Sebastian Köhler
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Sandra C. Doelken
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Christopher J. Mungall
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Sebastian Bauer
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Helen V. Firth
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Isabelle Bailleul-Forestier
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Graeme C. M. Black
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Danielle L. Brown
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Michael Brudno
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Jennifer Campbell
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
David R. FitzPatrick
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Janan T. Eppig
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Andrew P. Jackson
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Kathleen Freson
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Marta Girdea
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Ingo Helbig
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Jane A. Hurst
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Johanna Jähn
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Laird G. Jackson
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Anne M. Kelly
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
David H. Ledbetter
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Sahar Mansour
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Christa L. Martin
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Celia Moss
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Andrew Mumford
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Willem H. Ouwehand
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Soo-Mi Park
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Erin Rooney Riggs
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Richard H. Scott
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Sanjay Sisodiya
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Steven Van Vooren
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Ronald J. Wapner
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Andrew O. M. Wilkie
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Caroline F. Wright
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Anneke T. Vulto-van Silfhout
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Nicole de Leeuw
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Bert B. A. de Vries
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Nicole L. Washingthon
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Cynthia L. Smith
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Monte Westerfield
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Paul Schofield
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Barbara J. Ruef
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Georgios V. Gkoutos
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Melissa Haendel
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Damian Smedley
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Suzanna E. Lewis
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany
Peter N. Robinson
1Institute for Medical Genetics and Human Genetics, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 2Berlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, 3Lawrence Berkeley National Laboratory, Mail Stop 84R0171, Berkeley, CA 94720, USA, 4The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridgeshire CB10 1SA, UK, 5Department of Medical Genetics, Cambridge University Addenbrooke’s Hospital, Cambridge CB2 2QQ, UK, 6Université Paul Sabatier, Faculté de Chirurgie Dentaire, CHU Toulouse, France, 7Centre for Genomic Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), Manchester, UK, 8Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, MAHSC, Manchester M13 9WL, UK, 9Institute of Genetic Medicine. Newcastle University, Central Parkway, Newcastle upon Tyne, NE1 3BZ, UK, 10Department of Computer Science, University of Toronto, Ontario, Canada, 11Centre for Computational Medicine, Hospital for Sick Children, Toronto, Ontario, Canada, 12Department of Clinical Genetics, Leeds Teaching Hospitals NHS Trust, Leeds LS2 9NS, UK, 13MRC Human Genetics Unit, MRC Institute of Genetic and Molecular Medicine, University of Edinburgh, Edinburgh EH4 2XU, UK, 14The Jackson Laboratory, Bar Harbor, ME 04609, USA, 15Center for Molecular and Vascular Biology, University of Leuven, Belgium, 16Department of Neuropediatrics, University Medical Center Schleswig-Holstein, Kiel Campus, 24105 Kiel, Germany, 17NE Thames Genetics Service, Great Ormond Street Hospital, London WC1N 3JH, UK, 18Drexel University College of Medicine, Philadelphia, PA 19102, USA, 19Department of Haematology, University of Cambridge and NHS Blood and Transplant Cambridge, CB2 0PT Cambridge, UK, 20Autism and Developmental Medicine Institute, Geisinger Health System, Danville, PA 17822, USA, 21SW Thames Regional Genetics Service, St George’s Healthcare NHS Trust, Tooting, London SW17 0RE, UK, 22Department of Dermatology, Birmingham Children’s Hospital, Birmingham, UK, 23Bristol Heart Institute, University of Bristol, Bristol, UK, 24Department of Clinical Genetics, Great Ormond Street Hospital, London and Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK, 25Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, London, UK, 26Cartagenia, Leuven, Belgium, 27Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY 10032, USA, 28Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, UK, 29Department of Human Genetics, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands, 30ZFIN, University of Oregon, Eugene, OR 97403-5291, USA, 31Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK, 32Department of Computer Science, Aberystwyth University, Aberystwyth, Ceredigion, SY23 3DB, UK, 33Department of Medical Informatics & Clinical Epidemiology, Oregon Health & Science University, Portland, OR 97239, USA and 34Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany

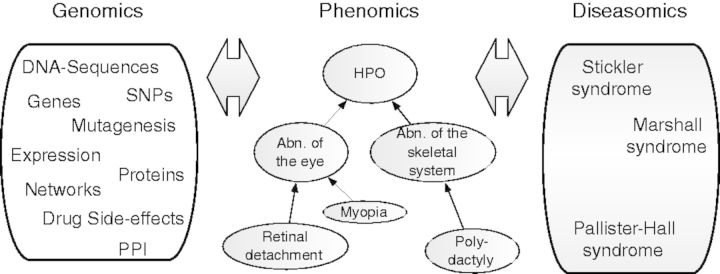

 ‘decreased concentration’
‘decreased concentration’


 Embryonal
Embryonal


 Fetal
Fetal


 Neonatal
Neonatal


 Infantile
Infantile


 Childhood
Childhood


 Juvenile
Juvenile


 Young adult
Young adult


 Mid adult
Mid adult


 Old age
Old age


 Phenomizer
Phenomizer


 BOQA
BOQA


 Exomiser
Exomiser


 Cartagenia
Cartagenia


 ECARUCA
ECARUCA


 DECIPHER
DECIPHER


 PhenoTips
PhenoTips


 PhenoDigm
PhenoDigm


 MouseFinder
MouseFinder


 Monarch
Monarch


 PhenomeNet
PhenomeNet


 Uberpheno
Uberpheno




