Abstract
Free full text

The aryl hydrocarbon receptor promotes IL-10 production by natural killer cells
Associated Data
Abstract
The cytokine IL-10 has an important role in limiting inflammation in many settings, including toxoplasmosis. In these studies, an IL-10 reporter mouse was used to identify the sources of this cytokine following challenge with Toxoplasma gondii. During infection, multiple cell types expressed the IL-10 reporter but natural killer cells were a major early source of this cytokine. These IL-10 reporter+ NK cells expressed high levels of the IL-12 target genes T-bet, KLRG1, and IFN-γ, and IL-12 depletion abrogated reporter expression. However, IL-12 signaling alone was not sufficient to promote NK cell IL-10 and activation of the aryl hydrocarbon receptor (AHR) was also required for maximal IL-10 production. NK cells basally expressed the AHR, relevant chaperone proteins, and the AHR nuclear translocator (ARNT), which heterodimerizes with the AHR to form a competent transcription factor. In vitro studies revealed that IL-12 stimulation increased NK cell AHR levels, and the AHR and ARNT were required for optimal production of IL-10. Additionally, NK cells isolated from T. gondii-infected Ahr-/- mice had impaired expression of IL-10, which was associated with increased resistance to this infection. Together, these data identify the AHR as a critical cofactor involved in NK cell production of IL-10.
Introduction
Toxoplasma gondii is an apicomplexan parasite that induces highly Th1 polarized immune responses characterized by the production of IL-12 and IFN-γ, which are required to control parasite growth (1, 2). Appropriate regulation of this Th1 response is critical for surviving infection, which has been illustrated by reports that IL-10-/- mice infected with T. gondii control parasite burdens but succumb to immune-mediated pathology (3, 4). Although CD4+ T cells contribute to this pathology these cells are also a critical source of IL-10 during toxoplasmosis. Consequently mice in which T cells are unable to express IL-10 also develop immune-mediated tissue pathology when challenged with T. gondii (5). Additionally, IL-10-/- RAG2-/- mice reconstituted with CD4+ T cells that are capable of producing IL-10 survive T. gondii infection while their counterparts given IL-10-/- CD4+ T cells do not (6). Although these results indicate that CD4+ T cells are an important source of IL-10 that protects against fatal immune-mediated pathology during toxoplasmosis, a number of other cell types produce IL-10 during this infection. The biological relevance of innate sources of IL-10 was suggested by the finding that IL-10-/- SCID mice, which lack B and T cells, exhibit improved survival following T. gondii infection compared to SCID mice (7). Recent studies have shown that natural killer cells can produce IL-10 and are a biologically relevant source of this cytokine during toxoplasmosis (8). NK cells are also a source of IL-10 in other murine models of infection, as NK cell IL-10 promotes increased parasite burdens during visceral leishmaniasis and limits the magnitude of the CD8+ T cell response during murine cytomegalovirus infection (8-10). Together, these reports indicate major biological functions for NK cell derived IL-10 in a variety of viral, bacterial, and parasitic infections.
Recent studies have identified effects of aryl hydrocarbon receptor (AHR) signaling on multiple aspects of the immune response, including IL-10 production (11). The AHR is a ligand-activated transcription factor that interacts with a structurally diverse array of ligands, which comprise synthetic compounds such as 2,3,7,8-tetrachlorodibenzo-p-dioxin and endogenous molecules, which include certain tryptophan and arachidonic acid metabolites (12). AHR activity was initially studied for its role in mediating tetrachlorodibenzo-p-dioxin-induced toxicity. However a number of recent studies have identified multiple effects of AHR signaling on the immune system, most notably in Th17 cells and innate lymphoid cells (13-18). In contrast to its effects in promoting the expression of the effector cytokines IL-22 or IL-17 in these cells, the AHR has also been shown to promote the production of IL-10. Thus, in type 1 regulatory T cells, the AHR interacts with the transcription factor c-Maf to promote IL-10 expression (11). Ahr-/- dendritic cells and macrophages also exhibit defects in IL-10 production (19, 20). Importantly, AHR activity appears to play a role in the response to T. gondii as Ahr-/- mice show increased susceptibility to this challenge, possibly due to immune-mediated pathology (21).
The studies presented here utilize an IL-10 reporter mouse to characterize the cell types that express IL-10 during toxoplasmosis and identified NK cells as a major source of this regulatory factor. During acute infection, NK cells that expressed the IL-10 reporter had higher levels of the IL-12 target genes IFN-γ, T-bet, and KLRG1 than reporter negative NK cells and IL-12 depletion abrogated NK cell IL-10 reporter expression. However, in vitro studies using NK cells suggested that IL-12 was not sufficient to induce IL-10 and that AHR activation contributed to optimal IL-10 production. NK cells basally expressed Ahr transcripts and these were increased following stimulation with IL-12. IL-10 production by in vitro expanded NK cells (lymphokine activated killer cells, or LAKs) was enhanced by augmenting AHR activity and decreased in the presence of AHR inhibitors. LAKs genetically deficient for the AHR or the AHR nuclear translocator (ARNT), which dimerizes with the AHR to form a competent transcription factor, were impaired in their ability to produce IL-10. Finally, NK cells isolated from Ahr-/- mice that had been infected with T. gondii exhibited defects in IL-10 expression. These data identify the AHR as a critical cofactor involved in the ability of IL-12 to promote NK cell production of IL-10, suggesting that AHR ligands can serve as signals that allow NK cells to sense and respond to their environment.
Materials and Methods
Mice and infections
Vert-X mice were provided by Dr. Christopher L. Karp (previously at the University of Cincinnati College of Medicine, Cincinnati, OH). Ahr-/- mice that had been backcrossed onto a C57/Bl6 background for 21 generations were obtained from Dr. Christopher A. Bradfield (University of Wisconsin School of Medicine and Public Health, Madison, WI). Tissues from Vav-Cre Arntfl/fl mice and control mice were provided by M. Celeste Simon (University of Pennsylvania, Philadelphia, PA). RAG1-/- mice and C57/Bl6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CBA/J mice were purchased from the National Cancer Institute Animal Production Program (Frederick, MD). All mice were bred and housed in specific pathogen-free facilities at the University of Pennsylvania in accordance with institutional guidelines. For infections, Me49 cysts were harvested from the brains of chronically infected CBA/J mice and experimental animals were injected i.p. with 20 cysts. For IL-12 depletion, mice were injected i.p. with 1mg anti-IL-12p40 (clone C17.8) or control rat IgG (Sigma, St. Louis, MO) one day before infection and 3 days post-infection.
