Abstract
Background
Although the transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR) are commonly used to differentiate heart failure patients with pulmonary vascular disease from those with passive pulmonary hypertension (PH), elevations in TPG and PVR may not always reflect pre-capillary PH. Recently, it has been suggested an elevated diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient (DPG) may be a better indicator of pulmonary vascular remodeling, and therefore, may be of added prognostic value in patients with PH being considered for cardiac transplantation.Methods
Using the United Network for Organ Sharing (UNOS) database, we retrospectively reviewed all primary adult (age > 17 years) orthotropic heart transplant recipients between 1998 and 2011. All patients with available pre-transplant hemodynamic data and PH (mean pulmonary artery pressure ≥ 25 mm Hg) were included (n = 16,811). We assessed the prognostic value of DPG on post-transplant survival in patients with PH and an elevated TPG and PVR.Results
In patients with PH and a TPG > 12 mm Hg (n = 5,827), there was no difference in survival at up to 5 years post-transplant between high DPG (defined as ≥3, ≥5, ≥7, or ≥10 mm Hg) and low DPG (<3, <5, <7, or <10 mm Hg) groups. Similarly, there was no difference in survival between high and low DPG groups in those with a PVR > 3 Wood units (n = 6,270). Defining an elevated TPG as > 15 mm Hg (n = 3,065) or an elevated PVR > 5 (n = 1,783) yielded similar results.Conclusions
This large analysis investigating the prognostic value of DPG found an elevated DPG had no effect on post-transplant survival in patients with PH and an elevated TPG and PVR.Free full text

Prognostic Value of the Pre-Transplant Diastolic Pulmonary Artery Pressure to Pulmonary Capillary Wedge Pressure Gradient (DPG) in Cardiac Transplant Recipients with Pulmonary Hypertension
Associated Data
Abstract
Background
Although the transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR) are commonly used to differentiate heart failure patients with pulmonary vascular disease from those with passive pulmonary hypertension (PH), elevations in TPG and PVR may not always reflect pre-capillary PH. Recently, it has been suggested an elevated diastolic pulmonary artery pressure to pulmonary capillary wedge pressure gradient (DPG) may be better indicator of pulmonary vascular remodeling, and therefore, may be of added prognostic value in patients with PH being considered for cardiac transplantation.
Methods
Utilizing the United Network for Organ Sharing (UNOS) database, we retrospectively reviewed all primary adult (age >17 years) orthotropic heart transplant recipients between 1998–2011. All patients with available pre-transplant hemodynamic data and PH (mean pulmonary artery pressure ≥ 25mmHg were included (n=16,811). We assessed the prognostic value of DPG on post-transplant survival in patients with PH and an elevated TPG and PVR.
Results
In patients with PH and a TPG > 12mmHg (n=5,827), there was no difference in survival at up to 5 years post-transplant between high (defined as ≥3, ≥5, ≥7, or ≥10mmHg) and low DPG groups (<3, <5, <7, or <10mmHg). Similarly, there was no difference in survival between high and low DPG groups in those with a PVR > 3 wood units (n=6,270). Defining an elevated TPG as > 15mmHg (n=3,065) or an elevated PVR > 5 (n=1783) yielded similar results.
Conclusions
In the largest analysis to date investigating the prognostic value of DPG, an elevated DPG had no impact on post-transplant survival in patients with PH and an elevated TPG and PVR.
Introduction
Significant pre-capillary pulmonary hypertension (PH) is a relative contraindication to cardiac transplantation due to the risk of post-operative right heart failure.1 The transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR) are commonly utilized by clinicians to determine the degree of pre-capillary PH and suitability of potential heart recipients.1 However, these metrics are not perfect surrogates for pulmonary vascular remodeling. In particular, the TPG varies with differences in cardiac output and left atrial pressure and neither measure clearly differentiates fixed pulmonary vascular remodeling from reversible changes in pulmonary vascular smooth muscle tone. 2–4 For this reason, acute and chronic vasodilator response is often tested to determine the reversibility of the pre-capillary PH; yet, even when reversibility is demonstrated, post-transplant mortality remains higher than that seen in patients without PH.5 Because of these limitations, a metric to better differentiate high and low risk among patients with PH is needed. There has been growing interest in using the diastolic pulmonary gradient (DPG, diastolic pulmonary artery pressure minus pulmonary capillary wedge pressure) as a means to identify those left heart failure patients with clinically significant pre-capillary PH.4,6 A recent analysis of patients with left heart disease and PH suggested that a TPG > 12mmHg and DPG ≥ 7mmHg was associated with worse survival compared to a TPG > 12 mmHg and DPG < 7.6 Our purpose was to use the United Network of Organ Sharing (UNOS) database to explore whether differences in DPG define high and low risk subpopulations among patients with PH being considered for orthotopic heart transplant (OHT).
Methods
Data Source
UNOS provided Standard Transplant Analysis and Research (STAR) files with donor-specific data from December 1988 to June 2011. The data set included prospectively collected metrics from all patients who received thoracic transplantation in the United States. The current study was granted an exemption by the institutional review board at the Johns Hopkins because none of the investigators had access to datasets containing protected health information.
Study Design
We retrospectively examined all primary, adult (>17 years) OHT patients (1988 to 2011) with a complete set of pre-transplant hemodynamic data, which at minimum included systolic pulmonary artery pressure (sPAP), diastolic pulmonary artery pressure (dPAP), mean pulmonary artery pressure (mPAP), pulmonary capillary wedge pressure (PCWP), and cardiac output (CO). Only patients with PH (defined as mPAP ≥ 25mmHg) were included in the analysis. Patients with multi-organ transplants and re-do transplants were excluded. Outcomes of interest included survival at 30 days, one and five years.
