Abstract
Free full text

Quantitative control of the stationary phase-specific sigma factor, sigma S, in Escherichia coli: involvement of the nucleoid protein H-NS.
Abstract
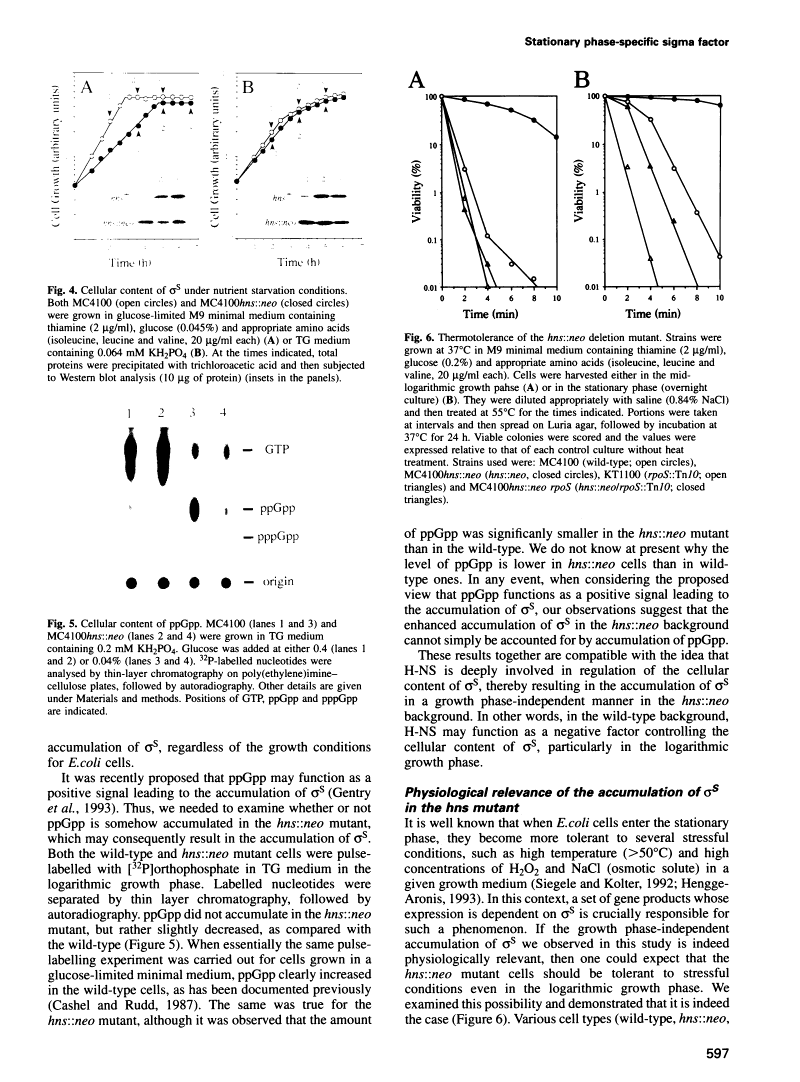
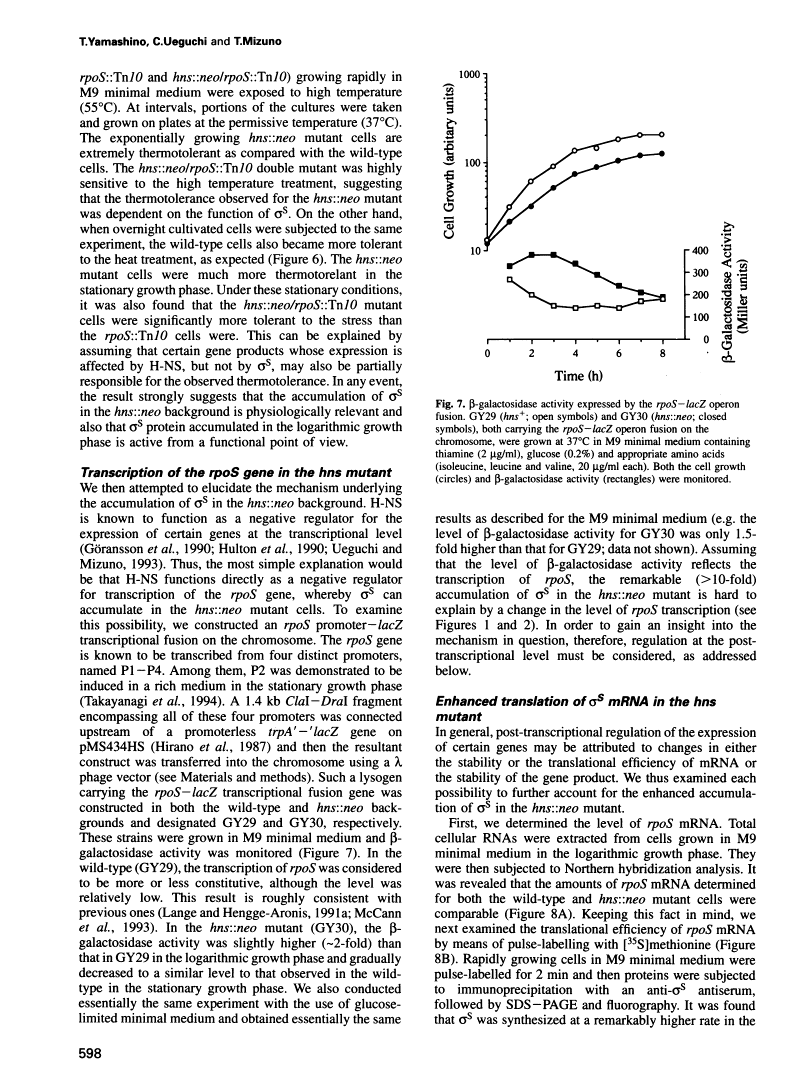
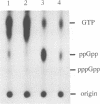
In Escherichia coli, recent intensive studies revealed that expression of a certain subset of genes is under the control of the stationary phase-specific sigma factor, sigma S, which is encoded by the rpoS gene. Since sigma S functions predominantly under certain growth conditions, its activity and/or cellular content has accordingly to be tightly controlled, however, the underlying molecular mechanism is at present unclear. We previously demonstrated that expression of the cbpA gene, encoding an analogue of the DnaJ molecular chaperone, is largely dependent on sigma S function. Here we have found that a mutational lesion of the hns gene, which encodes one of the well-characterized nucleoid proteins, H-NS, affects the cellular content of sigma S remarkably and consequently affects the expression of cbpA. Enhanced accumulation of sigma S in hns deletion cells was particularly observed in the logarithmic growth phase and was demonstrated to result from an elevated translational efficiency of rpoS mRNA and also from an increased stability of newly synthesized sigma S. Although H-NS is known to influence the transcription of a number of apparently unlinked genes on the chromosome, in this study we provide a novel instance in which H-NS is deeply implicated in post-transcriptional regulation(s) of the expression of rpoS. As to physiological relevance, it was also demonstrated that hns deletion cells exhibit an extreme thermotolerance even in the logarithmic growth phase, presumably because of the enhanced accumulation of sigma S.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [Abstract] [Google Scholar]
- Arnqvist A, Olsén A, Normark S. Sigma S-dependent growth-phase induction of the csgBA promoter in Escherichia coli can be achieved in vivo by sigma 70 in the absence of the nucleoid-associated protein H-NS. Mol Microbiol. 1994 Sep;13(6):1021–1032. [Abstract] [Google Scholar]
- Bochner BR, Ames BN. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [Abstract] [Google Scholar]
- Casadaban MJ. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. [Abstract] [Google Scholar]
- Chamberlain JP. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. [Abstract] [Google Scholar]
- Dersch P, Schmidt K, Bremer E. Synthesis of the Escherichia coli K-12 nucleoid-associated DNA-binding protein H-NS is subjected to growth-phase control and autoregulation. Mol Microbiol. 1993 May;8(5):875–889. [Abstract] [Google Scholar]