Cell isolation
Lymphocytes were isolated from the liver as described previously (22). Spleens were dissociated through a 40μm filter and red blood cells were lysed with 0.86% ammonium chloride (Sigma) in sterile water. PECs (peritoneal exudate cells) were collected by lavaging the peritoneal cavity with 7ml PBS. Bone marrow was isolated by flushing femurs and tibias with PBS, followed by red blood cell lysis.
LAK generation
For the production of LAKs from RAG1-/- mice, bone marrow cells were plated at 1×106 cells/ml in 10ml complete RPMI 1640 (10% heat inactivated FCS, 2mM glutamine, 10U/ml penicillin, 10μg/ml streptomycin, 1mM sodium pyruvate, 1% nonessential amino acids, 5×10-5M 2-ME) and 4×103U/ml recombinant human IL-2 (proleukin, Novartis, Basel, Switzerland). Cells were fed with IL-2 on days 3 and 5 or days 4 and 6 after plating. LAKs were collected and stimulated on day 7. For the generation of LAKs from Vert-X mice, Ahr-/- mice, Vav-Cre Arntfl/fl mice, or controls, NK cells were enriched from the bone marrow or spleen using the EasySep mouse NK cell enrichment kit (STEMCELL Technologies, Vancouver, Canada). CD3- NK1.1+ cells were sorted on a FACSAria (BD Biosciences, San Jose, CA) and cultured for 1 week in complete RPMI with recombinant human IL-2.
Stimulation of LAKs or NK cells isolated from infected mice
LAKs were harvested and plated at a concentration of 1-2.5×106 cells/ml on 96 well plates (BD Biosciences). The cells were cultured in complete RPMI (or complete IMDM where indicated). LAKs were stimulated with a concentration of 5ng/ml IL-12 (eBioscience, San Diego, CA) unless otherwise stated, 2000U/ml proleukin, 30μM CH-223191 (Calbiochem, Darmstadt, Germany), 0.625μM flavone (Sigma), 0.31μM α-napthoflavone (Sigma), 300nM FICZ (Enzo Life Sciences, Farmingdale, NY), or DMSO (Sigma) as a vehicle control. After 48 hours, supernatants were collected and levels of IL-10 or IFN-γ were assayed by ELISA. For analysis of IL-10/GFP expression, cells were surface stained and run on a FACSCanto II (BD Biosciences). For analysis of IL-10 production from NK cells from infected mice, DX5+ NK 1.1+CD3- cells were sorted from spleens. The purified NK cells were plated at a concentration of 2×106 cells/ml on 96 well plates, stimulated for 48 hours with 50ng/ml PMA and 1μM ionomycin, and cytokine levels were measured by ELISA.
ELISAs
For IL-10 ELISAs, Immulon 4HBX plates (Thermo Fisher Scientific, Waltham, MA) were coated with anti-IL-10 (clone JES5-2A5) (BD Pharmingen San Diego, CA), blocked in 5% FBS in PBS, and loaded with samples. Biotinylated anti-IL-10 (clone JES5-16E3) was used for detection followed by peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA), SureBlue (KPL, Gaithersburg, MD) and TMB Stop Solution (KPL). For IFN-γ ELISAs, plates were coated with anti- IFN-γ (clone AN-18) (eBioscience) and loaded with samples. Biotinylated anti- IFN-γ (clone R4-6A2) (eBioscience) was used for detection, followed by peroxidase-conjugated streptavidin and ABTS (KPL). For IL-12p40 ELISAs, plates were coated with anti-IL-12p40 (clone C17.8), followed by detection using biotinylated anti-IL-12p40 (clone C15.6).
RNA preparation and PCR
To evaluate the expression of the AHR and its chaperone proteins, LAKs were stimulated for 6 hours or NK1.1+DX5+ cells were sorted from the spleens of RAG1-/- mice. To analyze cytokine expression by NK cells from infected wild type or Ahr-/- mice, DX5+NK1.1+CD3- cells were sorted from liver lymphocyte preparations. All sorting was performed on a FACSAria or FACSVantage SE (BD Biosciences). RNA was isolated using the RNAeasy Mini Kit (Qiagen, Valencia, CA). Samples were treated with DNAse (Promega, Madison, WI), and cDNA was generated using reagents from Invitrogen (Grand Island, NY). For RT-PCR analysis, cDNA samples were amplified with SYBR Green (Applied Biosystems, Carlsbad, CA) on the 7500 Fast Real-Time PCR System (Applied Biosystems). Primers for the AHR and β-actin were purchased from Qiagen. Primers for the AHR chaperone proteins and ARNT were generated using Primer 3 and obtained from Integrated DNA Technologies (Coralville, IA). The following sequences were used for p23 (5′-TGATTCCATGAGGCAGTTGA-3′ and 5′-AATTTGTTTCCGCCTCCTTT-3′), hsp90 (5′-AAGGCAGAGGCTGACAAGAA-3′ and 5′-ACAGCAGCACTGGTGTCATC-3′), Arnt (5′-TGCCTCATCTGGTACTGCTG-3′ and 5′-GAACATGCTGCTCACTGGAA-3′) and Ara9 (5′-GCTAGGAGTTGCCGAAACAG-3′ and 5′-GAAAGTGGAACGTGGCCTTA-3′). The following sequences were used for IL-10 (5′-ACCTGCTCCACTGCCTTGCT-3′ and 5′-GGTTGCCAAGCCTTATCGGA-3′), IFN-γ (5′-CTTCTTCAGCAACAGCAAGG-3′ and 5′-TGAGCTCATTGAATGCTTGG-3′), and HPRT (5′-AACTTTTATGTCCCCGGTTGA-3′ and 5′-GGCTATAAGTTCTTTGCTGACCTG-3′). Relative gene expression was calculated using the 2-ΔΔCT method (23).
T cell polarization
CD4+ T cells were sorted from the spleens and lymph nodes of C57/Bl6 mice. Cells were cultured in complete IMDM for 4 days on plates coated with 1μg/ml anti-CD3 (eBioscience), with soluble 1μg/ml anti-CD28 (eBioscience). Th17 cells were cultured under previously described conditions (24). Th1 cells were stimulated with 20ng/ml IL-12 and 10μg/ml anti-IL-4 (clone 11B11).
