Abstract
Free full text

The effect of lifelong exercise dose on cardiovascular function during exercise
Abstract
An increased “dose” of endurance exercise training is associated with a greater maximal oxygen uptake ( o2max), a larger left ventricular (LV) mass, and improved heart rate and blood pressure control. However, the effect of lifelong exercise dose on metabolic and hemodynamic response during exercise has not been previously examined. We performed a cross-sectional study on 101 (69 men) seniors (60 yr and older) focusing on lifelong exercise frequency as an index of exercise dose. These included 27 who had performed ≤2 exercise sessions/wk (sedentary), 25 who performed 2–3 sessions/wk (casual), 24 who performed 4–5 sessions/wk (committed) and 25 who performed ≥6 sessions/wk plus regular competitions (Masters athletes) over at least the last 25 yr. Oxygen uptake and hemodynamics [cardiac output, stroke volume (SV)] were collected at rest, two levels of steady-state submaximal exercise, and maximal exercise. Doppler ultrasound measures of LV diastolic filling were assessed at rest and during LV loading (saline infusion) to simulate increased LV filling. Body composition, total blood volume, and heart rate recovery after maximal exercise were also examined.
o2max), a larger left ventricular (LV) mass, and improved heart rate and blood pressure control. However, the effect of lifelong exercise dose on metabolic and hemodynamic response during exercise has not been previously examined. We performed a cross-sectional study on 101 (69 men) seniors (60 yr and older) focusing on lifelong exercise frequency as an index of exercise dose. These included 27 who had performed ≤2 exercise sessions/wk (sedentary), 25 who performed 2–3 sessions/wk (casual), 24 who performed 4–5 sessions/wk (committed) and 25 who performed ≥6 sessions/wk plus regular competitions (Masters athletes) over at least the last 25 yr. Oxygen uptake and hemodynamics [cardiac output, stroke volume (SV)] were collected at rest, two levels of steady-state submaximal exercise, and maximal exercise. Doppler ultrasound measures of LV diastolic filling were assessed at rest and during LV loading (saline infusion) to simulate increased LV filling. Body composition, total blood volume, and heart rate recovery after maximal exercise were also examined.  o2max increased in a dose-dependent manner (P < 0.05). At maximal exercise, cardiac output and SV were largest in committed exercisers and Masters athletes (P < 0.05), while arteriovenous oxygen difference was greater in all trained groups (P < 0.05). At maximal exercise, effective arterial elastance, an index of ventricular-arterial coupling, was lower in committed exercisers and Masters athletes (P < 0.05). Doppler measures of LV filling were not enhanced at any condition, irrespective of lifelong exercise frequency. These data suggest that performing four or more weekly endurance exercise sessions over a lifetime results in significant gains in
o2max increased in a dose-dependent manner (P < 0.05). At maximal exercise, cardiac output and SV were largest in committed exercisers and Masters athletes (P < 0.05), while arteriovenous oxygen difference was greater in all trained groups (P < 0.05). At maximal exercise, effective arterial elastance, an index of ventricular-arterial coupling, was lower in committed exercisers and Masters athletes (P < 0.05). Doppler measures of LV filling were not enhanced at any condition, irrespective of lifelong exercise frequency. These data suggest that performing four or more weekly endurance exercise sessions over a lifetime results in significant gains in  o2max, SV, and heart rate regulation during exercise; however, improved SV regulation during exercise is not coupled with favorable effects on LV filling, even when the heart is fully loaded.
o2max, SV, and heart rate regulation during exercise; however, improved SV regulation during exercise is not coupled with favorable effects on LV filling, even when the heart is fully loaded.
healthy aging is characterized by a progressive decline in maximal oxygen uptake ( o2max) (2, 20), which increases functional disability and risk of all-cause mortality (6). In longitudinal and cross-sectional studies, a smaller maximal cardiac output (
o2max) (2, 20), which increases functional disability and risk of all-cause mortality (6). In longitudinal and cross-sectional studies, a smaller maximal cardiac output (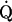 c) and arteriovenous oxygen difference [(a-v)Do2] contribute to the decline in
c) and arteriovenous oxygen difference [(a-v)Do2] contribute to the decline in  o2max with senescence (4, 9, 28, 36).
o2max with senescence (4, 9, 28, 36).
The “dose” of exercise consists of a number of factors, including intensity (how hard), duration (how long), and frequency (how often). A greater frequency of exercise is associated with a greater  o2max (23), a larger left ventricular (LV) mass (23, 56), and improved heart rate and blood pressure regulation (37, 51). To date, no studies have examined the effect of lifelong exercise dose on physical fitness and exercise cardiovascular function. A greater depth of knowledge describing the metabolic and hemodynamic adaptations to different doses of lifelong exercise would be beneficial to determining the optimal exercise dose for minimizing the reduction in physical performance with aging.
o2max (23), a larger left ventricular (LV) mass (23, 56), and improved heart rate and blood pressure regulation (37, 51). To date, no studies have examined the effect of lifelong exercise dose on physical fitness and exercise cardiovascular function. A greater depth of knowledge describing the metabolic and hemodynamic adaptations to different doses of lifelong exercise would be beneficial to determining the optimal exercise dose for minimizing the reduction in physical performance with aging.
The augmentation of stroke volume (SV) with endurance exercise training is primarily attributed to a larger end-diastolic volume (26, 46). In combination with LV compliance, rapid active relaxation and vigorous diastolic suction would enhance end-diastolic volume, while minimizing increases in filling pressure during exercise. However, compelling evidence of enhanced LV filling properties, including active relaxation and diastolic suction, are still lacking in endurance-trained individuals, even in those who perform near daily exercise (3, 8, 11, 31, 41). The apparent absence of a training effect may be related to methodological issues, including small sample size, heterogeneous exercise histories, or, importantly, an inability to comprehensively assess differences in LV loading conditions between trained and untrained participants.
Therefore, the purpose of this study was to examine the relationship between lifelong (>25 yr) exercise dose as tracked by exercise frequency and 1) metabolic and hemodynamic variables at rest and during exercise; 2) dynamic LV diastolic filling parameters at rest and during LV volume loading to simulate increased LV filling during exercise; and 3) postexercise heart rate recovery (HRR). We hypothesized that  o2max, exercise SV, dynamic LV diastolic filling properties, and HRR would be improved in a dose-dependent manner.
o2max, exercise SV, dynamic LV diastolic filling properties, and HRR would be improved in a dose-dependent manner.
METHODS
Participant Recruitment
Participants were recruited primarily from the Cooper Center Longitudinal Study (58), a cohort of over 80,000 individuals in whom physical activity and cardiovascular risk factors have been quantified and followed for greater than 40 yr. The Masters athlete population was enriched through recruitment of top performers at regional and national endurance events. Participants were grouped based on lifelong exercise frequency [exercise session(s)/wk], with exercise being defined as periods of aerobic activity lasting at least 30 min. Sedentary participants exercised no more than once per week; “casual” exercisers engaged in 2–3 sessions per week; “committed” exercisers performed 4–5 sessions per week; and Masters athletes trained 6–7 times per week and participated in regular competitions. All participants were nonsmokers, were not taking any cardiovascular medications, had a 24-h ambulatory blood pressure <140/90 mmHg, body mass index ≤ 30 kg/m2, and a normal ECG and exercise stress echocardiogram. All participants were informed of the purpose and procedures used in the study and gave their written, informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center in Dallas and Texas Health Presbyterian Hospital Dallas.