Statistical Analysis
Continuous variables were compared via Student’s t-test (parametric) or Wilcoxon rank-sum test (non-parametric) as appropriate. Categorical variables were compared with chi-squared or Fisher’s exact test. Receiver operator characteristic (ROC) curves were constructed for DPG, TPG, and PVR to assess their utility in discriminating survivors from non-survivors post-transplant. Total area under the curve (AUC) was considered to assess the value of these measures. ROC cutpoints were defined using the Youden’s index. Survival was estimated by the Kaplan Meier method and compared using the log-rank test. A P-value (two-tailed) of < 0.05 was considered significant. Means are presented with standard deviations. Because the cause of death was not available, a sensitivity analysis was performed to ascertain the impact of cause of death on our findings. All statistical analyses were performed using STATA version 12.1 software (StataCorp, LP, College Station, TX).
Results
Cohort Statistics
From December 1988 to June 2011, 43,494 patients > 17 years old underwent primary OHT. After excluding 18,041 patients without complete hemodynamic data and 8,642 patients without PH (mPAP <25mmHg), the final study cohort consisted of 16,811 patients.
ROC Curve analyses
When considering all patients with PH (mPAP ≥ 25mmHg), DPG, TPG, and PVR all had poor ability to discriminate survivors from non-survivors as evidenced by the AUC values near 0.5(Table 1). The optimal cut points for DPG in those patients with PH and an elevated TPG, PVR, or both were determined (Table 2). DPG did not discriminate survivors from non-survivors significantly better than chance in any of these groups at any of the three time points (p>0.05 for all).
Table 1
All patients with mean pulmonary artery pressure ≥ 25mmHg
| Area Under Curve (AUC) | |
|---|---|
| DPG | |
| 30 day survival | 0.52 |
| 1 year survival | 0.51 |
| 5 year survival | 0.52 |
| TPG | |
| 30 day survival | 0.54 |
| 1 year survival | 0.52 |
| 5 year survival | 0.52 |
| PVR | |
| 30 day survival | 0.53 |
| 1 year survival | 0.52 |
| 5 year | 0.51 |
Table 2
All patients with mean pulmonary artery pressure ≥ 25mmHg
| Pre-Capillary Pulmonary Hypertension Parameter | DPG Cut Point | Area Under Curve AUC) | P value |
|---|---|---|---|
| TPG > 12 mmHg | |||
| 30 day survival | 11.5 | 0.50 | 0.89 |
| 1 year survival | 1.2 | 0.51 | 0.77 |
| 5 year survival | 8.5 | 0.50 | 0.90 |
| TPG > 15 mmHg | |||
| 30 day survival | 5.9 | 0.50 | 0.98 |
| 1 year survival | 10.1 | 0.50 | 0.91 |
| 5 year survival | 8.5 | 0.51 | 0.70 |
| PVR > 3 Wood units | |||
| 30 day survival | 2.9 | 0.52 | 0.25 |
| 1 year survival | 6 | 0.51 | 0.54 |
| 5 year survival | 2.9 | 0.51 | 0.64 |
| PVR > 5 Wood units | |||
| 30 day survival | 8.5 | 0.52 | 0.69 |
| 1 year survival | 4 | 0.52 | 0.52 |
| 5 year survival | 8.5 | 0.52 | 0.57 |
| TPG > 12 mmHg And PVR > 3 Wood units | |||
| 30 day survival | 11.5 | 0.50 | 0.94 |
| 1 year survival | 1.70 | 0.50 | 0.97 |
| 5 year survival | 8.5 | 0.50 | 0.73 |
| TPG > 15 mmHg And PVR > 5 Wood units | |||
| 30 day survival | 8.5 | 0.51 | 0.77 |
| 1 year survival | 14 | 0.50 | 0.94 |
| 5 year survival | 8.5 | 0.51 | 0.67 |
Pulmonary Hypertension with an Elevated TPG
Demographic, acuity, and hemodynamic data for patients with PH and a TPG > 12mmHg in strata of DPG (n=5,827) are presented in Table 3. Given the variable ROC cut points for DPG in our analyses as well as various cut points proposed in the literature, we explored four cut points for DPG chosen a priori: 3 mmHg, 5mmHg, 7 mmHg, and 10mmHg. Compared with the lower DPG groups (defined as < 3, <5, <7, or <10mmHg), higher DPG groups (as ≥3, ≥5, ≥7, or ≥10mmHg) were younger and were more likely to be of black race, have a diagnosis of idiopathic cardiomyopathy, and have a ventricular assist device. The lower DPG groups were more likely to have an ischemic cardiomyopathy and carry a diagnosis of diabetes. There were no differences in terms of gender, creatinine, bilirubin, ventilator support, or cardiac output. PCWP and pulmonary pulse pressure were higher in the lower DPG groups than the higher DPG groups. TPG and PVR were higher in the high DPG groups. Thirty-day, 1 year, and 5 year post-transplant survival was similar between low DPG and high DPG groups, p>0.05 for all (Table 4, Figure 1). Defining an elevated TPG as > 15 mmHg (n=3,065) had no affect on the ability of the DPG cutoffs to predict survival at any time point (Table 4).