IL-12 expression in infected mice
Serum was collected 5 days post-infection and IL-12p40 levels were assayed by ELISA. Splenocytes were cultured at 1×106 cells/ml in complete RPMI with or without 62μg/ml soluble Toxoplasma antigen (STAg). Supernatants were collected after 48 hours and IL-12p40 was detected by ELISA. PECs were incubated with brefeldin A (Sigma) and Golgi Stop (BD Biosciences) for 6 hours, surface stained, and fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Cells were permeabilized with 0.5% saponin (Sigma) and stained for IL-12.
Antibodies and flow cytometry
For surface staining, samples were washed in flow cytometry buffer containing 1% BSA (Sigma) and 2mM EDTA (Invitrogen) in PBS, Fc blocked with 2.4G2 and normal rat IgG (Invitrogen), and stained with monoclonal antibodies. For the detection of IFN-γ expression by NK cells, splenocytes were surface stained and stimulated with PMA and ionomycin with brefeldin A (Sigma) for 4 hours. The cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% saponin, and stained for IFN-γ. For the detection of T-bet and Ki67, cells were surface stained and then stained intracellularly for T-bet and Ki67 using the FoxP3/transcription factor staining buffer set (eBioscience). For the detection of GFP in fixed cells from Vert-X mice, cells were stained intracellularly for GFP using an anti-GFP antibody from eBioscience followed by staining with a secondary antibody (Alexa Fluor 488 conjugated AffiniPure goat anti-rabbit from Jackson ImmunoResearch). NK1.1 FITC, CD8 PeCy7, CD11c PeCy7, CD4 APC, CD3 APC-eFluor 780, CD69 PerCP Cy5.5, IFN-γ PeCy7, T-bet AF647, KLRG1 APC, IL-12p40 PE and CD19 eFluor 450 were purchased from eBioscience. CD19 PerCP Cy5.5, NK1.1 Pacific Blue, and CD3 Pacific Blue were obtained from BioLegend. CD49b PE was purchased from BD Biosciences, and Ki67 AF647 was obtained from BD Pharmingen. Samples were run on a FACSCanto II and data was analyzed using Flowjo software (Tree Star, Inc., Ashland, OR).
Parasite burdens
DNA was isolated from the PECs using the High Pure PCR Template Preparation kit (Roche, Indianapolis, IN). Parasite DNA levels were determined by RT-PCR as previously described (25).
Statistical analysis
Statistical significance was determined using paired or unpaired Student's t tests, which were performed using Prism software (GraphPad software, Inc. La Jolla, CA). For Figure 3B, 5C, 5H, 5I, 5J and and6I6I paired t tests were used to determine significance using pooled data from multiple experiments. In this analysis, the results from each individual experiment were paired.

(A) IL-10 and IFN-γ production by LAKs, 48 hours after they were stimulated with varying concentrations of IL-12 in the presence or absence of IL-2. Results are representative of three independent experiments. (B) IL-10 and IFN-γ production by LAKs that were stimulated with IL-2, IL-12 or a combination of the two cytokines in RPMI or IMDM for 48 hours. (*p<0.05 based on a Student's t test with replicates in the representative experiment that is shown. These results were also significant (p<.05) in a paired Student's t test with pooled data from 4 independent experiments.) All error bars represent standard error.
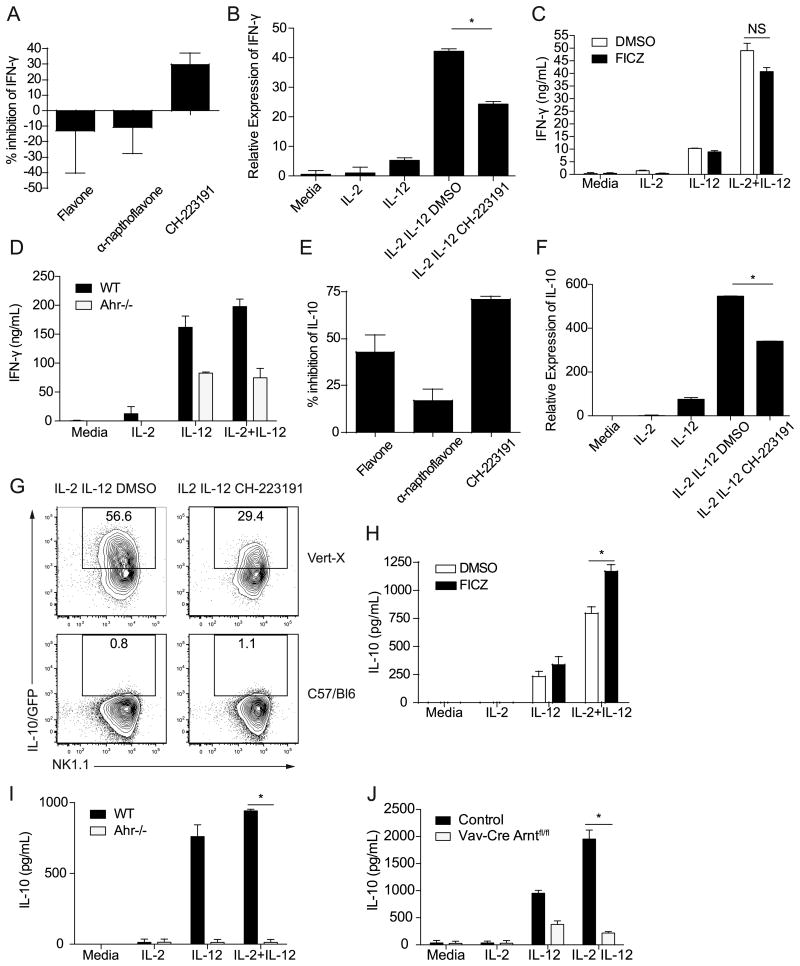
(A, E) Percent inhibition by AHR inhibitors (flavone, α–napthoflavone and CH-223191) of IFN-γ and IL-10 production by LAKs that were stimulated with IL-2 and IL-12 compared to vehicle control (DMSO) treated cells. The graphs show the average inhibition from 3-9 pooled independent experiments. (B, F) IFN-γ and IL-10 transcript expression by LAKs stimulated for 6 hours or 27 hours respectively. Sample transcript levels were normalized based on HPRT expression, and calibrated to the IL-2 treated sample. Results are representative of three similar experiments, and error bars are based on technical replicates (*p<0.05 based on a Student's t test on technical replicates. Similar results were seen in 3 separate experiments). (C, H) IFN-γ or IL-10 production by LAKs stimulated with IL-2 and IL-12 in the presence or absence of FICZ for 48 hours. Graphs show representative data from one experiment. (*p<0.05 based on a Student's t test with replicates in the representative experiment that is shown. These results were also significant (p<.05) in a paired Student's t test with pooled data from 4 independent experiments.) (D, I) IFN-γ or IL-10 production by LAKs generated from Ahr-/- or wild type mice, 48 hours after stimulation with IL-2 and IL-12. Graphs show representative data from one experiment. (*p<0.005 based on a Student's t test with replicates in the representative experiment that is shown. These results were also significant (p<.05) in a paired Student's t test with pooled data from 3 independent experiments.) (G) IL-10/GFP expression by LAKs generated from Vert-X mice 48 hours post-stimulation. LAKs from C57/Bl6 mice were used as controls to gate for IL-10/GFP+ cells. Representative plots from 3 independent experiments are shown. (J) IL-10 production by LAKs generated from a Vav-Cre Arntfl/fl mouse or control 48 hours post-stimulation. Results from one of two independent experiments are shown. (*p<0.0001 based on a Student's t test with replicates in the representative experiment that is shown.) All error bars represent standard error.