Measurement of Resting and Exercise Metabolic and Hemodynamic Variables
Oxygen uptake ( o2) (Douglas bag technique), hemodynamics, and blood pressures were determined at the following treadmill conditions: 1) quiet rest, 2) low-intensity (≈30–45% of
o2) (Douglas bag technique), hemodynamics, and blood pressures were determined at the following treadmill conditions: 1) quiet rest, 2) low-intensity (≈30–45% of  o2max) steady-state submaximal exercise, 3) moderate-intensity (≈60–75% of
o2max) steady-state submaximal exercise, 3) moderate-intensity (≈60–75% of  o2max) steady-state submaximal exercise, and 4) maximal exercise. A small number of participants (n = 4) were tested on an upright cycle at the same conditions because of orthopedic concerns or participant request. Gas fractions were analyzed by mass spectrometry and ventilatory volumes by a Tissot spirometer, as previously reported (1).
o2max) steady-state submaximal exercise, and 4) maximal exercise. A small number of participants (n = 4) were tested on an upright cycle at the same conditions because of orthopedic concerns or participant request. Gas fractions were analyzed by mass spectrometry and ventilatory volumes by a Tissot spirometer, as previously reported (1).  o2max was defined as the highest
o2max was defined as the highest  o2 measured from at least a 30-s Douglas bag.
o2 measured from at least a 30-s Douglas bag.  c was measured with the acetylene rebreathing method, which has been previously validated in this laboratory (29). Heart rate was measured via a 12-lead ECG, and SV was calculated as
c was measured with the acetylene rebreathing method, which has been previously validated in this laboratory (29). Heart rate was measured via a 12-lead ECG, and SV was calculated as 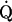 c/heart rate, while (a-v)Do2 was calculated from the Fick equation [(a-v)Do2 =
c/heart rate, while (a-v)Do2 was calculated from the Fick equation [(a-v)Do2 =  o2/
o2/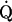 c]. As differences in body size and composition may influence cardiovascular variables (9), SV was scaled relative to body surface area (BSA) (stroke index) and fat-free mass (FFM). Typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility for
c]. As differences in body size and composition may influence cardiovascular variables (9), SV was scaled relative to body surface area (BSA) (stroke index) and fat-free mass (FFM). Typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility for  o2max and maximal
o2max and maximal 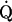 c, SV, and heart rate for 86 participants in the current cohort is 4.4% (2.15 ± 0.64 vs. 2.19 ± 0.64 l/min), 11.1% (15.9 ± 4.6 vs. 15.2 ± 4.8 l/min), 11.9% (99 ± 30 vs. 93 ± 30 ml), and 3.5% (163 ± 12 vs. 161 ± 12 beats/min), respectively.
c, SV, and heart rate for 86 participants in the current cohort is 4.4% (2.15 ± 0.64 vs. 2.19 ± 0.64 l/min), 11.1% (15.9 ± 4.6 vs. 15.2 ± 4.8 l/min), 11.9% (99 ± 30 vs. 93 ± 30 ml), and 3.5% (163 ± 12 vs. 161 ± 12 beats/min), respectively.
Resting and exercise blood pressures were measured on the left arm by ECG gated electrosphygmomanometry (Tango; SunTech Medical). Systemic vascular resistance (SVR) was calculated by the following formula: mean arterial pressure (MAP)/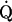 c × 80, where 80 is a conversion factor to dyn·s·cm−5. Effective arterial elastance (Ea), an index of ventricular-arterial coupling, was estimated as ESP/SV, in which ESP represents end-systolic blood pressure, by multiplying brachial systolic blood pressure by 0.9 (12). Ea was scaled relative to BSA, as previously reported (43).
c × 80, where 80 is a conversion factor to dyn·s·cm−5. Effective arterial elastance (Ea), an index of ventricular-arterial coupling, was estimated as ESP/SV, in which ESP represents end-systolic blood pressure, by multiplying brachial systolic blood pressure by 0.9 (12). Ea was scaled relative to BSA, as previously reported (43).
Measurement of HRR after Maximal Exercise
Immediately after the cessation of maximal exercise, participants recovered in an upright seated position. Heart rate was continuously recorded, and HRR was analyzed at 1-min periods from 1–5 min of recovery. HRR is presented as beats per minute and R-R interval, which was calculated from the following formula: 60/heart rate × 1,000.
Measurement of Body Composition
Body density and composition were determined by underwater weighing with correction for residual lung volume (59). Each subject performed at least three adequate measurements defined as a definite plateau in underwater weight, and the mean value was calculated.
Assessment of Dynamic LV Diastolic Filling
LV function was assessed using previously described techniques (1, 41). Briefly, a 6 Fr balloon-tipped fluid-filled catheter (Edwards Lifesciences) was placed using fluoroscopic guidance to measure pulmonary capillary wedge pressure (PCWP). PCWP was determined from three measurements obtained at end expiration, as previously described (1, 41). PCWP, 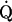 c, and Doppler measures of dynamic LV diastolic filling measurements were obtained after at least 20 min of supine rest. LV filling was increased through a rapid infusion of warm (37°C) isotonic saline solution at 200 ml/min via an antecubital intravenous line to achieve total volume infusion of 10–15 ml/kg (NS15). In this cohort, there was a positive association between SV at NS15 and during moderate intensity submaximal exercise (R = 0.69, P < 0.05) and maximal exercise (R = 0.58, P < 0.05) using the acetylene rebreathing method.
c, and Doppler measures of dynamic LV diastolic filling measurements were obtained after at least 20 min of supine rest. LV filling was increased through a rapid infusion of warm (37°C) isotonic saline solution at 200 ml/min via an antecubital intravenous line to achieve total volume infusion of 10–15 ml/kg (NS15). In this cohort, there was a positive association between SV at NS15 and during moderate intensity submaximal exercise (R = 0.69, P < 0.05) and maximal exercise (R = 0.58, P < 0.05) using the acetylene rebreathing method.
Echocardiographic images were digitally acquired using an iE33 (Philips,) and ATL HDI5000 (Advanced Technology Laboratories) and were measured offline in Xcelera cardiovascular image management system (Philips). LV end-diastolic volume was determined using a modified Simpson's method, as previously described (1), and then scaled relative to BSA (left ventricular end-diastolic volume index).
Mitral inflow.
A 2-mm sample volume placed at the tips of the mitral valve leaflets to determine peak early and late mitral inflow velocities, and the corresponding early-to-late ratio was calculated.
Tissue Doppler imaging.
In the apical four-chamber view, a 2-mm sample volume was placed at the septal and lateral side of the mitral annulus. A mean value of septal and lateral values of the peak early (mean Em) mitral annular velocity was calculated (41).
Propagation velocity of early mitral inflow.
A color M-mode image of LV inflow was obtained with the sampling area positioned to extend from mid-atrium to the apex, directly through the mitral valve orifice. The scale was reduced to produce a clear aliasing within the early portion of the mitral inflow. The slope of first aliasing velocity from the mitral plane to 4 cm into the ventricle was used to measure propagation velocity of early mitral inflow. Using a five-chamber apical view with a 4-mm sample volume, the interval between aortic valve closing and mitral valve opening [isovolumic relaxation time (IVRT)] was determined.
Measurement of Total Blood Volume
Total blood volume (TBV) was measured using a carbon monoxide rebreathing method modified from that described by Burge and Skinner (7) and has been described in detail elsewhere (25). Typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility for hemoglobin mass, which is used as a marker of carbon monoxide distribution, is ≈3% for repeated measures in our laboratory (25).
Statistical Analysis
One-factor ANOVA and repeated-measures ANOVAs were used to determine the effect of lifelong exercise frequency on subject characteristics, exercise metabolic and hemodynamics variables, Doppler measures of LV dynamic diastolic filling, and HRR. Tukey adjustments were used to control for multiple comparisons. Nonparametric data were analyzed via Kruskal-Wallis ANOVA on ranks. Pearson's correlations were used to establish the relationship between TBV and  o2max. All statistical analysis was performed using SigmaStat (Systat Software). P < 0.05 was considered statistically significant. Data are presented as means ± SD in Tables 1–4 and means ± SE in Figs. 1–4.
o2max. All statistical analysis was performed using SigmaStat (Systat Software). P < 0.05 was considered statistically significant. Data are presented as means ± SD in Tables 1–4 and means ± SE in Figs. 1–4.
