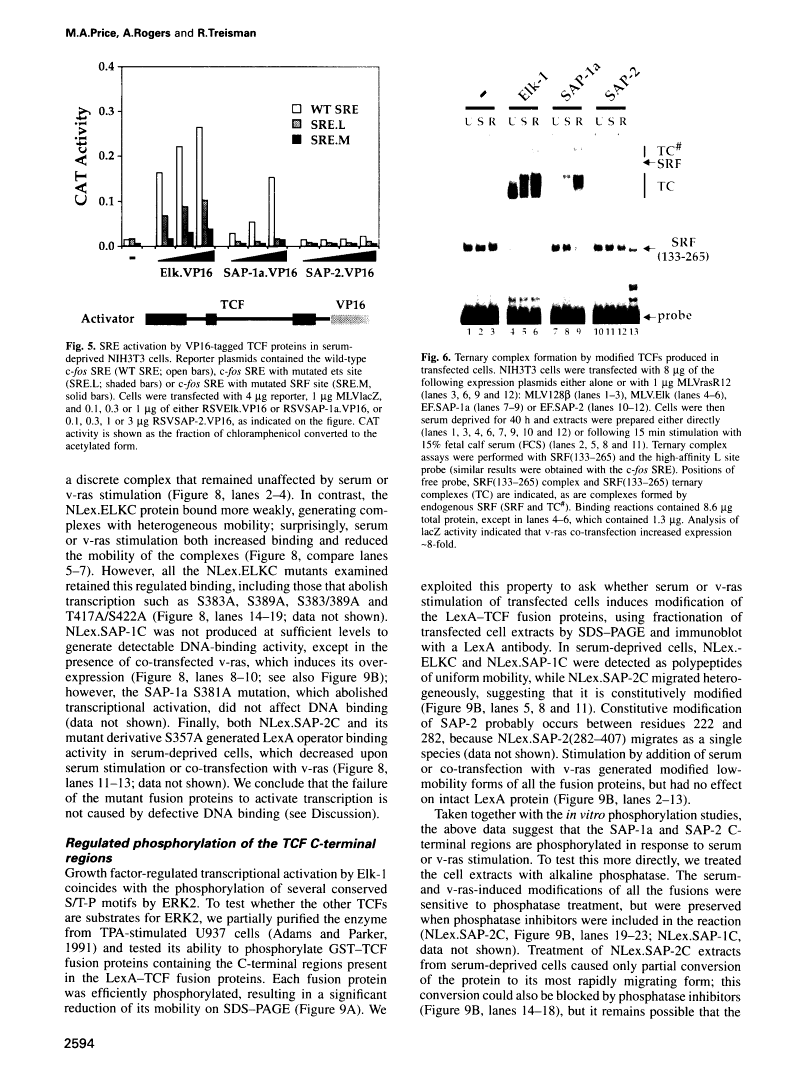
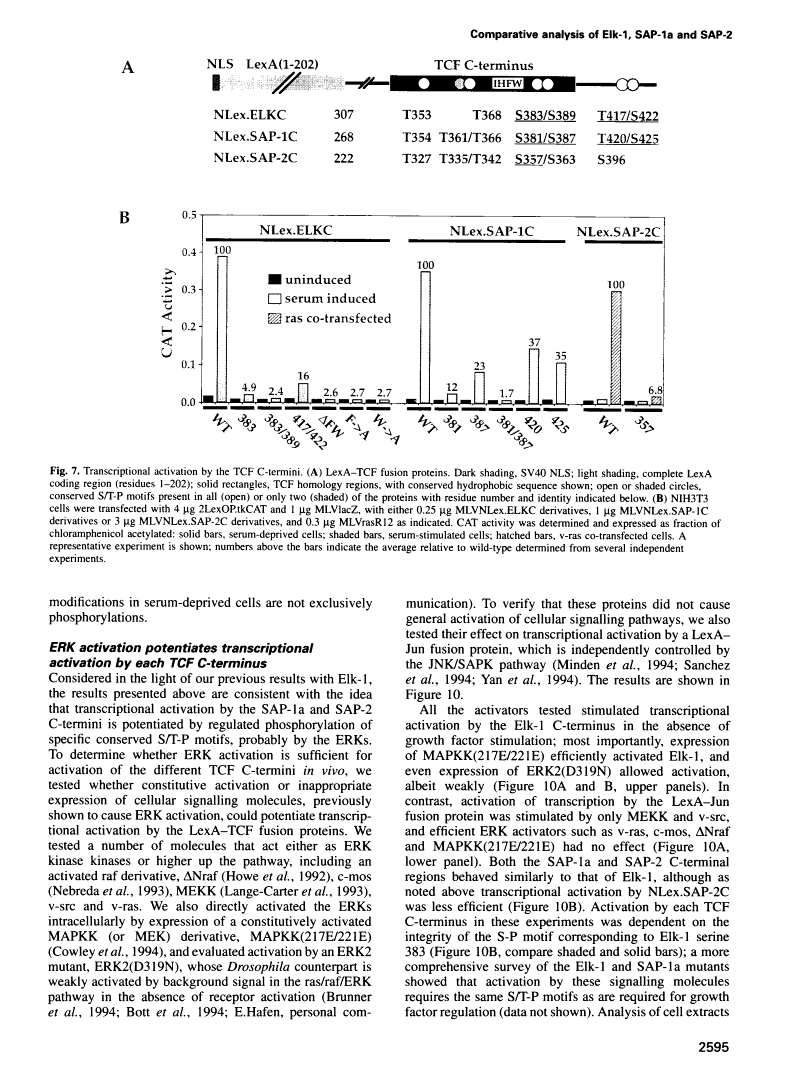
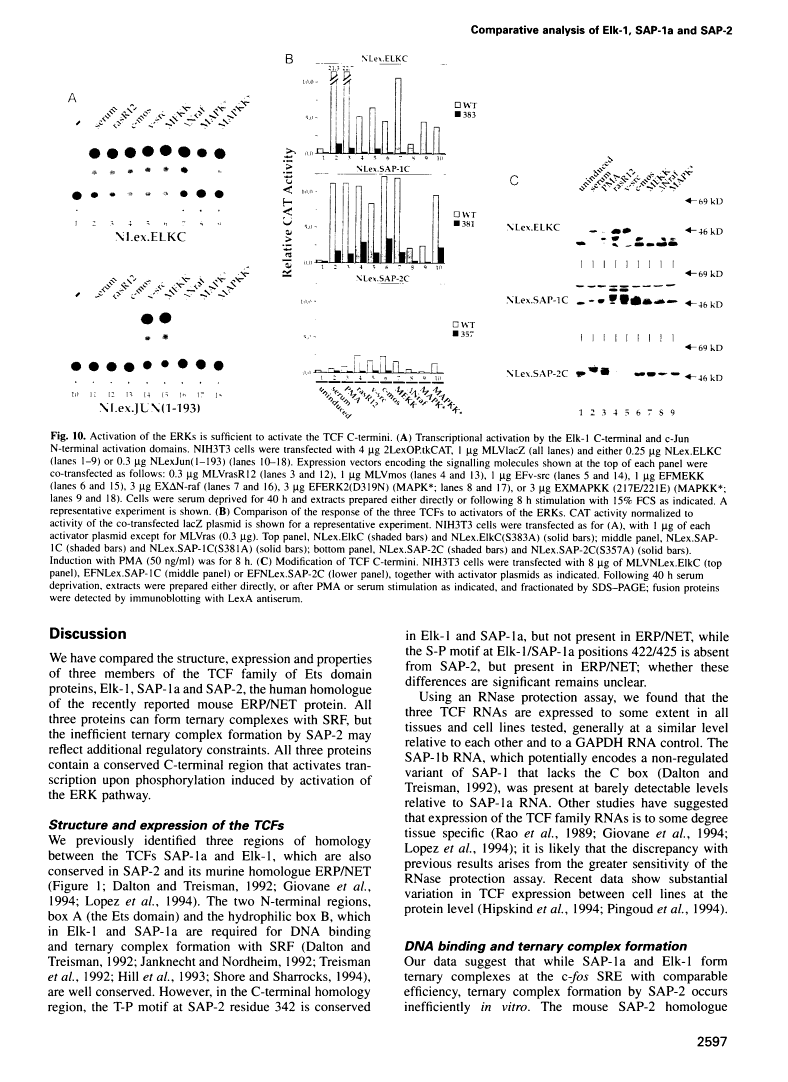
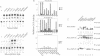