Wild type or Ahr-/- mice were infected for five days with T. gondii. (A) IL-12p40 levels in the serum of WT or Ahr-/- mice. Pooled data from 3 independent experiments with a total of 8-9 mice per group are shown. (B) IL-12p40 production by dendritic cells in the PECs (peritoneal exudate cells) following a 6 hour incubation with brefeldin A and Golgi Stop. Dendritic cells were gated as CD3-CD19-NK1.1-CD11c+ cells. Plots show representative data from 3 independent experiments with a total of 7-9 mice per group. (C) Frequency of dendritic cells that stained positive for IL-12p40 following a 6 hour incubation with brefeldin A and Golgi Stop. Data are pooled from three independent experiments. (D) IL-12p40 secretion by splenocytes from infected mice 48 hours post-incubation in media alone or STAg. Graphs show pooled averages from 2 independent experiments with a total of 5-6 mice per group. (E) Total numbers of NK cells in the spleens of infected WT or Ahr-/- mice. Data are pooled from three independent experiments with a total of 10 mice per group. NK cells were gated as DX5+ NK 1.1+CD3-CD19- cells. (F) CD69 and CD11c expression by splenic NK cells from infected wild type or Ahr-/- mice. Representative plots from three independent experiments are shown. (G) Expression of T-bet, KLRG1, and Ki67 by splenic NK cells from infected wild type or Ahr-/- mice. Numbers represent the mean ± the standard deviation from three separate experiments with a total of 10 mice per group. (H) IL-10 transcript expression by NK cells sorted from the livers of infected wild type or Ahr-/- mice. Sample transcript levels were normalized based on expression of the housekeeping gene β-actin, and calibrated using the wild type sample. Data are pooled from two separate experiments. (*p<.05 from a Student's t test). (I) Cytokine secretion by NK cells sorted from the spleens of infected wild type or Ahr-/- mice and stimulated ex vivo with PMA and ionomycin for 48 hours. The points show the paired results from 3 independent experiments. (*p<.05 based on a paired Student's t test with data pooled from 3 experiments). (J) Levels of parasite DNA in the PECs five days post-infection. The graph shows pooled data from 3 separate experiments with 7-9 mice per group. (*p<0.05 using pooled data from 3 independent experiments) All error bars represent standard error.
Results
Characterization of cellular sources of IL-10 during toxoplasmosis
While CD4+ T cells are an essential source of IL-10 during infection, studies in IL-10-/- SCID mice have suggested that the production of IL-10 by innate cell populations promotes susceptibility to T. gondii (6, 7). To identify innate and adaptive sources of IL-10, Vert-X IL-10/GFP reporter mice, which have been used previously to assess IL-10 expression (8, 26, 27), were challenged with the Me49 strain of T. gondii. Five days following infection, NK cells constituted the largest fraction of total IL-10 expressing cells in the spleen and liver, which are two of the main sites of mature NK cell localization (28) (Figure 1A). The numbers of splenic IL-10/GFP+ cells are shown in Supplementary Figure 1. These results indicated that NK cells are a major source of IL-10 during early infection, in agreement with previous findings (8).

Vert-X IL-10 reporter mice were infected with T. gondii for five days. (A). The percentages of different cell types within the total IL-10/GFP+ population in the spleen, PECs (peritoneal exudate cells) and liver of infected Vert-X mice, represented as pie graphs. Average percentages were calculated from 2-3 independent experiments, with a total of 8-10 mice. Various cell types were distinguished based on the expression of surface receptors; NK cells (DX5+NK1.1+CD3-CD19-), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), CD4-CD8- T cells (CD3+CD4-CD8-), and B cells (CD19+CD3-). (B) Expression of CD69 and CD11c on splenic NK cells from naïve and infected Vert-X mice. Results are representative of three independent experiments, with a total of 5 naïve and 11 infected mice. (C) The expression of Ki67, IFN-γ, T-bet, and KLRG1 by total splenic NK cells from naïve Vert-X mice and the IL-10/GFP+ or IL-10/GFP- NK cells from infected mice. Cells were re-stimulated with PMA/ionomycin to evaluate IFN-γ production. Data are representative of 2-3 independent experiments with a total of 8-9 infected mice and 4-5 naïve mice. (D) The frequency of splenic IL-10/GFP- and IL-10/GFP+ NK cells from infected Vert-X mice that produce IFN-γ following PMA/ionomycin restimulation. Data are pooled from two experiments with a total of 8 mice. (E) The MFI (mean fluorescence intensity) of T-bet in splenic IL-10/GFP- and IL-10/GFP+ NK cells from infected Vert-X mice. Data are pooled from three experiments with a total of 9 mice. (F) Frequencies of IL-10/GFP- NK cells or IL-10/GFP+ NK cells from infected Vert-X mice that are KLRG1+. Data are pooled from three independent experiments with a total of 9 mice.