Table 1.
Participant characteristics
| Sedentary | Casual | Committed | Masters Athletes | ANOVA P Value | |
|---|---|---|---|---|---|
| n | 27 | 25 | 24 | 25 | |
| No. men (%) | 15 (56) | 18 (72) | 19 (79) | 17 (68) | |
| Age, yr | 69 ± 5 | 71 ± 6 | 69 ± 6 | 68 ± 3 | 0.201 |
| Weight, kg | 75 ± 11 | 76 ± 14 | 73 ± 11 | 66 ± 12*† | 0.015 |
| Height, cm | 169 ± 10 | 174 ± 10 | 173 ± 8 | 171 ± 10 | 0.336 |
| BSA, m2 | 1.87 ± 0.19 | 1.91 ± 0.23 | 1.88 ± 0.18 | 1.76 ± 0.21 | 0.058 |
| Body fat, % | 32 ± 8 | 30 ± 7 | 29 ± 5 | 22 ± 7*† | <0.001 |
| FFM, kg | 51 ± 9 | 53 ± 10 | 52 ± 9 | 51 ± 8 | 0.699 |
| HgB concentration, g/dl | 14 ± 1 | 13 ± 1 | 14 ± 1 | 14 ± 1 | 0.153 |
| TBV, ml/kg | 66 ± 11 | 69 ± 8 | 73 ± 8* | 83 ± 8*† | <0.001 |
| LVMI g/m2 | 50 ± 8 | 52 ± 8 | 62 ± 11*† | 67 ± 12*† | <0.001 |
Values are means ± SD; n. no. of subjects.
BSA, body surface area; FFM, fat-free mass; HgB, hemoglobin; TBV, total blood volume; LVMI, left ventricular mass index.
Table 4.
Left ventricular volumes, Doppler parameters, and PCWP at supine rest and saline volume infusion (NS15)
| Sedentary | Casual | Committed | Masters Athletes | |
|---|---|---|---|---|
| Supine rest | ||||
| Heart rate, beats/min | 72 ± 12 | 65 ± 8* | 63 ± 9* | 60 ± 7* |
| E/A ratio | 0.97 ± 0.24 | 1.02 ± 0.20 | 1.14 ± 0.32 | 1.10 ± 0.24 |
| Mean Em, cm/s | 9.2 ± 1.8 | 9.0 ± 1.4 | 9.4 ± 1.3 | 10.4 ± 2.2† |
| IVRT, ms | 122 ± 27 | 108 ± 17 | 117 ± 25 | 126 ± 27† |
| VP, cm/s | 43 ± 8 | 44 ± 10 | 45 ± 9 | 38 ± 6 |
| PCWP, mmHg | 8.7 ± 2.3 | 8.5 ± 1.6 | 8.4 ± 1.5 | 8.3 ± 1.6 |
| LVEDVI, ml/m2 | 55 ± 12 | 59 ± 12 | 59 ± 12 | 75 ± 14*†‡ |
| NS15 | ||||
| Heart rate, beats/min | 80 ± 11 | 74 ± 10 | 68 ± 10* | 64 ± 7*† |
| E/A ratio | 1.28 ± 0.31 | 1.17 ± 0.26 | 1.36 ± 0.38 | 1.27 ± 0.32 |
| Mean Em, cm/s | 10.1 ± 1.9 | 9.4 ± 1.6 | 10.2 ± 1.4 | 11.1 ± 2.2† |
| IVRT, ms | 97 ± 23 | 90 ± 16 | 95 ± 18 | 108 ± 25† |
| VP, cm/s | 55 ± 14 | 60 ± 15 | 55 ± 12 | 48 ± 11† |
| PCWP, mmHg | 15.2 ± 1.8 | 15.2 ± 2.8 | 14.7 ± 2.5 | 13.4 ± 2.3*† |
| LVEDVI, ml/m2 | 59 ± 12 | 62 ± 12 | 62 ± 12 | 80 ± 18*†‡ |
Values are means ±SD.
E/A ratio, the ratio of peak early-to-late mitral inflow velocities; Mean Em, mean peak early mitral annular velocity; IVRT, isovolumic relaxation time; Vp, propagation velocity; PCWP, pulmonary capillary wedge pressure; LVEDVI, left ventricular end-diastolic volume index.
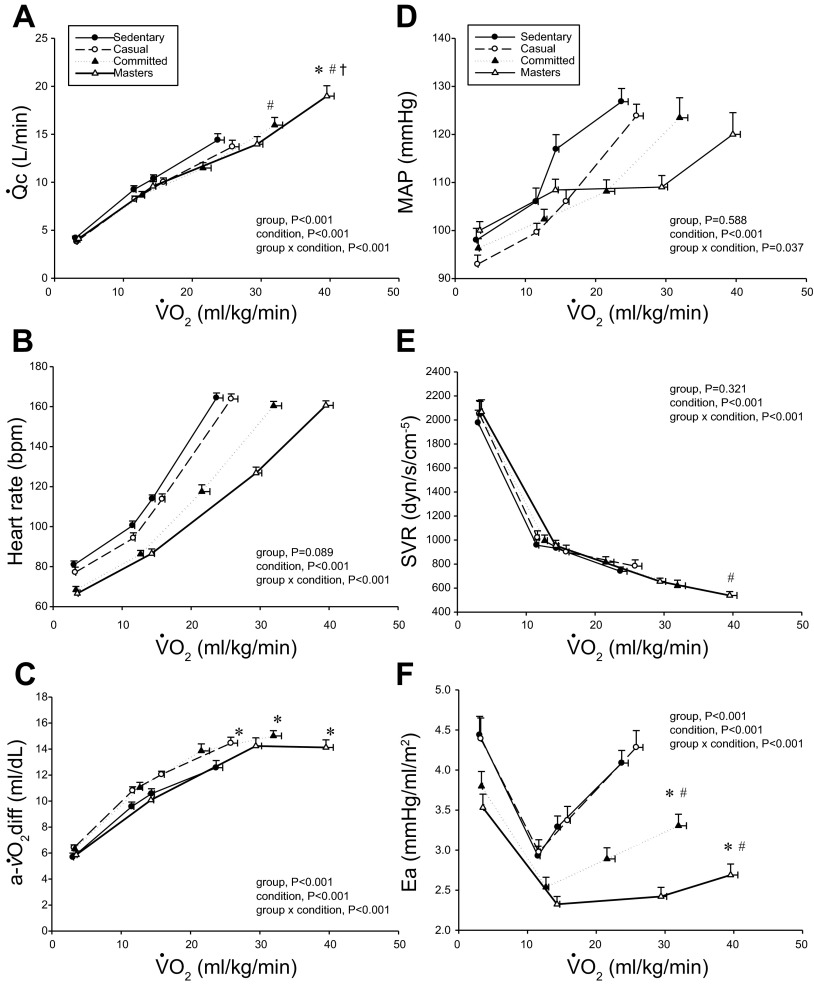
Cardiac output ( c; A), heart rate (B), arteriovenous oxygen difference [a-
c; A), heart rate (B), arteriovenous oxygen difference [a- O2diff; (a-v)Do2; C], mean arterial pressure (MAP; D), systemic vascular resistance (SVR; E), and effective arterial elastance (Ea; F) as a function of oxygen uptake (
O2diff; (a-v)Do2; C], mean arterial pressure (MAP; D), systemic vascular resistance (SVR; E), and effective arterial elastance (Ea; F) as a function of oxygen uptake ( o2) scaled relative to total body mass. Values are means ± SE. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
o2) scaled relative to total body mass. Values are means ± SE. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
RESULTS
Participants Characteristics
The LV pressure-volume curves and MRI measurements of cardiac morphology in this cohort have been reported (4a). The Masters athletes were lighter, had a smaller BSA, and were leaner, but were similar in terms of FFM (Table 1). TBV scaled relative to total body mass (ml/kg) and LV mass index were larger in Masters athletes and committed exercisers compared with sedentary exercisers (P < 0.05) (Table 1).