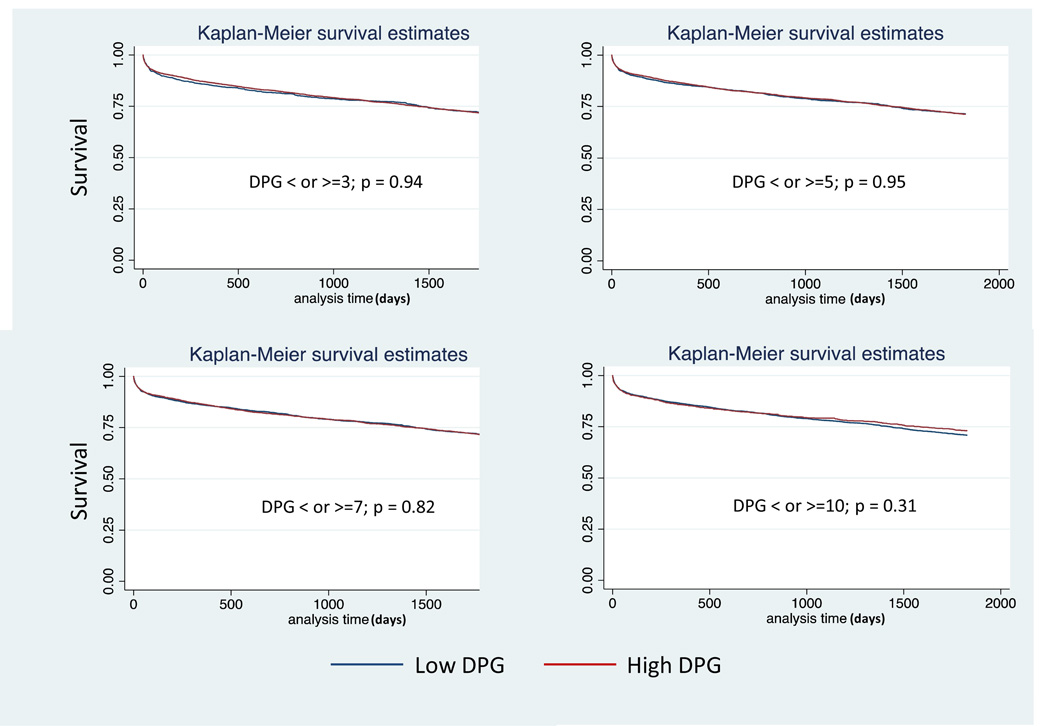
Kaplan Meier Plots for Diastolic Pulmonary Artery Pressure to Pulmonary Capillary Wedge Pressure Gradient (DPG) in patients with pulmonary hypertension (PH) and an elevated transpulmonary gradient > 12mmHg: a) 3mmHg, b) 5 mmHg, c) 7 mmHg, d) 10 mmHg.
Table 3
Transpulmonary Gradient > 12 mmHg
| DPG < 3 mmHg | DPG ≥ 3 mmHg | P value | DPG < 5 mmHg | DPG ≥ 5 mmHg | P value | DPG < 7 mmHg | DPG ≥ 7 mmHg | P value | DPG < 10 mmHg | DPG ≥ 10 mmHg | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (N) | 1605 | 4222 | 2506 | 3321 | 3542 | 2285 | 4679 | 1148 | ||||
| Demographics | ||||||||||||
| Age (years) | 53.9±10.7 | 52.6±11.0 | <0.001 | 53.8±10.7 | 52.3±11.0 | <0.001 | 53.6±10.7 | 52.0±11.2 | <0.001 | 53.3±10.8 | 51.7±11.2 | <0.001 |
| Female | 23.2% | 22.7% | 0.68 | 23.4% | 22.5% | 0.39 | 23.6% | 21.8% | 0.11 | 23.0% | 22.3% | 0.60 |
| White | 73.6% | 69.9% | 0.006 | 72.0% | 70.1% | 0.10 | 72.6% | 68.2% | <0.001 | 71.7% | 67.8% | 0.009 |
| Black | 16.1% | 20.9% | <0.001 | 17.7% | 21.0% | 0.001 | 17.7% | 22.5% | <0.001 | 18.9% | 22.3% | 0.01 |
| BMI (kg/m2) | 26.7±5.0 | 26.5±5.5 | 0.33 | 26.5±5.9 | 26.5±5.0 | 0.88 | 26.6±5.6 | 26.5±5.0 | 0.50 | 26.6±5.5 | 26.2±5.2 | 0.022 |
| Diagnosis | ||||||||||||
| Idiopathic | 35.1% | 44.4% | 38.0% | 44.8% | 39.7% | 45.2% | 41.1% | 45.2% | ||||
| Ischemic | 54.1% | 46.3% | 52.0% | 45.8% | 50.4% | 45.5% | 49.4% | 45.0% | ||||
| Congenital | 1.6% | 1.3% | 1.4% | 1.4% | 1.4% | 1.4% | 1.4% | 1.5% | ||||
| Other | 9.1% | 8.0% | <0.001 | 8.6% | 8.0% | <0.001 | 8.5% | 7.9% | 0.001 | 8.3% | 8.4% | 0.06 |
| Acuity | ||||||||||||
| Diabetes | 426/1502 | 991/3954 | 0.01 | 650/2342 | 767/3114 | 0.009 | 903/3318 | 514/2138 | 0.009 | 1169/4382 | 248/1074 | 0.02 |
| Isch. Time (hrs) | 3.1±1.0 | 3.0±1.0 | 0.03 | 3.1±1.0 | 3.0±1.1 | 0.047 | 3.1±1.0 | 3.0±1.0 | 0.02 | 3.1±1.0 | 3.0±1.0 | 0.12 |
| Creat. (mg/dL) | 1.5±1.7 | 1.4±1.5 | 0.55 | 1.5±1.6 | 1.5±1.5 | 0.94 | 1.5±1.5 | 1.5±1.6 | 0.94 | 1.5±1.6 | 1.5±1.2 | 0.98 |
| Bili. (mg/dL) | 1.5±3.8 | 1.3±2.7 | 0.06 | 1.5±3.8 | 1.3±2.3 | 0.06 | 1.4±3.5 | 1.3±2.2 | 0.25 | 1.4±3.2 | 1.3±2.7 | 0.71 |
| IABP | 5.7% | 6.1% | 0.55 | 5.4% | 6.4% | 0.08 | 5.4% | 6.9% | 0.20 | 5.6% | 7.6% | 0.01 |
| Assist Device | 11.7% | 14.0% | 0.02 | 11.7% | 14.5% | 0.002 | 12.3% | 14.8% | 0.006 | 13.1% | 14.2% | 0.33 |
| Ventilation | 2.5% | 2.3% | 0.74 | 2.6% | 2.3% | 0.46 | 2.4% | 2.4% | 0.93 | 2.4% | 2.3% | 0.77 |
| Hemodynamics | ||||||||||||