Further characterization of NK cells in the Vert-X mice revealed that infection led to a global increase in CD11c and CD69 expression and that these markers of NK cell activation were expressed similarly by IL-10/GFP+ and IL-10/GFP- NK cells (Figure 1B) (29, 30). The levels of Ki67, a marker of proliferating cells, also increased in IL-10/GFP+ and IL-10/GFP- NK cells from infected mice (Figure 1C). However, several other markers were differentially expressed by IL-10/GFP+ and IL-10/GFP- NK cells. Following PMA/ionomycin stimulation, a higher percentage of IL-10/GFP+ NK cells produced IFN-γ than IL-10/GFP- NK cells (Figure 1C, D). Most NK cells in naïve mice stained positive for the transcription factor T-bet, but T-bet expression increased following infection (Figure 1C) and IL-10/GFP+ NK cells expressed the highest levels of T-bet (Figure 1E). Both IL-10/GFP+ and IL-10/GFP- NK cells from infected mice expressed high levels of KLRG1, but a significantly higher percentage of IL-10/GFP+ cells were KLRG1+ (Figure 1C, F). The marked increases in NK cell expression of CD69 and CD11c following infection indicated that both IL-10/GFP+ and IL-10/GFP- NK cells had become activated, but IL-10/GFP+ NK cells expressed higher levels of IFN-γ, T-bet, and KLRG1, which are targets of IL-12 signaling (31, 32).
IL-12 induces NK cell IL-10 expression
The finding that NK cells are a major source of IL-10 in early infection raised the question of which signals induce IL-10 expression by this population. Previous work has shown that IL-12 promotes NK cell IL-10 production during toxoplasmosis (8), consistent with the finding that IL-10/GFP+ NK cells expressed high levels of IL-12 target genes. To examine the effects of IL-12 signaling on NK cells during infection, Vert-X mice were treated with IL-12 depleting antibodies and challenged with T. gondii. Depletion of IL-12 in infected Vert-X mice abrogated NK cell IL-10 reporter expression (Figure 2A), in agreement with previous studies (8). NK cells in infected control and anti-IL-12p40 treated mice upregulated expression of CD69 and CD11c (Figure 2B). However IL-12 depletion antagonized the infection-induced upregulation of KLRG1 and Ki67 (Figure 2C, D, E). T-bet MFIs (mean fluorescence intensities) were also reduced following IL-12 depletion (Figure 2C, F). These results suggested that IL-12 signaling during infection promoted NK cell IL-10 expression and proliferation. In agreement with this, infected mice that had been treated with IL-12 depleting antibodies had fewer total splenic NK cells than infected controls (data not shown).
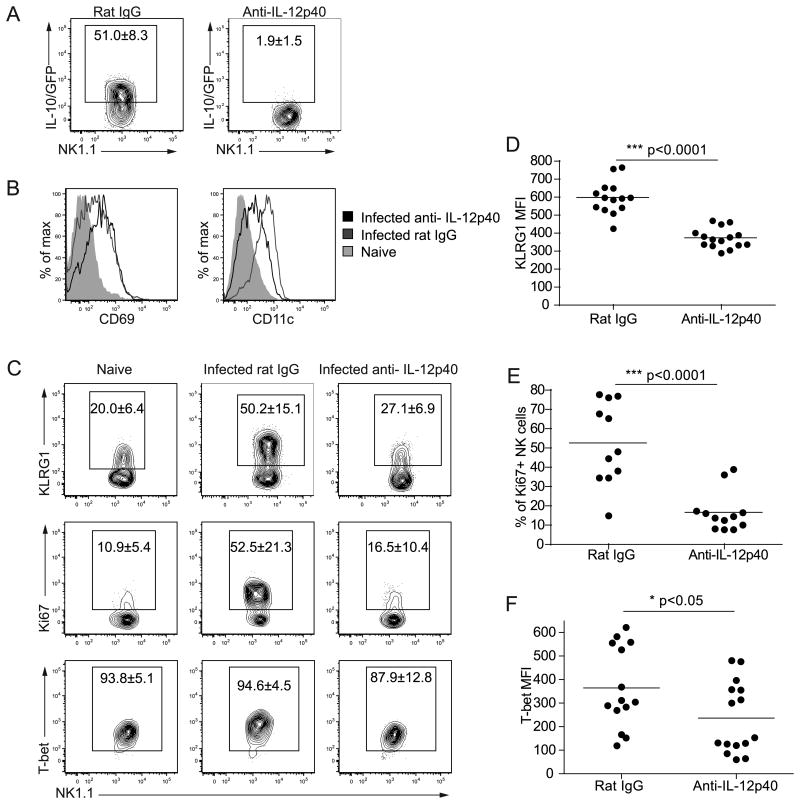
Vert-X IL-10 reporter mice were infected with T. gondii for five days and given control rat IgG or anti-IL-12p40. NK cells were gated as DX5+ NK1.1+CD3-CD19- cells. (A) IL-10/GFP expression by NK cells in the spleens of Vert-X mice that were given rat IgG or anti-IL-12p40. Numbers represent the mean ± the standard deviation from four independent experiments, with a total of 13-16 mice per group. (B&C) Expression of CD69, CD11c, KLRG1, Ki67 and T-bet by NK cells from naïve Vert-X mice or infected Vert-X mice that were given rat IgG or anti-IL-12p40. Numbers represent the mean ± the standard deviation from 2-4 independent experiments with a total of 5 naïve and 13-16 infected mice per group. (D) KLRG1 MFIs of KLRG1+ NK cells from infected Vert-X mice given rat IgG or anti-IL-12p40. Data are pooled from 4 independent experiments. (E) Frequency of Ki67+ NK cells from infected Vert-X mice given rat IgG or anti-IL-12p40. Data are pooled from 4 independent experiments. (F) T-bet MFIs of NK cells from infected Vert-X mice given rat IgG or anti-IL-12p40. Data are pooled from 4 independent experiments.
To study the factors that regulate NK cell expression of IL-10 in vitro, IL-2 activated lymphokine activated killer cells (LAKs) were utilized, which were generated as previously described (33). To evaluate IL-10 production by these cells, LAKs were stimulated with different combinations of cytokines for 48 hours. In the absence of stimulation, these cells did not produce detectable levels of IL-10 and stimulation with IL-2 or IL-12 alone induced little IL-10 secretion (Figure 3A). However, when treated with the combination of IL-2 and IL-12, LAKs produced high levels of IL-10 and IFN-γ as previously reported (34). Similar results were observed with NK cells freshly isolated from the spleens of RAG1-/- mice (data not shown). A number of other cytokines, including the IL-12 family members IL-23 and IL-27, did not induce IL-10 production from LAKs when used in various combinations with IL-2 or IL-12 (data not shown).