Resting Metabolic and Hemodynamics Variables
Resting metabolic and hemodynamic variables are presented in Table 2. Stroke index was larger, while heart rate and Ea were lower in the Masters athletes compared with sedentary and casual exercisers (P < 0.05). MAP, SVR, and (a-v)Do2 were not different among groups.
Table 2.
Resting parameters
| Sedentary | Casual | Committed | Masters Athletes | ANOVA P Value | |
|---|---|---|---|---|---|
 o2 o2 | |||||
    ml/min ml/min | 229 ± 56 | 214 ± 48 | 244 ± 63 | 230 ± 48 | 0.726 |
    ml·kg−1·min−1 ml·kg−1·min−1 | 3.0 ± 0.5 | 3.2 ± 0.4 | 3.3 ± 0.7 | 3.5 ± 0.7* | 0.037 |
    ml·kg FFM−1·min−1 ml·kg FFM−1·min−1 | 4.5 ± 0.8 | 4.6 ± 0.6 | 4.7 ± 1.0 | 4.6 ± 0.8 | 0.920 |
 c, l/min c, l/min | 4.2 ± 1.0 | 3.8 ± 0.8 | 3.9 ± 0.9 | 4.1 ± 1.2 | 0.619 |
| QI, l·m−2·min−1 | 2.2 ± 0.5 | 2.0 ± 0.3 | 2.1 ± 0.4 | 2.3 ± 0.5 | 0.130 |
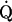 c, ml·kg FFM−1·min−1 c, ml·kg FFM−1·min−1 | 82.6 ± 15.0 | 72.8 ± 14.5 | 75.3 ± 14.6 | 81.4 ± 17.1 | 0.070 |
| Heart rate, beats/min | 81 ± 10 | 77 ± 13 | 68 ± 9*† | 67 ± 9*† | <0.001 |
| SV, ml | 53 ± 13 | 51 ± 16 | 59 ± 18 | 63 ± 19 | 0.056 |
| SI, ml/m2 | 28 ± 7 | 27 ± 7 | 31 ± 8 | 35 ± 9*† | 0.002 |
| SV, ml/kg FFM | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.3† | 0.007 |
| MAP, mmHg | 98 ± 13 | 93 ± 10 | 96 ± 10 | 100 ± 9 | 0.115 |
| Ea, mmHg·ml−1·m−2 | 4.4 ± 1.2 | 4.4 ± 1.3 | 3.8 ± 0.9 | 3.5 ± 0.8*† | 0.009 |
| SVR, dyn·s·cm−5 | 1,976 ± 547 | 2,051 ± 557 | 2,053 ± 505 | 2,067 ± 504 | 0.586 |
| (a-v)Do2, ml/dl | 6 ± 2 | 6 ± 1 | 6 ± 2 | 6 ± 1 | 0.206 |
Values are means ± SD.
 o2, oxygen uptake;
o2, oxygen uptake; 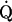 c, cardiac output; QI, cardiac index; SV, stroke volume; SI, stroke index; MAP, mean arterial pressure; Ea, effective arterial elastance index; SVR, systemic vascular resistance; (a-v)Do2, arteriovenous oxygen difference.
c, cardiac output; QI, cardiac index; SV, stroke volume; SI, stroke index; MAP, mean arterial pressure; Ea, effective arterial elastance index; SVR, systemic vascular resistance; (a-v)Do2, arteriovenous oxygen difference.
Steady-State Submaximal and Maximal Exercise
Maximal exercise metabolic variables are presented in Table 3. Irrespective of unit,  o2max was greater in committed exercisers and Master athletes compared with sedentary and casual exercisers (P < 0.05).
o2max was greater in committed exercisers and Master athletes compared with sedentary and casual exercisers (P < 0.05).
Table 3.
Maximal exercise parameters
| Sedentary | Casual | Committed | Masters Athletes | ANOVA P Value | |
|---|---|---|---|---|---|
 o2max o2max | |||||
    l/min l/min | 1.8 ± 0.5 | 2.0 ± 0.6 | 2.4 ± 0.6* | 2.6 ± 0.5*† | <0.001 |
    ml·kg−1·min−1 ml·kg−1·min−1 | 24 ± 5 | 26 ± 5 | 32 ± 6*† | 40 ± 5*†‡ | <0.001 |
    ml·kg FFM−1·min−1 ml·kg FFM−1·min−1 | 35 ± 7 | 37 ± 6 | 46 ± 6*† | 51 ± 7*† | <0.001 |
| Maximal heart rate, beats/min | 164 ± 13 | 164 ± 12 | 160 ± 11 | 161 ± 11 | 0.522 |
| RER | 1.21 ± 0.11 | 1.19 ± 0.06 | 1.17 ± 0.06 | 1.19 ± 0.08 | 0.564 |
Values are means ± SD.
 o2max, maximal oxygen uptake; RER, respiratory exchange ratio.
o2max, maximal oxygen uptake; RER, respiratory exchange ratio.
Figures 1 and and22 depict the relationship between  o2 expressed relative to total body mass (ml·kg−1·min−1) and as a percentage of
o2 expressed relative to total body mass (ml·kg−1·min−1) and as a percentage of  o2max (%
o2max (% o2max) (Fig. 3) vs.
o2max) (Fig. 3) vs. 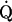 c, SV, and (a-v)Do2 at rest, both levels of submaximal exercise, and maximal exercise. Plots of
c, SV, and (a-v)Do2 at rest, both levels of submaximal exercise, and maximal exercise. Plots of 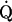 c vs.
c vs.  o2 were basically superimposable among the groups, with committed exercisers and Masters athletes achieving a greater maximal
o2 were basically superimposable among the groups, with committed exercisers and Masters athletes achieving a greater maximal 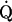 c compared with sedentary and casual exercise groups (Figs. 1A and and3A).3A). Heart rate response during submaximal exercise was lower in committed exercisers and Masters athletes relative to any
c compared with sedentary and casual exercise groups (Figs. 1A and and3A).3A). Heart rate response during submaximal exercise was lower in committed exercisers and Masters athletes relative to any  o2 expressed relative to total body mass (rightward shift) (Fig. 1B), but the response was not different among groups when depicted as a percentage of
o2 expressed relative to total body mass (rightward shift) (Fig. 1B), but the response was not different among groups when depicted as a percentage of  o2max (Fig. 3B). Absolute and scaled SV were larger (upward shift) in committed exercisers and Masters athletes during submaximal and maximal exercise, while the casual exercisers mirrored the sedentary group (Figs. 2, ,AA–C). While the response of MAP during exercise varied among the groups, MAP was not different at any condition (Fig. 1D).
o2max (Fig. 3B). Absolute and scaled SV were larger (upward shift) in committed exercisers and Masters athletes during submaximal and maximal exercise, while the casual exercisers mirrored the sedentary group (Figs. 2, ,AA–C). While the response of MAP during exercise varied among the groups, MAP was not different at any condition (Fig. 1D).