| SPAP (mmHg) | 60.9±12.6 | 55.4±12.8 | <0.001 | 59.1±12.2 | 55.2±13.3 | <0.001 | 57.8±12.2 | 55.4±14.0 | <0.001 | 56.9±12.4 | 56.9±15.2 | 0.85 |
| DPAP (mmHg) | 25.1±7.5 | 28.7±8.1 | <0.001 | 25.4±7.3 | 29.4±8.3 | <0.001 | 26.0±7.3 | 30.3±8.6 | <0.001 | 26.5±7.4 | 32.5±9.3 | <0.001 |
| mPAP (mmHg | 40.9±8.1 | 38.6±9.0 | <0.001 | 39.8±8.1 | 38.8±9.2 | <0.001 | 39.3±8.1 | 39.2±9.7 | 0.64 | 38.9±8.3 | 40.8±10.4 | <0.001 |
| PCWP (mmHg) | 25.7±7.4 | 20.7±7.7 | <0.001 | 24.6±7.4 | 20.1±7.8 | <0.001 | 23.8±7.4 | 19.3±7.9 | <0.001 | 22.9±7.5 | 18.4±8.4 | <0.001 |
| C. O. (L/min) | 4.38±1.5 | 4.35±1.5 | 0.54 | 4.3±1.6 | 4.4±1.5 | 0.47 | 4.4±1.5 | 4.4±1.5 | 0.94 | 4.4±1.5 | 4.3±1.5 | 0.11 |
| PVR (Woods) | 3.91±1.9 | 4.73±2.9 | <0.001 | 4.0±2.2 | 4.9±2.9 | <0.001 | 4.0±2.1 | 5.2±3.2 | <0.001 | 4.1±2.2 | 6.0±3.6 | <0.001 |
| TPG (mmHg) | 15.2±3.0 | 17.9±5.5 | <0.001 | 15.3±2.9 | 18.6±5.9 | <0.001 | 15.5±2.9 | 19.8±6.5 | <0.001 | 15.9±3.3 | 22.3±7.4 | <0.001 |
Mean ± Standard Deviation; BMI = Body Mass Index, Isch = Ischemic, Creat = Creatinine, Bili = Bilirubin, IABP = Intra-aortic Balloon Pump, SPAP = Systolic Pulmonary Artery Pressure, DPAP = Diastolic Pulmonary Artery Pressure, mPAP = Mean Pulmonary Artery Pressure, PCWP = Pulmonary Capillary Wedge Pressure, C.O. = Cardiac Output, PVR = Pulmonary Vascular Resistance, TPG = Transpulmonary Gradient
Table 4
Survival (Kaplan Meier Analysis) in all patients with mean pulmonary artery pressure ≥ 25mmHg
| Survival | Survival | Survival | Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPG < 3 mmHg | DPG ≥ 3 mmHg | P value | DPG < 5 mmHg | DPG ≥ 5 mmHg | P value | DPG < 7 mmHg | DPG ≥ 7 mmHg | P value | DPG < 10 mmHg | DPG ≥ 10 mmHg | P value | |
| TPG > 12 mmHg | ||||||||||||
| 30 day | 93.4% | 93.2% | 0.89 | 93.4% | 93.2% | 0.95 | 93.3% | 93.1% | 0.89 | 93.3% | 93.0% | 0.70 |
| 1 year | 84.3% | 85.7% | 0.21 | 84.9% | 85.6% | 0.41 | 85.2% | 85.4% | 0.76 | 85.4% | 85.0% | 0.65 |
| 5 year | 71.2% | 70.8% | 0.94 | 71.2% | 70.8% | 0.95 | 71.2% | 70.5% | 0.82 | 70.5% | 72.5% | 0.31 |
| TPG > 15 mmHg | ||||||||||||
| 30 day | 93.1% | 93.0% | 0.66 | 93.7% | 92.8% | 0.29 | 93.1% | 93.0% | 0.64 | 93.0% | 93.0% | 0.84 |
| 1 year | 85.3% | 85.4% | 0.90 | 85.2% | 85.5% | 0.87 | 85.5% | 85.4% | 0.81 | 85.4% | 85.4% | 0.83 |
| 5 year | 72.3% | 71.4% | 0.66 | 71.4% | 71.6% | 0.83 | 71.8% | 71.4% | 0.82 | 70.7% | 73.3% | 0.27 |
| PVR > 3 WU | ||||||||||||
| 30 day | 94.5% | 93.1% | 0.09 | 94.1% | 93.1% | 0.20 | 93.9% | 93.1% | 0.39 | 93.8% | 92.7% | 0.22 |