- Drlica K, Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. [Europe PMC free article] [Abstract] [Google Scholar]
- ECHOLS H, GAREN A, GAREN S, TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. [Abstract] [Google Scholar]
- Falconi M, Higgins NP, Spurio R, Pon CL, Gualerzi CO. Expression of the gene encoding the major bacterial nucleotide protein H-NS is subject to transcriptional auto-repression. Mol Microbiol. 1993 Oct;10(2):273–282. [Abstract] [Google Scholar]
- Gentry DR, Hernandez VJ, Nguyen LH, Jensen DB, Cashel M. Synthesis of the stationary-phase sigma factor sigma s is positively regulated by ppGpp. J Bacteriol. 1993 Dec;175(24):7982–7989. [Europe PMC free article] [Abstract] [Google Scholar]
- Göransson M, Sondén B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin BE. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. [Abstract] [Google Scholar]
- Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993 Jan 29;72(2):165–168. [Abstract] [Google Scholar]
- Higgins CF, Hinton JC, Hulton CS, Owen-Hughes T, Pavitt GD, Seirafi A. Protein H1: a role for chromatin structure in the regulation of bacterial gene expression and virulence? Mol Microbiol. 1990 Dec;4(12):2007–2012. [Abstract] [Google Scholar]
- Hirano M, Shigesada K, Imai M. Construction and characterization of plasmid and lambda phage vector systems for study of transcriptional control in Escherichia coli. Gene. 1987;57(1):89–99. [Abstract] [Google Scholar]
- Huisman GW, Kolter R. Sensing starvation: a homoserine lactone--dependent signaling pathway in Escherichia coli. Science. 1994 Jul 22;265(5171):537–539. [Abstract] [Google Scholar]
- Hulton CS, Seirafi A, Hinton JC, Sidebotham JM, Waddell L, Pavitt GD, Owen-Hughes T, Spassky A, Buc H, Higgins CF. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. [Abstract] [Google Scholar]
- Ito K, Bassford PJ, Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. [Abstract] [Google Scholar]
- Kolter R, Moreno F. Genetics of ribosomally synthesized peptide antibiotics. Annu Rev Microbiol. 1992;46:141–163. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Lammi M, Paci M, Pon CL, Losso MA, Miano A, Pawlik RT, Gianfranceschi GL, Gualerzi CO. Proteins from the prokaryotic nucleoid: biochemical and 1H NMR studies on three bacterial histone-like proteins. Adv Exp Med Biol. 1984;179:467–477. [Abstract] [Google Scholar]
- Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. [Abstract] [Google Scholar]
- Lange R, Hengge-Aronis R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J Bacteriol. 1991 Jul;173(14):4474–4481. [Europe PMC free article] [Abstract] [Google Scholar]
- Lange R, Hengge-Aronis R. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994 Jul 1;8(13):1600–1612. [Abstract] [Google Scholar]