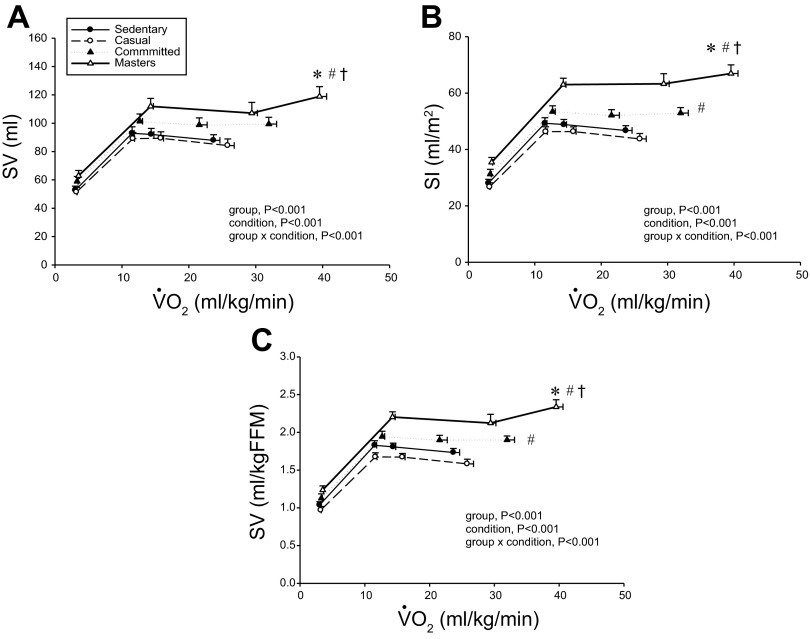
Absolute stroke volume (SV; A), stroke index (SI; B), and SV scaled relative to fat-free mass (FFM; C) as a function of  o2 scaled relative to total body mass. Values are means ± SE. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
o2 scaled relative to total body mass. Values are means ± SE. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
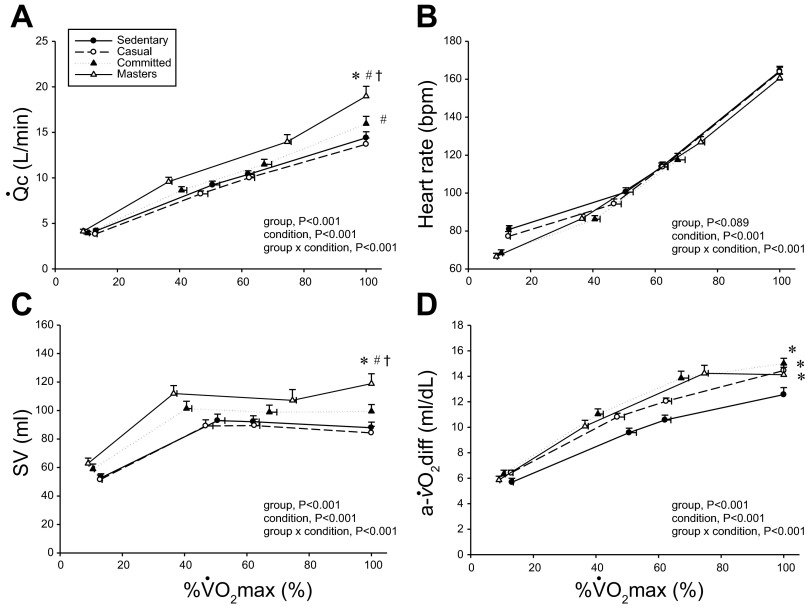
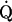 c (A), heart rate (B), absolute SV (C), and (a-v)Do2 (D) as a function of
c (A), heart rate (B), absolute SV (C), and (a-v)Do2 (D) as a function of  o2 as a percentage of maximum (%max;
o2 as a percentage of maximum (%max;  o2max). Values are means ± SE. bpm, Beats/min. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
o2max). Values are means ± SE. bpm, Beats/min. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
Plots of (a-v)Do2 vs.  o2 scaled to total body mass and as a percentage of
o2 scaled to total body mass and as a percentage of  o2max show that systemic oxygen extraction was greater (upward shift) in all trained groups compared with the sedentary group during moderate-intensity submaximal and maximal exercise (P < 0.05) (Figs. 1C and and3D).3D). Masters athletes had a lower SVR during moderate-intensity submaximal exercise (P < 0.05 vs. sedentary and casual) and at maximal exercise (P < 0.05 vs. casual exercisers) (Fig. 1E). Ea was lower in Masters athletes compared with the sedentary and casual groups during submaximal exercise (downward shift) and was lower in committed exercisers and Masters athletes compared with the other two groups at maximal exercise (P < 0.05) (Fig. 1F).
o2max show that systemic oxygen extraction was greater (upward shift) in all trained groups compared with the sedentary group during moderate-intensity submaximal and maximal exercise (P < 0.05) (Figs. 1C and and3D).3D). Masters athletes had a lower SVR during moderate-intensity submaximal exercise (P < 0.05 vs. sedentary and casual) and at maximal exercise (P < 0.05 vs. casual exercisers) (Fig. 1E). Ea was lower in Masters athletes compared with the sedentary and casual groups during submaximal exercise (downward shift) and was lower in committed exercisers and Masters athletes compared with the other two groups at maximal exercise (P < 0.05) (Fig. 1F).
HRR after Maximal Exercise
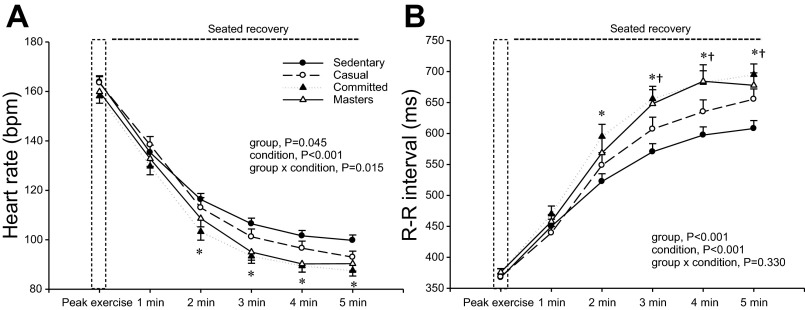
As shown in Fig. 4, ,AA and andB,B, heart rate was lower, while R-R interval was longer at 3–5 min of recovery in the committed exercisers and Masters athletes compared with sedentary subjects. The relative recovery of heart rate from maximal heart rate was only longer after 2 min of recovery in committed exercisers and Masters athletes when HRR was expressed as an R-R interval (P < 0.05 vs. sedentary).
LV Volumes, Filling Pressure, and Doppler Measures of LV Filling and Active Relaxation at Rest and During Volume Infusion
Doppler indexes of LV diastolic filling at supine rest and during LV loading are shown in Table 4. Left ventricular end-diastolic volume index was larger in Master athletes at supine rest and during saline infusion (P < 0.05). Except for a faster mean Em and a slower IVRT in the Masters athletes compared with casual exercisers (P < 0.05), there were no differences in Doppler measures at rest among the groups. These differences in mean Em and IVRT between casual exercisers and Masters athletes persisted during LV volume loading. Propagation velocity of early mitral inflow was slower in Masters athletes compared with the sedentary group (P < 0.05), which in part likely reflects a lower PCWP (P < 0.05 vs. sedentary) in this group at the same condition.
Blood Volume and  o2max
o2max
As depicted in Fig. 5A, there was a positive relationship between absolute levels of TBV and  o2max in all participants (R = 0.77, P < 0.001). Plots (Fig. 5, ,BB and andC)C) show that the direction of this relationship remained intact when TBV and
o2max in all participants (R = 0.77, P < 0.001). Plots (Fig. 5, ,BB and andC)C) show that the direction of this relationship remained intact when TBV and  o2max were scaled relative to total body mass (R = 0.69, P < 0.001) or FFM (R = 0.42, P < 0.001).
o2max were scaled relative to total body mass (R = 0.69, P < 0.001) or FFM (R = 0.42, P < 0.001).
