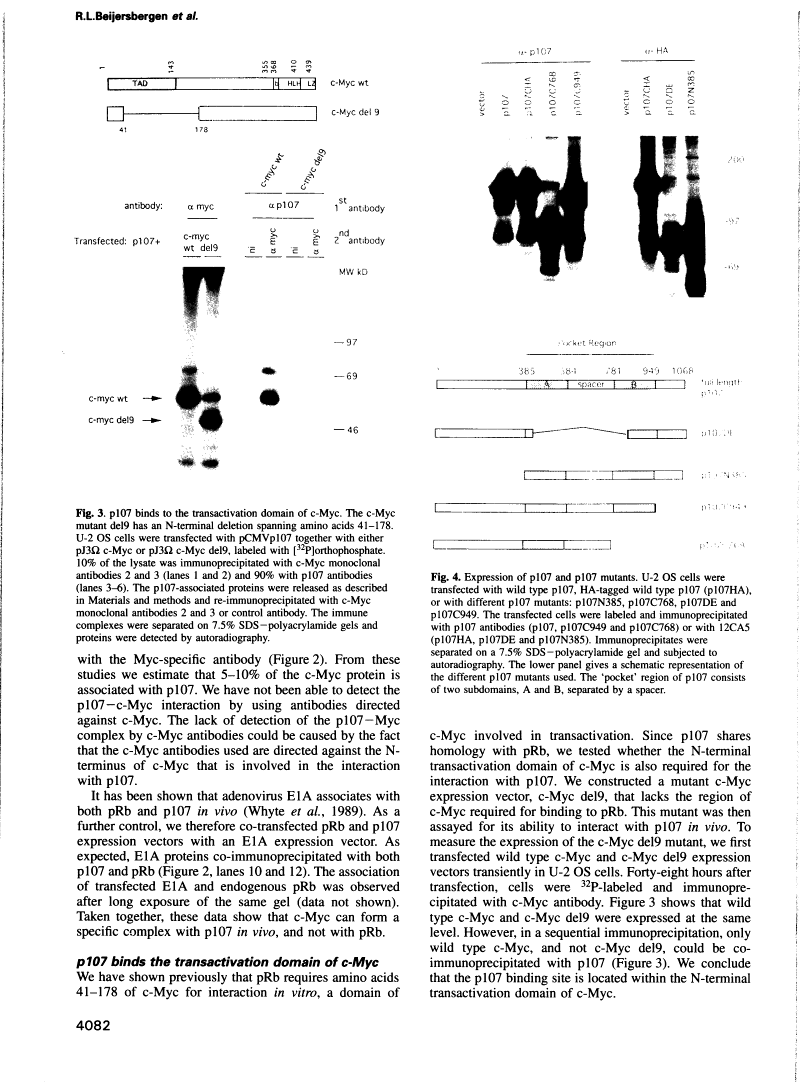
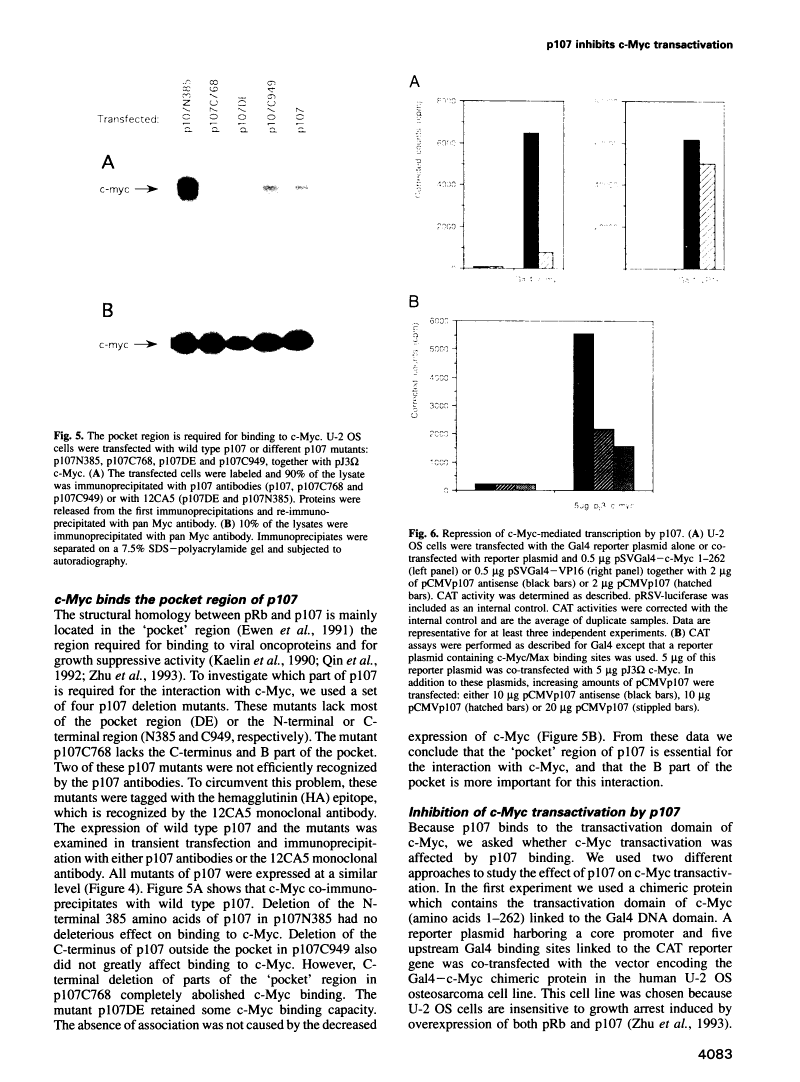
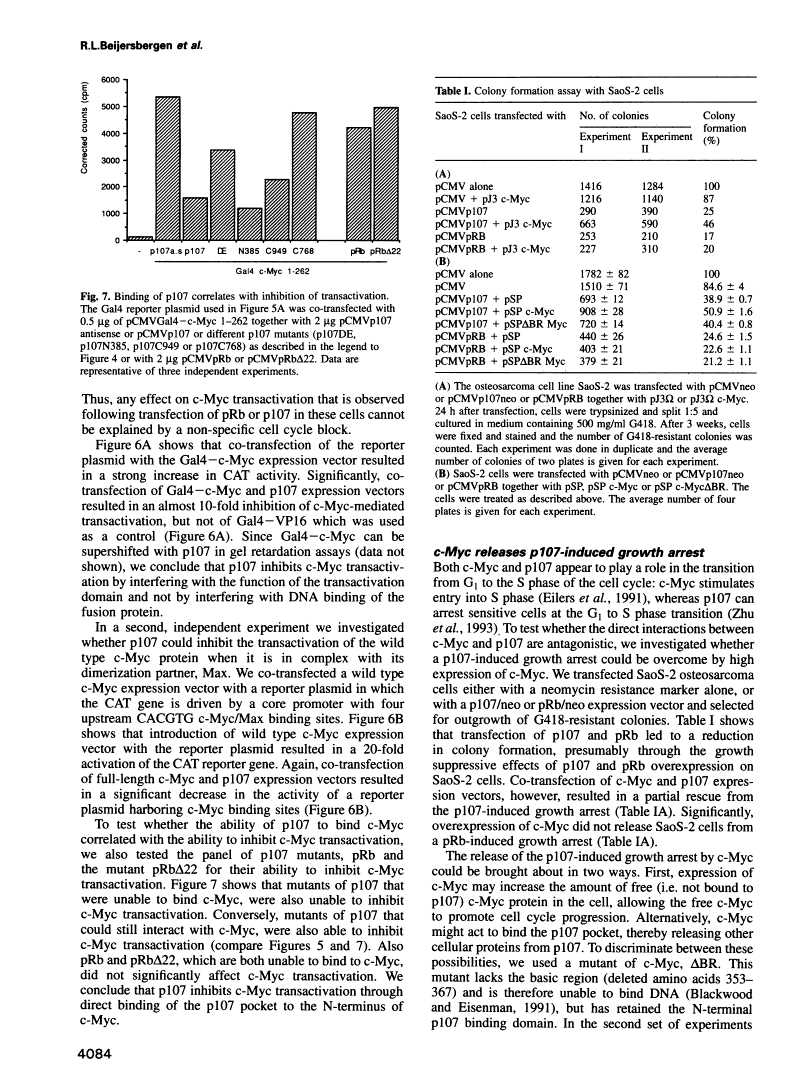
Abstract
Free full text

Interaction of c-Myc with the pRb-related protein p107 results in inhibition of c-Myc-mediated transactivation.