| 1 year | 86.4% | 85.6% | 0.44 | 86.1% | 85.6% | 0.66 | 86.2% | 85.2% | 0.44 | 86.1% | 84.9% | 0.31 |
| 5 year | 73.6% | 70.9% | 0.054 | 72.9% | 70.9% | 0.17 | 72.4% | 70.9% | 0.30 | 71.7% | 73.2% | 0.57 |
| PVR > 5 WU | ||||||||||||
| 30 day a | 93.1% | 93.0% | 0.58 | 92.7% | 93.2% | 0.50 | 92.6% | 93.4% | 0.31 | 92.9% | 93.2% | 0.65 |
| 1 year | 82.7% | 85.6% | 0.15 | 83.3% | 85.9% | 0.15 | 83.6% | 86.3% | 0.10 | 84.4% | 86.1% | 0.34 |
| 5 year | 71.0% | 72.9% | 0.36 | 71.9% | 72.7% | 0.56 | 71.0% | 73.8% | 0.15 | 70.7% | 75.9% | 0.04 |
| TPG > 12 mmHg & PVR > 3 WU | ||||||||||||
| 30 day | 93.4% | 92.9% | 0.97 | 93.2% | 92.9% | 0.97 | 93.1% | 93.0% | 0.88 | 93.1% | 92.7% | 0.73 |
| 1 year | 84.9% | 85.4% | 0.63 | 85.0% | 85.4% | 0.59 | 85.3% | 85.2% | 0.92 | 85.3% | 85.1% | 0.81 |
| 5 year | 72.0% | 71.2% | 0.80 | 71.6% | 71.3% | 0.95 | 71.6% | 71.1% | 0.90 | 70.8% | 73.2% | 0.24 |
| TPG > 15 mmHg & PVR > 5 WU | ||||||||||||
| 30 day | 91.3% | 93.1% | 0.49 | 92.7% | 92.9% | 0.93 | 92.7% | 92.9% | 0.94 | 92.6% | 93.1% | 0.81 |
| 1 year | 82.9% | 85.9% | 0.34 | 84.1% | 85.9% | 0.54 | 85.1% | 85.7% | 0.84 | 85.1% | 85.9% | 0.75 |
| 5 year | 69.3% | 74.0% | 0.25 | 70.7% | 74.1% | 0.30 | 71.9% | 74.1% | 0.47 | 71.4% | 75.7% | 0.15 |
DPG = Diastolic pulmonary artery pressure to pulmonary capillary wedge pressure gradient; TPG = Transpulmonary gradient; PVR = Pulmonary vascular resistance; WU = Wood units
Pulmonary Hypertension with an Elevated PVR
Demographic, acuity, and hemodynamic data for patients with a PVR > 3 wood units and PH (n=6,270) are presented in (Table 5). Compared with the lower DPG groups, higher DPG groups were on average younger, male predominant, and were more likely to be of black race. PCWP, pulmonary pulse pressure, TPG, PVR, and cardiac output were lower in the low DPG groups than the high DPG groups. Similar to the TPG analysis, there was no difference in survival at up to 5 years post transplant in the low vs. high DPG groups, p >0.05 for all (Table 4, Figure 2). Defining an elevated PVR as > 5 wood units (n=1783) had no affect on the ability of DPG to predict post-transplant survival (Table 4).
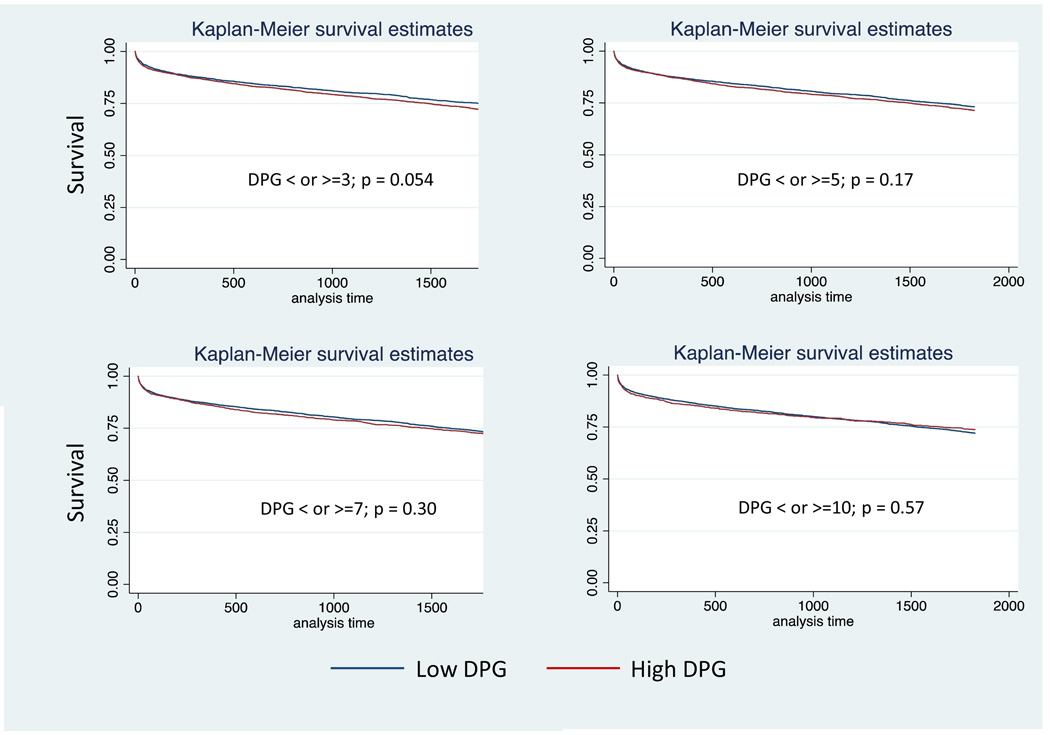
Kaplan Meier Plots for Diastolic Pulmonary Artery Pressure to Pulmonary Capillary Wedge Pressure Gradient (DPG) in patients with pulmonary hypertension (PH) and an elevated pulmonary vascular resistance > 3 Wood units: a) 3mmHg, b) 5 mmHg, c) 7 mmHg, d) 10 mmHg.