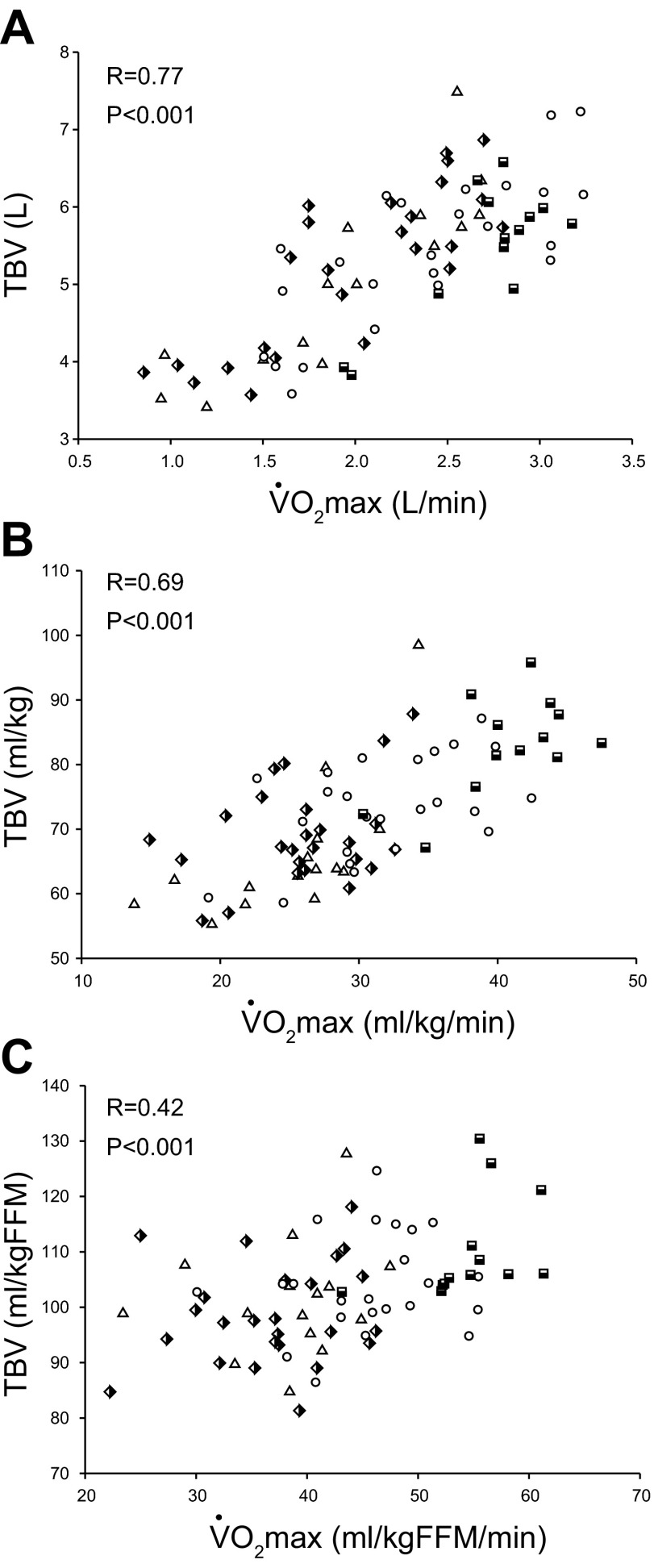
Absolute (A) and total blood volume (TBV) scaled relative to total body mass (B) and FFM (C) as a function of absolute and scaled  o2max. Triangles [sedentary, 15 subjects (9 men)]; semi-filled diamonds [casual exercisers, 25 subjects (18 men)]; circles [committed exercisers, 24 subjects (19 men)]; semi-filled squares [Masters athletes, 13 subjects (11 men)].
o2max. Triangles [sedentary, 15 subjects (9 men)]; semi-filled diamonds [casual exercisers, 25 subjects (18 men)]; circles [committed exercisers, 24 subjects (19 men)]; semi-filled squares [Masters athletes, 13 subjects (11 men)].
DISCUSSION
The primary novel finding from this study is that four or more weekly endurance exercise sessions for at least 25 yr resulted in enhanced O2 transport and utilization during maximal exercise compared with lower frequencies of lifelong exercise. Higher frequencies of lifelong endurance exercise also resulted in favorable effects on SV regulation (ventricular-arterial coupling) and heart rate control during and after exercise. By collecting measures of LV mass, TBV, and Doppler measures of LV filling at rest and during saline infusion, the findings of this present study indicate that a larger SV during exercise is coupled with cardiac growth and TBV expansion, but not necessarily with beneficial effects on LV filling properties, even when the heart is fully loaded. Thus the current findings suggest that four or more weekly exercise sessions over a lifetime appears to be an effective strategy to preserving cardiovascular structure and function during exercise in seniors.
The Effect of Lifelong Exercise Frequency on  o2max, Exercise Hemodynamics, and (a-v)Do2
o2max, Exercise Hemodynamics, and (a-v)Do2
An important observation of this study was a strong relationship between  o2max and lifelong exercise frequency, which is consistent with previous findings in men only (23). We extend these finding by showing that: 1) a low frequency of lifelong exercise (2–3 sessions/wk) exerts a modest effect on
o2max and lifelong exercise frequency, which is consistent with previous findings in men only (23). We extend these finding by showing that: 1) a low frequency of lifelong exercise (2–3 sessions/wk) exerts a modest effect on  o2max expressed relative to total body mass (≈8%), and 2) the addition of one to two exercise sessions above this level (4–5 sessions/wk) is sufficient stimulus to limit the reduction in
o2max expressed relative to total body mass (≈8%), and 2) the addition of one to two exercise sessions above this level (4–5 sessions/wk) is sufficient stimulus to limit the reduction in  o2max with aging. Our present findings complement a recent study in elite octogenarian athletes, who had performed at least 4 sessions/wk over 50 yr, resulting in an 80% greater
o2max with aging. Our present findings complement a recent study in elite octogenarian athletes, who had performed at least 4 sessions/wk over 50 yr, resulting in an 80% greater  o2max (38 ± 1 vs. 21 ± 1 ml·kg−1·min−1) compared with age- and sex-matched controls (57) and are comparable to values observed in healthy untrained adults 40–50 yr younger (10).
o2max (38 ± 1 vs. 21 ± 1 ml·kg−1·min−1) compared with age- and sex-matched controls (57) and are comparable to values observed in healthy untrained adults 40–50 yr younger (10).
Consistent with previous cross-sectional studies in men and women, the present study demonstrated that a high level of lifelong endurance exercise training is associated with a larger  c and SV during submaximal and maximal exercise (26, 33, 36, 46). However, the effect of endurance training on maximal SV when begun later in life (>60 yr) is less clear, with several (17, 22, 52, 53), but not all (4, 18), studies reporting improvements. The reason for the discrepancy in study findings is unclear, but may be due to methodological differences, including measurement techniques, the mode of training, the length and intensity of the training stimulus, and the overall health of study participants. Given this uncertainty, further research is needed to establish the role of exercise dose, including intensity, frequency, and duration on maximal SV when exercise training is started later in life.
c and SV during submaximal and maximal exercise (26, 33, 36, 46). However, the effect of endurance training on maximal SV when begun later in life (>60 yr) is less clear, with several (17, 22, 52, 53), but not all (4, 18), studies reporting improvements. The reason for the discrepancy in study findings is unclear, but may be due to methodological differences, including measurement techniques, the mode of training, the length and intensity of the training stimulus, and the overall health of study participants. Given this uncertainty, further research is needed to establish the role of exercise dose, including intensity, frequency, and duration on maximal SV when exercise training is started later in life.