Abstract
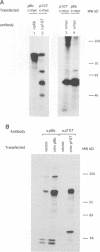
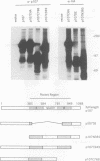
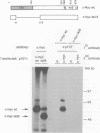
The product of the c-myc proto-oncogene, c-Myc, is a sequence-specific DNA binding protein with an N-terminal transactivation domain and a C-terminal DNA binding domain. Several lines of evidence indicate that c-Myc activity is essential for normal cell cycle progression. Since the abundance of c-Myc during the cell cycle is constant, c-Myc's activity may be regulated at a post-translational level. We have shown previously that the N-terminus of c-Myc can form a specific complex with the product of the retinoblastoma gene, pRb, in vitro. These data suggested a model in which pRb, or pRb-related proteins, regulate c-Myc activity through direct binding. We show here that the pRb-related protein p107, but not pRb itself, forms a specific complex with the N-terminal transactivation domain of c-Myc in vivo. Binding of p107 to c-Myc causes a significant inhibition of c-Myc transactivation. Expression of c-Myc releases cells from a p107-induced growth arrest, but not from pRb-induced growth arrest. Our data suggest that p107 can control c-Myc activity through direct binding to the transactivation domain and that c-Myc is a target for p107-mediated growth suppression.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. [Abstract] [Google Scholar]
- Cobrinik D, Whyte P, Peeper DS, Jacks T, Weinberg RA. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993 Dec;7(12A):2392–2404. [Abstract] [Google Scholar]
- Eilers M, Schirm S, Bishop JM. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 1991 Jan;10(1):133–141. [Europe PMC free article] [Abstract] [Google Scholar]
- Einat M, Resnitzky D, Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. [Abstract] [Google Scholar]
- Ewen ME, Xing YG, Lawrence JB, Livingston DM. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. [Abstract] [Google Scholar]
- Goodrich DW, Lee WH. Abrogation by c-myc of G1 phase arrest induced by RB protein but not by p53. Nature. 1992 Nov 12;360(6400):177–179. [Abstract] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. [Abstract] [Google Scholar]
- Gu W, Bhatia K, Magrath IT, Dang CV, Dalla-Favera R. Binding and suppression of the Myc transcriptional activation domain by p107. Science. 1994 Apr 8;264(5156):251–254. [Abstract] [Google Scholar]
- Gupta S, Seth A, Davis RJ. Transactivation of gene expression by Myc is inhibited by mutation at the phosphorylation sites Thr-58 and Ser-62. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3216–3220. [Europe PMC free article] [Abstract] [Google Scholar]
- Hann SR, Thompson CB, Eisenman RN. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. Nature. 314(6009):366–369. [Abstract] [Google Scholar]
- Harlow E, Franza BR, Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. [Europe PMC free article] [Abstract] [Google Scholar]
- Hateboer G, Timmers HT, Rustgi AK, Billaud M, van 't Veer LJ, Bernards R. TATA-binding protein and the retinoblastoma gene product bind to overlapping epitopes on c-Myc and adenovirus E1A protein. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8489–8493. [Europe PMC free article] [Abstract] [Google Scholar]
- Heikkila R, Schwab G, Wickstrom E, Loke SL, Pluznik DH, Watt R, Neckers LM. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature. 328(6129):445–449. [Abstract] [Google Scholar]
- Hu QJ, Bautista C, Edwards GM, Defeo-Jones D, Jones RE, Harlow E. Antibodies specific for the human retinoblastoma protein identify a family of related polypeptides. Mol Cell Biol. 1991 Nov;11(11):5792–5799. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaelin WG, Jr, Ewen ME, Livingston DM. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol. 1990 Jul;10(7):3761–3769. [Europe PMC free article] [Abstract] [Google Scholar]
- Kato GJ, Barrett J, Villa-Garcia M, Dang CV. An amino-terminal c-myc domain required for neoplastic transformation activates transcription. Mol Cell Biol. 1990 Nov;10(11):5914–5920. [Europe PMC free article] [Abstract] [Google Scholar]
- Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. [Abstract] [Google Scholar]
- Kretzner L, Blackwood EM, Eisenman RN. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992 Oct 1;359(6394):426–429. [Abstract] [Google Scholar]