LAKs and NK cells express the AHR
Although these studies highlighted the critical role of IL-12 in inducing NK cell IL-10 expression, optimal cytokine production by NK cells typically requires multiple signals. Therefore, additional pathways may have contributed to IL-10 induction in these cells. One candidate was signaling by the AHR, which promotes IL-10 production by macrophages, dendritic cells, and type 1 regulatory T cells cells (11, 19, 20). Indeed, LAKs that had been stimulated with IL-2 and IL-12 in the culture medium IMDM, which contains high levels of AHR ligands (35), secreted significantly more IL-10 than LAKs that were cultured in RPMI (Figure 3B). The production of IFN-γ was not affected by the type of media used (Figure 3B). Since this result suggested that AHR signaling promoted IL-10, NK cell expression of the AHR as well as the p23, hsp90 and ara9 chaperone proteins that associate with the AHR were then evaluated (Figure 4A). LAKs basally expressed transcripts for the AHR, p23, hsp90, ara9, and ARNT (Figure 4A), which heterodimerizes with the AHR in the nucleus to form a competent transcription factor. While the expression of most AHR chaperone proteins and ARNT was not affected by IL-12, this treatment did result in an approximately three-fold increase in AHR mRNA within six hours (Figure 4A). Transcripts for the AHR, p23, hsp90, ara9 and ARNT were also detected in NK cells that had been freshly isolated from the spleens of RAG1-/- mice (Figure 4B). Since Th17 cells express high levels of Ahr mRNA compared to other T cell subsets, polarized Th17 and Th1 cells were included in these experiments as controls (14, 15, 36). These results suggest that NK cells are able to respond to AHR signaling and that their ability to do so is enhanced by IL-12 stimulation. Interestingly, NK cells isolated from T. gondii infected mice 5 days post-infection expressed reduced levels of Ahr transcripts compared to cells from naïve mice (data not shown). These data are consistent with the idea that stimulation with AHR ligands can lead to a downregulation of AHR expression (37).
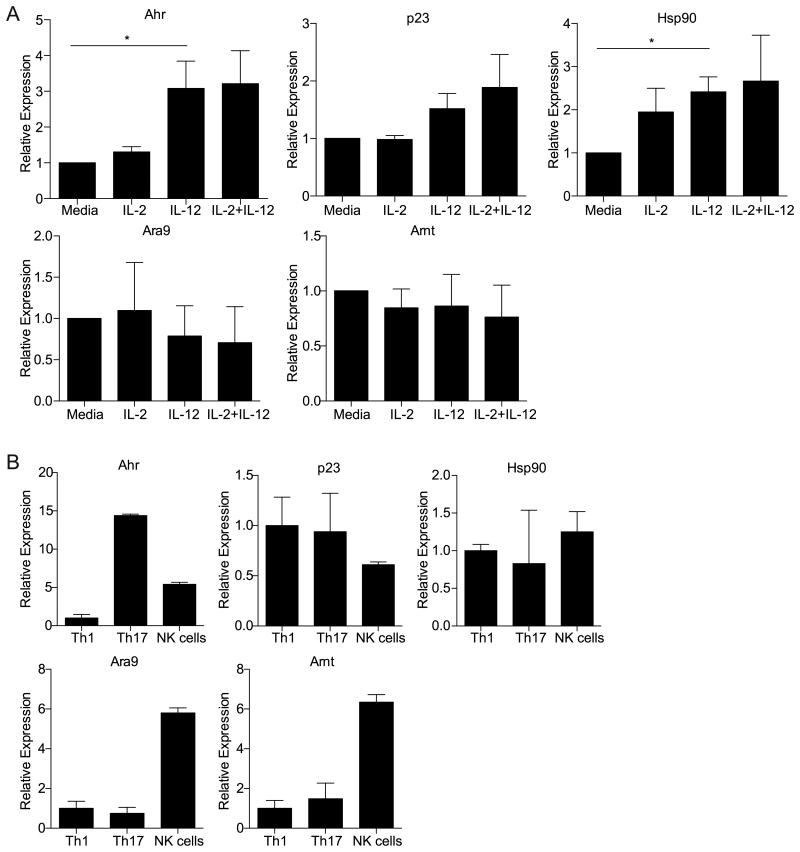
(A) The expression of the Ahr and AHR related genes (p23, Hsp90, Ara9 and Arnt) in LAKs that were stimulated with IL-2, IL-12 or the combination of IL-2 and IL-12 for 6 hours. Sample transcript levels were normalized to the expression of the housekeeping gene HPRT, and relative levels are shown using the media sample as a calibrator. Graphs show the pooled averages from three independent experiments. (*p<0.05 based on a Student's t test). (B) Ahr and AHR related gene (p23, Hsp90, Ara9 and Arnt) expression in NK cells sorted from the spleens of RAG1-/- mice and in vitro polarized Th17 or Th1 CD4+ T cells. Sample transcript levels were normalized to the expression of the housekeeping gene HPRT, and relative levels are shown calibrated to the Th1 sample. Results are representative of three similar experiments, and error bars are based on technical replicates. All error bars represent standard error.