Irrespective of age or sex, increases in exercise SV with endurance-type training are primarily attributed to a larger end-diastolic volume, resulting from a combination of LV remodeling with increased compliance, hypervolemia, and potentially faster diastolic filling rates (1, 24, 26). In this present study, a greater lifelong exercise frequency was associated with a larger TBV and LV mass. A similar observation for LV mass was reported in men aged 18–77 yr (23), suggesting that LV growth and hypervolemia are influenced by frequency of endurance exercise training.
Coupled with enhanced LV compliance (1, 4a, 30), rapid LV active relaxation and vigorous diastolic suction would maximize LV end-diastolic volume, while attenuating increases in filling pressure in highly trained individuals (55). Previous work, including from our laboratory, has examined the effect of lifelong exercise training on early diastolic filling properties, including active relaxation and diastolic suction at rest and during exercise in seniors, with the majority reporting only modest improvements (3, 8, 31, 40, 41). Similar finding have been reported in young athletes compared with age-matched controls (3, 8), suggesting that any potential training-related improvements in echocardiographic parameters are not diminished with age. The apparent lack of a training effect on Doppler measures of LV filling at any age may reflect small sample sizes, large variations in subject's exercise histories, and notably an inability to comprehensively assess differences in LV loading conditions between trained and untrained individuals. The latter factor is particularly relevant issue given the load-dependency of Doppler measures of dynamic LV diastolic filling (19).
One previous study from our laboratory (41) collected invasive measures of LV filling conditions and reported minimal improvements in dynamic LV filling parameters in Masters athletes compared with sedentary controls. This present study in a much larger cohort shows that resting noninvasive measures of LV relaxation and suction are not improved at any frequency of lifelong training. Collectively, these findings seem to suggest that training-related improvements in SV during exercise are not coupled with significant enhancements in LV relaxation and diastolic suction at rest or during LV volume loading, a model analogous to increased preload during exercise. However, a key limitation to our findings includes that Doppler measures were collected while the subjects were supine, which may not reflect 1) hemodynamics and LV filling response that occur during upright exercise (39), or 2) changes in LV contractility during exercise, which would facilitate the ventricle contracting below its equilibrium volume engaging diastolic suction (35). Supporting this contention is previous work in our laboratory that showed that early diastolic intraventricular pressure gradients, an index of diastolic suction, increased progressively with incremental exercise workloads in Masters athletes, but only initially increased with low-intensity exercise in healthy, age-matched controls (40). Therefore, future studies should assess intraventricular pressure gradients and ventricular twist mechanics during exercise in a large cohort of senior athletes and controls to clearly determine the effect of lifelong exercise training on LV diastolic filling.
Early studies in small cohorts (21, 45) reported that measures of systolic function (ejection fraction, end-systolic volume) are improved during exercise in trained seniors, although this is not a consistent finding (46). A reduction in aortic impedance via a reduction in aortic stiffness (51) and SVR, improved ventricular-arterial coupling (22, 50), and potentially enhanced cardiac β-adrenergic sensitivity (54) would effectively augment LV emptying during exercise in trained seniors. Unfortunately, systolic volumes were not collected in this present study; thus we can only speculate on the effect of systolic function on SV regulation in our trained participants. However, Ea, an index that characterizes the total arterial load imposed on the LV, was lower during submaximal and maximal exercise in committed exercisers and Masters athletes, pointing toward improved arterial compliance and ventricular-arterial coupling in trained seniors, which is consistent with a previous observation in young trained men (38) and after 1 yr of training in previously untrained seniors (22). Therefore, these results highlight that improved ventricular-arterial coupling is a notable training-related adaptation for enhancing SV regulation during exercise in seniors.
We also found that maximal (a-v)Do2 was significantly greater in trained seniors, which has been reported in a prior study (36). Interestingly, we observed that even a low frequency of lifelong exercise (2–3 sessions/wk) improved oxygen extraction by metabolically active tissue during maximal exercise. These findings are important, as maximal (a-v)Do2 is reported to decline with aging (4, 9, 36), which probably in part reflects physical deconditioning in addition to the aging process itself, as maximal (a-v)Do2 is normalized in seniors with several months of exercise training (4, 53). Several mechanisms appear to underlie this improved oxygen extraction in trained seniors, including the preservation of FFM, increased number of mitochondria and enzymes activity (14), increased vasodilator response (44), and enhanced blood flow redistribution to exercising muscle mass (4).
The Effect of Lifelong Exercise Frequency on Heart Rate Control During and Recovery From Exercise
An important observation in this present study is that the relationship between  o2 relative to maximum effort (i.e., at the same relative workload) and heart rate was superimposable between our groups during exercise (Fig. 3B). Our results suggest that heart rate is precisely regulated in response to changes in SV during exercise to maintain the linear relationship between
o2 relative to maximum effort (i.e., at the same relative workload) and heart rate was superimposable between our groups during exercise (Fig. 3B). Our results suggest that heart rate is precisely regulated in response to changes in SV during exercise to maintain the linear relationship between  c and metabolic demand, as depicted in Fig. 1A. The current findings and previous data (36, 42) demonstrate that the slope of the relationship between blood flow and metabolic demand during exercise is not influenced by age, sex, or fitness in healthy individuals. In some conditions, such as postural orthostatic tachycardia syndrome, a compensatory heart rate response is demonstrated due to a smaller SV to “normalize” the slope relationship between
c and metabolic demand, as depicted in Fig. 1A. The current findings and previous data (36, 42) demonstrate that the slope of the relationship between blood flow and metabolic demand during exercise is not influenced by age, sex, or fitness in healthy individuals. In some conditions, such as postural orthostatic tachycardia syndrome, a compensatory heart rate response is demonstrated due to a smaller SV to “normalize” the slope relationship between  c and O2 delivery during exercise (49); however, this compensation may not be sufficient to “normalize” this slope in populations with metabolic disease or heart failure, with or without preserved ejection fraction (5, 13, 32).
c and O2 delivery during exercise (49); however, this compensation may not be sufficient to “normalize” this slope in populations with metabolic disease or heart failure, with or without preserved ejection fraction (5, 13, 32).
In contrast to the regulation of HR during exercise, HRR immediately after the cessation of exercise is exclusively due to restoration of vagal tone, while longer recovery durations reflect the balance between vagal tone restoration and sympathetic tone withdrawal (16). Because of its clear association with vagal activity, HRR may be used as a measure of “global cardiovascular health” in healthy individuals and patients with cardiovascular disease (15, 48). We observed that ≈97% of our participants achieved a HRR of >12 beats at 1 min (15) and >22 beats at 2 min (48), indicators of an appropriate HRR after maximal exercise.
Heart rate was slower, while R-R interval was longer at 3–5 min of recovery in highly trained seniors (Fig. 5, ,AA and B). Cross-sectional (15, 48) and longitudinal training studies (49) have reported that a greater fitness level is associated with either a slower heart rate or a faster relative decline in heart rate at recovery periods ranging from 30 s to 5 min. Studies (37, 51) from our laboratory have reported that weekly exercise frequency influences heart rate and blood pressure regulation in younger adults and seniors over a 1-yr training period. Of particular note, improvements were noted after 3–6 mo of training, suggesting that exercise training-related improvements in autonomic function may be observed after a relatively short period of training. We were, therefore, surprised that we did not observe a more marked decay in the early phase (1 min) of HRR, a period highly influenced by vagal reactivation, in our trained seniors. It is possible that the beneficial effects of lifelong exercise frequency on heart rate “recovery” are more evident before our earliest postexercise assessment, or, more likely, that a “normal” HRR before 2 min is more difficult to improve at any dose of lifelong exercise training.