- Loewen PC, von Ossowski I, Switala J, Mulvey MR. KatF (sigma S) synthesis in Escherichia coli is subject to posttranscriptional regulation. J Bacteriol. 1993 Apr;175(7):2150–2153. [Europe PMC free article] [Abstract] [Google Scholar]
- McCann MP, Fraley CD, Matin A. The putative sigma factor KatF is regulated posttranscriptionally during carbon starvation. J Bacteriol. 1993 Apr;175(7):2143–2149. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulvey MR, Loewen PC. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulvey MR, Switala J, Borys A, Loewen PC. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. [Europe PMC free article] [Abstract] [Google Scholar]
- Olsén A, Arnqvist A, Hammar M, Sukupolvi S, Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993 Feb;7(4):523–536. [Abstract] [Google Scholar]
- Owen-Hughes TA, Pavitt GD, Santos DS, Sidebotham JM, Hulton CS, Hinton JC, Higgins CF. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992 Oct 16;71(2):255–265. [Abstract] [Google Scholar]
- Pettijohn DE. Histone-like proteins and bacterial chromosome structure. J Biol Chem. 1988 Sep 15;263(26):12793–12796. [Abstract] [Google Scholar]
- Pon CL, Calogero RA, Gualerzi CO. Identification, cloning, nucleotide sequence and chromosomal map location of hns, the structural gene for Escherichia coli DNA-binding protein H-NS. Mol Gen Genet. 1988 May;212(2):199–202. [Abstract] [Google Scholar]
- Schmid MB. More than just "histone-like" proteins. Cell. 1990 Nov 2;63(3):451–453. [Abstract] [Google Scholar]
- Siegele DA, Kolter R. Life after log. J Bacteriol. 1992 Jan;174(2):345–348. [Europe PMC free article] [Abstract] [Google Scholar]
- Spurio R, Dürrenberger M, Falconi M, La Teana A, Pon CL, Gualerzi CO. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol Gen Genet. 1992 Jan;231(2):201–211. [Abstract] [Google Scholar]
- Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3511–3515. [Europe PMC free article] [Abstract] [Google Scholar]
- Takayanagi Y, Tanaka K, Takahashi H. Structure of the 5' upstream region and the regulation of the rpoS gene of Escherichia coli. Mol Gen Genet. 1994 Jun 3;243(5):525–531. [Abstract] [Google Scholar]
- Ueguchi C, Mizuno T. The Escherichia coli nucleoid protein H-NS functions directly as a transcriptional repressor. EMBO J. 1993 Mar;12(3):1039–1046. [Europe PMC free article] [Abstract] [Google Scholar]
- Ueguchi C, Kakeda M, Mizuno T. Autoregulatory expression of the Escherichia coli hns gene encoding a nucleoid protein: H-NS functions as a repressor of its own transcription. Mol Gen Genet. 1993 Jan;236(2-3):171–178. [Abstract] [Google Scholar]