Effect of AHR signaling on IL-10 and IFN-γ production by LAKs
To determine the effects of AHR signaling on NK cell cytokine production, LAKs were stimulated with AHR inhibitors in the presence of IL-2 and IL-12 and supernatants were assayed for IL-10 and IFN-γ. The effects of these inhibitors on LAK IFN-γ expression were variable; two of the inhibitors, flavone and α-napthoflavone, had no significant effect on IFN-γ production, while treatment with CH-223191 led to decreased IFN-γ secretion (Figure 5A). No difference in survival was seen between LAKs treated with DMSO as a vehicle control or cells stimulated with CH-223191 (data not shown). IFN-γ transcript expression also decreased following stimulation with CH-223191 (Figure 5B). To further investigate the effects of AHR signaling on NK cells, LAKs were stimulated in vitro with the high-affinity AHR ligand 6-formylindolo[2,3-b]carbazole (FICZ), which can form intracellularly from tryptophan following exposure to ultraviolet light (38, 39). LAKs stimulated with FICZ in the presence of IL-2 and IL-12 produced similar levels of IFN-γ as LAKs treated with the vehicle control (Figure 5C). Since possible off-target effects with the use of pharmacological agents as AHR agonists or antagonists were a concern, LAKs were generated from mice genetically deficient for the AHR and wild type controls (Figure 5D). Following stimulation with IL-2 and IL-12, Ahr-/- LAKs secreted less IFN-γ than LAKs from wild-type mice. This reduction was statistically significant (p<0.02) when comparing the pooled percentages of Ahr-/- LAK IL-10 production relative to wild type LAKs from three separate experiments. No survival differences were observed between wild type and Ahr-/- LAKs (data not shown). Together, the results from these studies suggest a role for AHR signaling in the ability of LAKs to produce IFN-γ.
The effects of AHR signaling on IL-10 production by LAKs were also evaluated. All three of the AHR inhibitors significantly reduced LAK IL-10 secretion in response to IL-2 and IL-12 (Figure 5E). Flavone and α-napthoflavone have been shown to act as AHR agonists at high concentrations (40), and their effects on IL-10 expression were concentration dependent, with maximal inhibition of IL-10 seen when LAKs were treated with intermediate concentrations of these inhibitors (data not shown). Similarly, stimulation with CH-223191, the most effective AHR inhibitor used in these studies (40), also led to a decrease in the level of IL-10 mRNA expressed by LAKs (Figure 5F). This was corroborated by using LAKs generated from Vert-X mice, which expressed reduced levels of the IL-10 reporter following treatment with CH-223191 compared to cells treated with the vehicle control (Figure 5G). While stimulation with the AHR agonist FICZ was not sufficient to induce detectable secretion of IL-10, FICZ treatment led to an increase in the amount of IL-10 expressed by LAKs in the presence of IL-2 and IL-12 (Figure 5H). Thus, augmenting AHR activity in culture promoted cytokine mediated NK cell production of IL-10. However stimulation with the AHR ligands tetrachlorodibenzo-p-dioxin or L-kynurenine did not lead to significant differences in IL-10 production (data not shown), suggesting that AHR ligands have different effects, in agreement with previous studies (14). In order to evaluate LAK IL-10 secretion in the complete absence of AHR activity, LAKs were generated from Ahr-/- mice or wild-type controls. When treated with IL-2 and IL-12, Ahr-/- LAKs exhibited significant defects in IL-10 production (Figure 5I). To determine whether ARNT activity also contributed to NK cell IL-10 expression, LAKs were generated from Vav-Cre Arntfl/fl mice, in which ARNT is conditionally deleted in hematopoietic cells. Following stimulation with IL-2 and IL-12, ARNT deficient LAKs exhibited impaired IL-10 production (Figure 5J), suggesting that the effects of the AHR on NK cell IL-10 expression were dependent on its interaction with ARNT. Collectively, these studies indicated that AHR activity is required for optimal IL-10 expression by LAKs in response to stimulation with IL-2 and IL-12.
Role of the AHR in the innate response to T. gondii
To determine the role of the AHR on NK cell responses in vivo, Ahr-/- mice were challenged with T. gondii and their innate immune responses were evaluated. Analysis of IL-12 production revealed that infection led to comparable serum levels of IL-12 in wild type and Ahr-/- mice (Figure 6A). Dendritic cells from these mice also expressed similar levels of IL-12 (Figure 6B, C), and splenocytes isolated from infected wild type and Ahr-/- mice produced comparable levels of IL-12 upon stimulation with soluble Toxoplasma antigen (STAg) (Figure 6D), indicating that infected Ahr-/- mice had no early defect in IL-12 production. Infected Ahr-/- and wild type mice also had similar numbers of NK cells (Figure 6E). These populations expressed comparable levels of CD69, CD11c, T-bet, KLRG1, and Ki67 (Figure 6F, G). However NK cells isolated from Ahr-/- mice had marked reductions in their levels of IL-10 mRNA compared to cells from wild-type mice (Figure 6H). Ahr-/- NK cells isolated from infected mice were also impaired in their ability to secrete IL-10 following stimulation with PMA/ionomycin ex vivo (Figure 6I). While IL-10 plays a critical role in limiting infection-induced immunopathology, IL-10 signaling can also promote increased pathogen burdens by attenuating inflammatory responses and suppressing antimicrobial activity. Accordingly, infected Ahr-/- mice had decreased parasite burdens during the acute phase of infection (Figure 6J). Collectively these results indicated that although T. gondii infection induced AHR-independent activation of NK cells, optimal NK cell expression of IL-10 was dependent on AHR activity.
Discussion
Multiple studies have identified effects of AHR signaling on cells of the immune system, including Th17 cells, regulatory T cells, γδ T cells, innate lymphoid cells, dendritic cells, and macrophages (14-20, 36, 41, 42). In many of these instances, AHR activity affects cytokine production, but AHR signaling can also affect the survival or maintenance of certain immune cell populations (16-18). The studies presented here establish that AHR signaling also influences NK cell production of IL-10. NK cells basally expressed Ahr transcripts, and Ahr expression in LAKs increased following stimulation with IL-12. AHR signaling in turn promoted NK cell IL-10 production in vitro and contributed to their IL-12 dependent production of IL-10 during infection with T. gondii. Although previous work has suggested that NK cell IL-10 limits IL-12 production during experimental toxoplasmosis (8), in the studies presented here Ahr-/- mice had similar levels of IL-12p40 as wild type mice. Importantly Ahr-/- mice also had lower parasite burdens than wild type mice, which likely impacted their IL-12 levels.
While this report has focused on the impact of the AHR on NK cell production of IL-10, its effects on IFN-γ expression were variable. However, a recent study has shown that the AHR promotes NK cell cytolytic activity and IFN-γ production, and that NK cells with deficient AHR activity are impaired in their ability to control tumor growth (43). Collectively these studies indicate that the AHR has a number of important effects on NK cell function in settings of infection and cancer. Thus AHR expression likely contributes to the ability of NK cells to sense environmental signals. NK cells express a number of receptors that allow them to detect external cues, including cytokine receptors and activating and inhibitory receptors (44). AHR activity may provide these cells with an additional means of responding to the environment, in this case, by promoting the production of IL-10. NK cells have been shown to produce IL-10 following infection with Y. pestis, Listeria monocytogenes, and murine cytomegalovirus (8, 10). Additionally, during visceral leishmaniasis, NK cell IL-10 promotes increased parasite burdens (9). Indeed in the studies presented here, the defect in NK cell IL-10 expression in Ahr-/- mice correlated with decreased levels of T. gondii at five days post-infection. Thus, it seems likely that AHR signaling affects NK cell function during challenge with other pathogens and thereby affects outcomes of infection.