Table 5
Pulmonary Vascular Resistance > 3 Wood units
| DPG < 3 mmHg | DPG ≥ 3 mmHg | P value | DPG < 5 mmHg | DPG ≥ 5 mmHg | P value | DPG < 7 mmHg | DPG ≥ 7 mmHg | P value | DPG < 10 mmHg | DPG ≥ 10 mmHg | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (N) | 2439 | 3831 | 3391 | 2879 | 4287 | 1983 | 5227 | 1043 | ||||
| Demographics | ||||||||||||
| Age (years) | 52.9±11.5 | 52.2±11.4 | 0.02 | 52.8±11.5 | 52.1±11.3 | 0.01 | 52.8±11.5 | 51.9±11.3 | 0.01 | 52.6±11.5 | 51.9±11.2 | 0.05 |
| Female | 29.2% | 26.5% | 0.02 | 29.3% | 25.4% | 0.001 | 29.0% | 24.4% | <0.001 | 28.2% | 24.2% | 0.008 |
| White | 70.6% | 68.1% | 0.03 | 69.5% | 68.7% | 0.51 | 70.0% | 67.2% | 0.03 | 69.5% | 66.9% | 0.10 |
| Black | 18.6% | 21.5% | 0.005 | 19.2% | 21.7% | 0.015 | 19.2% | 22.8% | 0.001 | 19.9% | 22.5% | 0.06 |
| BMI (kg/m2) | 25.4±5.6 | 25.8±5.5 | 0.001 | 25.4±6.1 | 25.9±4.8 | <0.001 | 25.5±5.8 | 26.0±4.9 | <0.001 | 25.6±5.7 | 25.8±4.9 | 0.26 |
| Diagnosis | ||||||||||||
| Idiopathic | 42.4% | 46.3% | 43.7% | 46.1% | 44.3% | 45.8% | 44.8% | 44.9% | ||||
| Ischemic | 45.4% | 43.7% | 44.8% | 43.9% | 44.4% | 44.4% | 44.4% | 44.6% | ||||
| Congenital | 1.2% | 1.5% | 1.2% | 1.5% | 1.2% | 1.6% | 1.3% | 1.7% | ||||
| Other | 11.0% | 8.6% | 0.001 | 10.3% | 8.5% | 0.03 | 10.1% | 8.1% | 0.048 | 9.6% | 8.8% | 0.60 |
| Acuity | ||||||||||||
| Diabetes | 495/2287 | 810/3588 | 0.40 | 683/3179 | 622/2696 | 0.15 | 881/4022 | 424/1853 | 0.40 | 1082/4899 | 223/976 | 0.60 |
| Isch. Time (hrs) | 3.1±1.0 | 3.0±1.0 | 0.36 | 3.1±1.0 | 3.0±1.0 | 0.08 | 3.1±1.0 | 3.0±1.0 | 0.03 | 3.1±1.0 | 3.0±1.0 | 0.19 |
| Creat. (mg/dL) | 1.4±1.0 | 1.4±1.5 | 0.09 | 1.36±1.2 | 1.44±1.5 | 0.04 | 1.4±1.2 | 1.4±1.6 | 0.08 | 1.4±1.4 | 1.5±1.2 | 0.15 |
| Bili. (mg/dL) | 1.5±3.6 | 1.4±2.5 | 0.02 | 1.5±3.3 | 1.4±2.5 | 0.06 | 1.5±3.2 | 1.3±2.4 | 0.11 | 1.4±3.0 | 1.3±2.8 | 0.30 |
| I ABP | 5.0% | 6.5% | 0.02 | 5.1% | 6.8% | 0.004 | 5.3% | 7.2% | 0.004 | 5.5% | 7.9% | 0.003 |
| Assist Device | 10.9% | 12.5% | 0.06 | 10.9% | 13.0% | 0.009 | 11.3% | 13.0% | 0.06 | 11.7% | 12.9% | 0.28 |
| Ventilation | 1.9% | 2.7% | 0.06 | 2.2% | 2.5% | 0.45 | 2.3% | 2.6% | 0.39 | 2.4% | 2.4% | 0.96 |
| Hemodynamics | ||||||||||||
| SPAP (mmHg) | 58.0±12.2 | 56.0±12.9 | <0.001 | 57.1±12.0 | 56.4±13.4 | 0.03 | 56.7±12.0 | 56.9±14.1 | 0.59 | 56.5±12.1 | 58.2±15.2 | <0.001 |
| DPAP (mmHg) | 26.3±7.0 | 29.7±8.0 | <0.001 | 26.6±6.9 | 30.5±8.3 | <0.001 | 27.0±6.9 | 31.3±8.7 | <0.001 | 27.4±7.1 | 33.1±9.5 | <0.001 |
| mPAP (mmHg) | 39.8±7.8 | 39.4±8.8 | 0.05 | 39.3±7.8 | 39.8±9.2 | 0.01 | 39.2±7.8 | 40.3±9.7 | <0.001 | 39.1±8.0 | 41.6±10.3 | <0.001 |
| PCWP (mmHg) | 27.2±7.1 | 21.7±7.7 | <0.001 | 26.3±7.1 | 21.0±7.9 | <0.001 | 25.6±7.2 | 20.1±8.1 | <0.001 | 24.9±7.4 | 18.8±8.6 | <0.001 |
| C. O. (L/min) | 3.1±0.9 | 3.7±1.1 | <0.001 | 3.2±0.9 | 3.8±1.1 | <0.001 | 3.3±0.9 | 3.9±1.1 | <0.001 | 3.4±1.0 | 4.0±1.2 | <0.001 |
| PVR (Woods) | 4.2±1.5 | 5.2±2.9 | <0.001 | 4.3±1.9 | 5.4±3.0 | <0.001 | 4.3±1.8 | 5.7±3.3 | <0.001 | 4.5±2.0 | 6.4±3.8 | <0.001 |
| TPG (mmHg) | 12.6±3.8 | 17.7±6.1 | <0.001 | 13.0±3.8 | 18.8±6.4 | <0.001 | 13.6±3.9 | 20.3±6.8 | <0.001 | 14.3±4.3 | 22.8±7.6 | <0.001 |
Mean ± Standard Deviation; BMI = Body Mass Index, Isch = Ischemic, Creat = Creatinine, Bili = Bilirubin, IABP = Intra-aortic Balloon Pump, SPAP = Systolic Pulmonary Artery Pressure, DPAP = Diastolic Pulmonary Artery Pressure, mPAP = Mean Pulmonary Artery Pressure, PCWP = Pulmonary Capillary Wedge Pressure, C.O. = Cardiac Output, PVR = Pulmonary Vascular Resistance, TPG = Transpulmonary Gradient
Pulmonary Hypertension with an Elevated TPG and Elevated PVR
In patients with a TPG > 12mmHg and PVR > 3 wood units (n=4419) as well as those with a TPG > 15mmHg and PVR > 5 wood units (n=1290), there was no difference in survival between low and high DPG groups at up to 5 years post transplant (Table 4).
Discussion