- Lees E, Faha B, Dulic V, Reed SI, Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992 Oct;6(10):1874–1885. [Abstract] [Google Scholar]
- Li Y, Graham C, Lacy S, Duncan AM, Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993 Dec;7(12A):2366–2377. [Abstract] [Google Scholar]
- Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990 Feb 25;18(4):1068–1068. [Europe PMC free article] [Abstract] [Google Scholar]
- Qin XQ, Chittenden T, Livingston DM, Kaelin WG., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992 Jun;6(6):953–964. [Abstract] [Google Scholar]
- Rustgi AK, Dyson N, Bernards R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1991 Aug 8;352(6335):541–544. [Abstract] [Google Scholar]
- Schwarz JK, Devoto SH, Smith EJ, Chellappan SP, Jakoi L, Nevins JR. Interactions of the p107 and Rb proteins with E2F during the cell proliferation response. EMBO J. 1993 Mar;12(3):1013–1020. [Europe PMC free article] [Abstract] [Google Scholar]
- Seed B, Sheen JY. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. [Abstract] [Google Scholar]
- Seth A, Alvarez E, Gupta S, Davis RJ. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [Abstract] [Google Scholar]
- Shirodkar S, Ewen M, DeCaprio JA, Morgan J, Livingston DM, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. [Abstract] [Google Scholar]
- Stone J, de Lange T, Ramsay G, Jakobovits E, Bishop JM, Varmus H, Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. [Europe PMC free article] [Abstract] [Google Scholar]
- Thompson CB, Challoner PB, Neiman PE, Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. Nature. 314(6009):363–366. [Abstract] [Google Scholar]
- van Zonneveld AJ, Curriden SA, Loskutoff DJ. Type 1 plasminogen activator inhibitor gene: functional analysis and glucocorticoid regulation of its promoter. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5525–5529. [Europe PMC free article] [Abstract] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. [Abstract] [Google Scholar]
- Whyte P, Williamson NM, Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. [Abstract] [Google Scholar]
- Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. [Abstract] [Google Scholar]
- Ayer DE, Kretzner L, Eisenman RN. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993 Jan 29;72(2):211–222. [Abstract] [Google Scholar]
- Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. [Abstract] [Google Scholar]
- Barrett J, Birrer MJ, Kato GJ, Dosaka-Akita H, Dang CV. Activation domains of L-Myc and c-Myc determine their transforming potencies in rat embryo cells. Mol Cell Biol. 1992 Jul;12(7):3130–3137. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1994.tb06725.x
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1994.tb06725.x
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1994.tb06725.x
Article citations
Retinoblastoma Protein Is Required for Epstein-Barr Virus Replication in Differentiated Epithelia.
J Virol, 97(2):e0103222, 31 Jan 2023
Cited by: 2 articles | PMID: 36719239 | PMCID: PMC9972952
The functions and mechanisms of prefoldin complex and prefoldin-subunits.
Cell Biosci, 10:87, 20 Jul 2020
Cited by: 17 articles | PMID: 32699605 | PMCID: PMC7370476
Review Free full text in Europe PMC
The HDAC-Associated Sin3B Protein Represses DREAM Complex Targets and Cooperates with APC/C to Promote Quiescence.
Cell Rep, 25(10):2797-2807.e8, 01 Dec 2018
Cited by: 20 articles | PMID: 30517867 | PMCID: PMC6324198
RB-loss puts focus on Myc.
Nat Cell Biol, 17(8):968-969, 01 Aug 2015
Cited by: 2 articles | PMID: 26239529
Myc and its interactors take shape.
Biochim Biophys Acta, 1849(5):469-483, 14 Jun 2014
Cited by: 69 articles | PMID: 24933113
Review
Go to all (69) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regulation of cellular genes in a chromosomal context by the retinoblastoma tumor suppressor protein.
Mol Cell Biol, 18(8):4565-4576, 01 Aug 1998
Cited by: 16 articles | PMID: 9671466 | PMCID: PMC109042
Independent regions of adenovirus E1A are required for binding to and dissociation of E2F-protein complexes.
Mol Cell Biol, 13(12):7267-7277, 01 Dec 1993
Cited by: 92 articles | PMID: 8246949 | PMCID: PMC364797
Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins.
EMBO J, 16(17):5322-5333, 01 Sep 1997
Cited by: 135 articles | PMID: 9311992 | PMCID: PMC1170164
pRB, p107 and the regulation of the E2F transcription factor.
J Cell Sci Suppl, 18:81-87, 01 Jan 1994
Cited by: 28 articles | PMID: 7883798
Review