The finding that only a subset of NK cells expressed IL-10/GFP at five days post-infection raises the question of what accounted for the differential expression of this cytokine. These subsets may reflect possible variability in AHR expression or access to AHR ligands. Alternatively, subsets may be distinguished by differences in cytokine responsiveness. NK cells exhibit variable expression of IL-12Rβ2, and those cells expressing higher levels of IL-12Rβ2 could be more prone to producing IL-10 in response to IL-12 stimulation (45). Interestingly, IL-10/GFP+ NK cells also expressed high levels of T-bet and KLRG1, mirroring the phenotype of short-lived effector CD8+ T cells, which express higher levels of T-bet and KLRG1 than the CD8+ T cells that give rise to long term memory (32). Several reports have indicated that NK cells can also form memory (46-49), and the expression of T-bet and KLRG1 may distinguish short-lived and long-lived populations of NK cells, paralleling the differential expression of these markers in different populations of CD8+ T cells.
This report also raises the question of what the sources of AHR ligands are that affect NK cell function. AHR ligands are present in cell culture medium, which is consistent with the finding that LAKs stimulated in IMDM, which contains relatively high levels of AHR ligands, produced the highest levels of IL-10. In vivo, AHR ligands may be obtained through the diet, as a number of plant compounds are thought to activate the AHR. Diets low in AHR ligands have been shown to affect immune activity in the gut (18, 50), but dietary AHR ligands may have more systemic effects on the immune response. Alternatively, AHR ligands may be produced by NK cells themselves or other host cells in response to infection. An additional possibility is that AHR ligands are produced directly by the parasite during infection. T. gondii can catalyze the production of lipoxin A4, a putative AHR ligand that can also be produced by host cells (51). Another potential source of parasite-derived ligands is based on the shikimate pathway, which is a biochemical pathway found in T. gondii that provides precursors for the synthesis of a number of aromatic compounds (52), which may serve as AHR ligands. The shikimate pathway is active in a number of pathogens, including other apicomplexans such as Plasmodium falciparum and bacteria such as Mycobacterium tuberculosis (52). The generation of potential AHR ligands by T. gondii raises the possibility that the AHR may act as a sensor that can detect pathogen-derived metabolic products during infection. Indeed, it has been suggested that the innate immune system can distinguish between live and dead microbes through the detection of viability-associated pathogen associated molecular patterns, such as bacterial mRNA (53). By acting through the AHR, parasite-derived metabolites could potentially function as another class of viability-associated pathogen associated molecular patterns.
Acknowledgments
Ahr-/- mice were kindly provided by Christopher A. Bradfield.
This work was made possible by funding from the following grants from the National Institutes of Health: R21-AI090234 and R01-AI-42334. Support was also provided by the Howard Hughes Medical Institute (to M.C.S.).
Abbreviations
| in this article | AHR, aryl hydrocarbon receptor |
| ARNT | AHR nuclear translocator |
| FICZ | 6-formylindolo[2,3-b]carbazole |
| KLRG1 | killer cell lectin-like receptor subfamily G, member 1 |
| LAKs | lymphokine activated killer cells |
| MFI | mean fluorescence intensity |
| PECs | peritoneal exudate cells |
| STAg | soluble Toxoplasma antigen |
| WT | wild type |
References
Full text links
Read article at publisher's site: https://doi.org/10.4049/jimmunol.1300497
Read article for free, from open access legal sources, via Unpaywall:
https://www.jimmunol.org/content/jimmunol/192/4/1661.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4049/jimmunol.1300497
Article citations
Reprogramming the immunosuppressive tumor microenvironment through nanomedicine: an immunometabolism perspective.
EBioMedicine, 107:105301, 22 Aug 2024
Cited by: 0 articles | PMID: 39178747 | PMCID: PMC11388279
Review Free full text in Europe PMC
Decidual natural killer cells dysfunction is caused by IDO downregulation in dMDSCs with Toxoplasma gondii infection.
Commun Biol, 7(1):669, 31 May 2024
Cited by: 1 article | PMID: 38822095 | PMCID: PMC11143278
Targeting aryl hydrocarbon receptor to prevent cancer in barrier organs.
Biochem Pharmacol, 223:116156, 20 Mar 2024
Cited by: 1 article | PMID: 38518996 | PMCID: PMC11144369
Review Free full text in Europe PMC
Commensal bacteria promote type I interferon signaling to maintain immune tolerance in mice.
J Exp Med, 221(1):e20230063, 12 Dec 2023
Cited by: 10 articles | PMID: 38085267 | PMCID: PMC10716256
Petiveria alliacea Reduces Tumor Burden and Metastasis and Regulates the Peripheral Immune Response in a Murine Myeloid Leukemia Model.
Int J Mol Sci, 24(16):12972, 19 Aug 2023
Cited by: 2 articles | PMID: 37629156 | PMCID: PMC10454792
Go to all (68) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii.
J Immunol, 158(5):2285-2293, 01 Mar 1997
Cited by: 54 articles | PMID: 9036976
Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii.
J Immunol, 165(5):2619-2627, 01 Sep 2000
Cited by: 58 articles | PMID: 10946290
The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis.
J Biomed Biotechnol, 2010:505694, 11 Jan 2010
Cited by: 29 articles | PMID: 20111744 | PMCID: PMC2810477
The role of IL-12 in stimulating NK cells against Toxoplasma gondii infection: a mini-review.
Parasitol Res, 120(7):2303-2309, 10 Jun 2021
Cited by: 17 articles | PMID: 34110502
Review
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NHLBI NIH HHS (1)
Grant ID: R01 HL066310
NIAID NIH HHS (5)
Grant ID: R01 AI110201
Grant ID: R21 AI090234
Grant ID: R01 AI042334
Grant ID: R01-AI-42334
Grant ID: R21-AI090234