In cardiac transplant candidates with pulmonary hypertension, determining the non-reversible component is vital for proper patient selection and good outcomes. Recent studies have suggested the diastolic pulmonary artery pressure to pulmonary capillary wedge pressure gradient may be useful in this regard4,6, but this has not been confirmed by large, multicenter studies. Using the UNOS database, we show that the DPG does not meaningfully delineate risk among patients with elevated TPG and PVR undergoing orthotopic heart transplant.
An elevated TPG or PVR does not always reflect irreversible pulmonary vascular disease. In left heart failure, the TPG can be elevated as a result of pulmonary vascular remodeling but also due to the effects of elevated left sided filling pressures. As pressures in the left atrium increase, this pressure is passively transmitted back to the pulmonary vasculature resulting in elevation of the diastolic pulmonary artery pressure. This increased venous pressure also leads to more vascular stiffness (or lower vascular compliance) than one would predict based on the pulmonary vascular resistance alone.3 The lower compliance leads to enhanced pulmonary arterial wave reflections which result in an increased systolic pulmonary artery pressure, and therefore mean pulmonary artery pressure. This occurs without an increase in diastolic pulmonary pressure, thereby raising the TPG. PVR is subject to the same effects because TPG is in the numerator of its calculation. Both parameters may also be affected by cardiac output as elegantly described by Naeije and colleagues.4
Given known limitations of TPG and PVR measurements, the DPG would appear an attractive alternative since dPAP is presumably not affected by the above mechanisms. However, other factors must be considered. First, from a technical measurement standpoint, the dPAP is particularly prone to the effects of both catheter whip and catheter ‘ringing’ when using fluid-filled catheters.7 Even a small error in the measured dPAP may lead to a significant change in the DPG. The use of high fidelity catheters could eliminate or reduce this error, yet these are not commonly used in clinical practice. Using computer generated mean values for hemodynamic measurements (averaged over the entire respiratory cycle) rather than manually determined end-expiration values may lead to inaccuracies, especially in situations of marked respiratory excursion.8 Incomplete catheter wedging may lead to an overestimation of PCWP and therefore a falsely low DPG. While these types of error may also affect the TPG and PVR, the relative effects on DPG may be more profound. Additionally, and most importantly, it is known that the DPG can change acutely in situations such as sepsis9,10, after bypass surgery11, acute respiratory distress syndrome12,13, acidosis2, and hypoxia2,14. The mechanism behind an elevated DPG in sepsis remains uncertain, but theories include microthrombi15, effects of endogenous prostaglandins16, acidosis2, and/or serotonin release.12 Utilizing intracardiac pacing, Enson et al. demonstrated that increases in heart rate alone lead to an increase in DPG.17 This later phenomenon may be particularly relevant to the heart failure population where tachycardia is common, compensating for a low cardiac output or as a result of inotropic medications.
Gerges and colleagues recently reported that a DPG ≥ 7 mmHg in heart failure patients with an elevated TPG was associated with worse long term prognosis compared to those with a DPG <7 mmHg.6 These findings may simply suggest that elevated DPG identifies a sicker patient population as evidenced by their faster heart rates, higher pulmonary pressures, and overall worse hemodynamic profiles compared to the lower DPG group. Once patients have resolution of their left heart failure (i.e. through cardiac transplantation), many of these ‘reversible’ factors that may elevate the DPG are no longer present.