- Ueguchi C, Kakeda M, Yamada H, Mizuno T. An analogue of the DnaJ molecular chaperone in Escherichia coli. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1054–1058. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamada H, Muramatsu S, Mizuno T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J Biochem. 1990 Sep;108(3):420–425. [Abstract] [Google Scholar]
- Yamada H, Yoshida T, Tanaka K, Sasakawa C, Mizuno T. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet. 1991 Nov;230(1-2):332–336. [Abstract] [Google Scholar]
- Yamashino T, Kakeda M, Ueguchi C, Mizuno T. An analogue of the DnaJ molecular chaperone whose expression is controlled by sigma s during the stationary phase and phosphate starvation in Escherichia coli. Mol Microbiol. 1994 Aug;13(3):475–483. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1995.tb07035.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc398118?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Growth Phase-Dependent Chromosome Condensation and Heat-Stable Nucleoid-Structuring Protein Redistribution in Escherichia coli under Osmotic Stress.
J Bacteriol, 201(23):e00469-19, 05 Nov 2019
Cited by: 9 articles | PMID: 31481544 | PMCID: PMC6832063
BAH1 an E3 Ligase from Arabidopsis thaliana Stabilizes Heat Shock Factor σ32 of Escherichia coli by Interacting with DnaK/DnaJ Chaperone Team.
Curr Microbiol, 75(4):450-455, 20 Dec 2017
Cited by: 3 articles | PMID: 29260303
Considerations on bacterial nucleoids.
Appl Microbiol Biotechnol, 101(14):5591-5602, 29 Jun 2017
Cited by: 7 articles | PMID: 28664324
Review
The Subtleties and Contrasts of the LeuO Regulator in Salmonella Typhi: Implications in the Immune Response.
Front Immunol, 5:581, 12 Dec 2014
Cited by: 9 articles | PMID: 25566242 | PMCID: PMC4264507
Review Free full text in Europe PMC
Silencing by H-NS potentiated the evolution of Salmonella.
PLoS Pathog, 10(11):e1004500, 06 Nov 2014
Cited by: 59 articles | PMID: 25375226 | PMCID: PMC4223078
Go to all (112) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Escherichia coli SOS gene sbmC is regulated by H-NS and RpoS during the SOS induction and stationary growth phase.
Biochem Biophys Res Commun, 288(4):1052-1058, 01 Nov 2001
Cited by: 8 articles | PMID: 11689018
The RNA-binding protein HF-I, known as a host factor for phage Qbeta RNA replication, is essential for rpoS translation in Escherichia coli.
Genes Dev, 10(9):1143-1151, 01 May 1996
Cited by: 186 articles | PMID: 8654929
An analogue of the DnaJ molecular chaperone whose expression is controlled by sigma s during the stationary phase and phosphate starvation in Escherichia coli.
Mol Microbiol, 13(3):475-483, 01 Aug 1994
Cited by: 38 articles | PMID: 7997164
Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli.
Mol Microbiol, 21(5):887-893, 01 Sep 1996
Cited by: 194 articles | PMID: 8885260
Review