Study Limitations
First, factors other than those measured in this study (genetics, lifelong physical activity levels) may influence  o2max and central hemodynamics; therefore, we cannot exclude the possibility that the improved metabolic and hemodynamic responses in our trained subjects may be related in part to factors other than exercise training. Second, this present study used a simple approach to allocate participants into lifelong exercise groups. The usage of such an approach limits any conclusions based on other components of exercise training program, including intensity and duration or mode, all of which may have an important impact on the metabolic and hemodynamic response during exercise. Third, our trained cohort was predominantly men, because we could only find a limited number of women whose lifelong exercise volume met our criteria. Further studies in a larger cohort of women are required to confirm these present results, or alternatively to elucidate whether sex may influence exercise function in response to different frequencies of lifelong exercise. Finally, our senior subjects were nonobese and normotensive and were screened for occult cardiovascular disease; therefore, it is unclear whether the present findings are applicable to the larger population.
o2max and central hemodynamics; therefore, we cannot exclude the possibility that the improved metabolic and hemodynamic responses in our trained subjects may be related in part to factors other than exercise training. Second, this present study used a simple approach to allocate participants into lifelong exercise groups. The usage of such an approach limits any conclusions based on other components of exercise training program, including intensity and duration or mode, all of which may have an important impact on the metabolic and hemodynamic response during exercise. Third, our trained cohort was predominantly men, because we could only find a limited number of women whose lifelong exercise volume met our criteria. Further studies in a larger cohort of women are required to confirm these present results, or alternatively to elucidate whether sex may influence exercise function in response to different frequencies of lifelong exercise. Finally, our senior subjects were nonobese and normotensive and were screened for occult cardiovascular disease; therefore, it is unclear whether the present findings are applicable to the larger population.
Perspectives
One of the debilitating effects of aging is a progressive reduction in functional capacity as assessed by  o2max. While it has been clearly established that vigorous lifelong exercise training minimizes the reduction in physical function and exercise function in seniors, there is very limited information on the optimal “dose” of lifetime exercise to obtain such benefits. This current study examined one component of the overall exercise “dose” and demonstrated that performing four or more endurance exercise sessions weekly over a lifetime is associated with a greater
o2max. While it has been clearly established that vigorous lifelong exercise training minimizes the reduction in physical function and exercise function in seniors, there is very limited information on the optimal “dose” of lifetime exercise to obtain such benefits. This current study examined one component of the overall exercise “dose” and demonstrated that performing four or more endurance exercise sessions weekly over a lifetime is associated with a greater  o2max, oxygen delivery and extraction, and improved ventricular-arterial coupling and heart rate control during exercise. It is important to note that, while the largest values of
o2max, oxygen delivery and extraction, and improved ventricular-arterial coupling and heart rate control during exercise. It is important to note that, while the largest values of  o2max and exercise function were observed in Master athletes, their level of training and competition are likely beyond the feasibility of most individuals. Instead, seniors who had consistently performed 4–5 exercise sessions/wk, and therefore likely reflect the current recommendations for weekly physical activity (≈150 min/wk) (34), still exhibited significant improvements in
o2max and exercise function were observed in Master athletes, their level of training and competition are likely beyond the feasibility of most individuals. Instead, seniors who had consistently performed 4–5 exercise sessions/wk, and therefore likely reflect the current recommendations for weekly physical activity (≈150 min/wk) (34), still exhibited significant improvements in  o2max, exercise function, and cardiovascular control variables compared with healthy controls and, therefore, should be viewed as the minimum frequency of lifelong exercise to attenuate the loss of functional capacity and exercise function with aging. Our results showed that aging effects on resting Doppler measures of LV relaxation and diastolic suction are not prevented even when subjected to many decades of stimulus.
o2max, exercise function, and cardiovascular control variables compared with healthy controls and, therefore, should be viewed as the minimum frequency of lifelong exercise to attenuate the loss of functional capacity and exercise function with aging. Our results showed that aging effects on resting Doppler measures of LV relaxation and diastolic suction are not prevented even when subjected to many decades of stimulus.
Conclusion
In summary, these present findings emphasize that lifelong exercise training frequency favorably influences  o2max, SV, systemic oxygen extraction, and ventricular-arterial coupling during exercise in healthy seniors, with the most prominent effect observed when four or more weekly endurance exercise sessions are performed throughout a lifetime.
o2max, SV, systemic oxygen extraction, and ventricular-arterial coupling during exercise in healthy seniors, with the most prominent effect observed when four or more weekly endurance exercise sessions are performed throughout a lifetime.
AUTHOR CONTRIBUTIONS
Author contributions: G.C.C.-R., J.L.H., P.S.B., N.F., S.S., M.D.P., K.B., S.L., E.D., and B.D.L. performed experiments; G.C.C.-R. and B.D.L. analyzed data; G.C.C.-R., J.L.H., P.S.B., N.F., S.S., M.D.P., K.B., S.L., E.D., and B.D.L. interpreted results of experiments; G.C.C.-R. prepared figures; G.C.C.-R. drafted manuscript; G.C.C.-R., J.L.H., P.S.B., N.F., S.S., and B.D.L. edited and revised manuscript; J.L.H., P.S.B., and B.D.L. conception and design of research; B.D.L. approved final version of manuscript.
REFERENCES
 o2max. J Gerentol A Biol Sci Med Sci
68: 608–616, 2013 [Europe PMC free article] [Abstract] [Google Scholar]
o2max. J Gerentol A Biol Sci Med Sci
68: 608–616, 2013 [Europe PMC free article] [Abstract] [Google Scholar] o2 relationships during submaximal cycle ergometer. J Appl Physiol
84: 599–605, 1998 [Abstract] [Google Scholar]
o2 relationships during submaximal cycle ergometer. J Appl Physiol
84: 599–605, 1998 [Abstract] [Google Scholar]Articles from Journal of Applied Physiology are provided here courtesy of American Physiological Society
Full text links
Read article at publisher's site: https://doi.org/10.1152/japplphysiol.00342.2013
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3972744
Citations & impact
Impact metrics
Article citations
Lifelong physiology of a former marathon world-record holder: the pros and cons of extreme cardiac remodeling.
J Appl Physiol (1985), 137(3):461-472, 27 Jun 2024
Cited by: 1 article | PMID: 38935800
Impaired longitudinal systolic-diastolic coupling and cardiac response to exercise in patients with hypertrophic cardiomyopathy.
Echocardiography, 41(6):e15857, 01 Jun 2024
Cited by: 0 articles | PMID: 38895911
The effect of cardiorespiratory fitness and habitual physical activity on cardiovascular responses to 2 h of uninterrupted sitting.
J Appl Physiol (1985), 136(5):1087-1096, 14 Mar 2024
Cited by: 1 article | PMID: 38482575
The effect of chronic habitual exercise on oxygen carrying capacity and blood compartment volumes in older adults.
J Appl Physiol (1985), 136(4):984-993, 29 Feb 2024
Cited by: 0 articles | PMID: 38420680
The athlete's heart: insights from echocardiography.
Echo Res Pract, 10(1):15, 18 Oct 2023
Cited by: 3 articles | PMID: 37848973 | PMCID: PMC10583359
Review Free full text in Europe PMC
Go to all (64) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Impact of lifelong exercise "dose" on left ventricular compliance and distensibility.
J Am Coll Cardiol, 64(12):1257-1266, 01 Sep 2014
Cited by: 105 articles | PMID: 25236519 | PMCID: PMC4272199
Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age.
Circulation, 122(18):1797-1805, 18 Oct 2010
Cited by: 137 articles | PMID: 20956204 | PMCID: PMC3730488
The effect of lifelong endurance exercise on cardiovascular structure and exercise function in women.
J Physiol, 598(13):2589-2605, 21 May 2020
Cited by: 12 articles | PMID: 32347540 | PMCID: PMC7347229
Funding
Funders who supported this work.
NIA NIH HHS (2)
Grant ID: R01 AG017479
Grant ID: R01AG-17479
 2,3
2,3