There are several limitations of this retrospective study that merit discussion. First, data on vasodilator testing prior to transplant was not available in the UNOS database, and thus, the reversibility of PH in patients with significant PH is not known. It is likely that some patients who did not demonstrate reversibility were excluded as transplant candidates and are therefore not included in this database. These excluded patients may have had an elevated DPG. However, the large numbers of patients with an elevated DPG in this database help to lessen this potential impact. Similarly, post-transplant hemodynamic data was not available, preventing us from determining if the DPG normalized after transplantation or if those with persistently elevated DPG had worse outcomes. Goland et al. have shown that failure to normalize PVR to < 3 wood units after transplantation (i.e, those with truly fixed pulmonary hypertension) is a risk factor for long term survival.18 In our analysis, pre-transplant DPG did not discriminate long term (5 year) survival. The cause of death was not available in post-transplant patients and not all patients died as a consequence of PH and right heart failure. Therefore, it remains possible that the low DPG groups died of different causes (i.e. rejection or infection) than the high DPG group (i.e. right heart failure), diluting the true ability of DPG to discriminate right heart failure death in our mixed population. However, by selectively analyzing groups of patients at highest risk of right heart failure (i.e. PVR>3, PVR >5, TPG>12, TPG >15) we should have enriched the proportion of deaths from right heart failure. Furthermore, given the large number of patients within each group, we should have had ample power to detect even small differences introduced through dilution of a hypothesized narrow relationship between right heart failure and DPG (illustrated in sensitivity analysis in the online supplement). The fact that we still did not detect any such significant difference in mortality along a gradient of DPG minimizes the impact of this limitation on our results.
It is also possible that factors that affect the DPG (i.e. heart rate, sepsis, hypoxia, etc) could have a confounding influence on the DPG to discriminate survivors from non-survivors. Importantly, these factors that may alter DPG are not routinely considered (in part because their relative impact on DPG is not known) by clinicians when reporting pre-transplant hemodynamics. Therefore, the analysis is similar to the real world practice in which the measured DPG is taken as “true DPG”. Additionally, acuity data was relatively similar in low and high DPG groups. Finally, in order to address the many cut-points already described in the literature, we performed many analyses. The multiple significance tests performed in our work increased the chance that we would see a “significant” association by chance alone. The fact that we saw no such association despite this propensity may further support the poor ability of DPG to discriminate survivors from non-survivors when applied broadly to a pre-transplant population.
In conclusion, in this large population of heart failure patients with evidence of pre-capillary PH (elevated TPG and PVR) undergoing cardiac transplantation, the diastolic pulmonary gradient did not predict post-transplant survival. Coupled with our previous knowledge that factors other than pulmonary vascular remodeling may contribute to an elevated DPG, the findings of this study urge caution before DPG is routinely incorporated into pre-transplant or PH-related clinical decision making.
Acknowledgment
Funding Sources
The authors wish to acknowledge funding from the National Heart, Lung, and Blood Institute [Grants: 1R01HL114910-01 and L30 HL110304].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report related to this manuscript.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.healun.2013.11.008
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3955214?pdf=render
Citations & impact
Impact metrics
Article citations
Post-capillary pulmonary hypertension in heart failure: impact of current definition in the PH-HF multicentre study.
Eur Heart J, 45(35):3274-3288, 01 Sep 2024
Cited by: 1 article | PMID: 39056467 | PMCID: PMC11400736
Effect of Preoperative Mitral Regurgitation on LVAD Outcomes in Patients with Elevated Pulmonary Vascular Resistance.
Cardiovasc Drugs Ther, 20 Aug 2024
Cited by: 0 articles | PMID: 39162770
Haemodynamic phenotypes of pulmonary hypertension associated with left heart disease: a moving target.
Eur Respir J, 63(3):2302280, 14 Mar 2024
Cited by: 0 articles | PMID: 38359964
Impact of diastolic pulmonary gradient and pulmonary vascular remodeling on survival after left ventricular assist device implantation and heart transplantation.
Egypt Heart J, 75(1):102, 20 Dec 2023
Cited by: 0 articles | PMID: 38117355 | PMCID: PMC10733249
The sine transform is the sine qua non of the pulmonary and systemic pressure relationship.
Front Cardiovasc Med, 10:1120330, 26 May 2023
Cited by: 0 articles | PMID: 37304951 | PMCID: PMC10250723
Go to all (66) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease.
JACC Heart Fail, 3(1):9-16, 12 Nov 2014
Cited by: 89 articles | PMID: 25453535 | PMCID: PMC4289416
Diastolic Pulmonary Gradient as a Predictor of Right Ventricular Failure After Left Ventricular Assist Device Implantation.
J Am Heart Assoc, 8(16):e012073, 14 Aug 2019
Cited by: 13 articles | PMID: 31411097 | PMCID: PMC6759881
Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease.
Int J Cardiol, 290:138-143, 13 May 2019
Cited by: 10 articles | PMID: 31126737
Revisiting Pulmonary Hypertension in the Era of Temporary Mechanical Circulatory Support - Literature Review and Case-Based Discussion.
Transplant Proc, 55(10):2462-2469, 18 Nov 2023
Cited by: 2 articles | PMID: 37980253
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: KL2 TR000421
NHLBI NIH HHS (4)
Grant ID: L30 HL110304
Grant ID: 1R01HL114910-01
Grant ID: L30HL110304
Grant ID: R01 HL114910