Abstract
Free full text

Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET).
Abstract
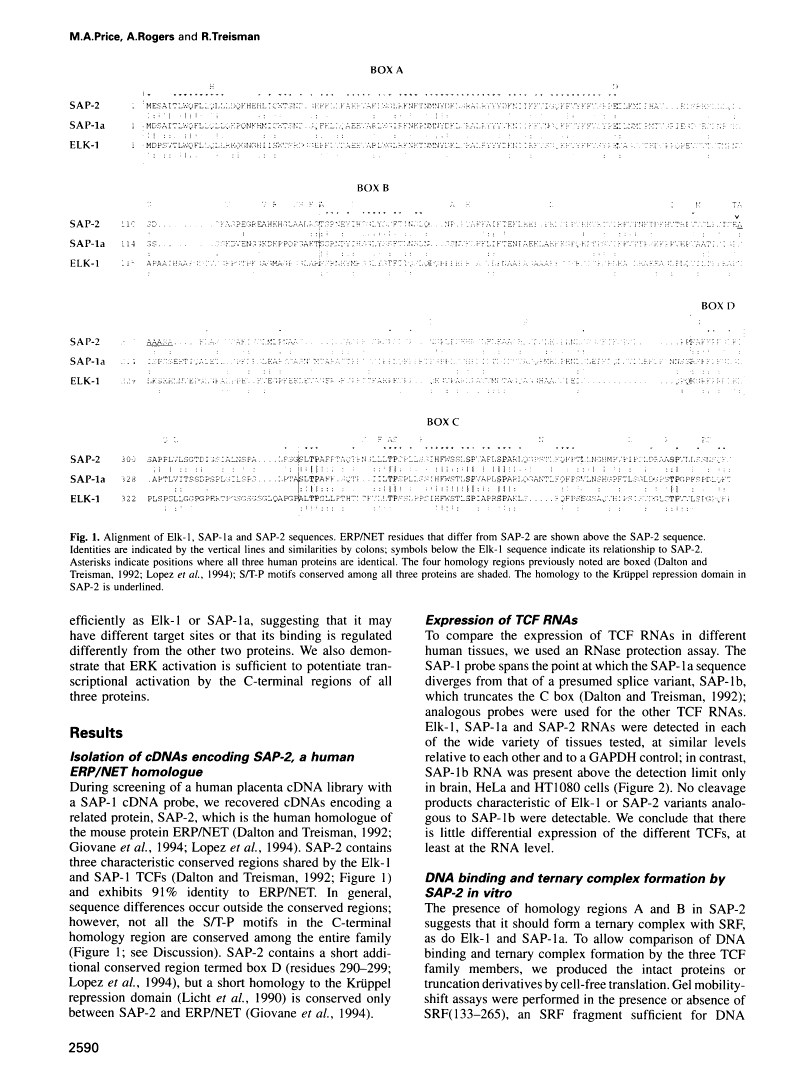
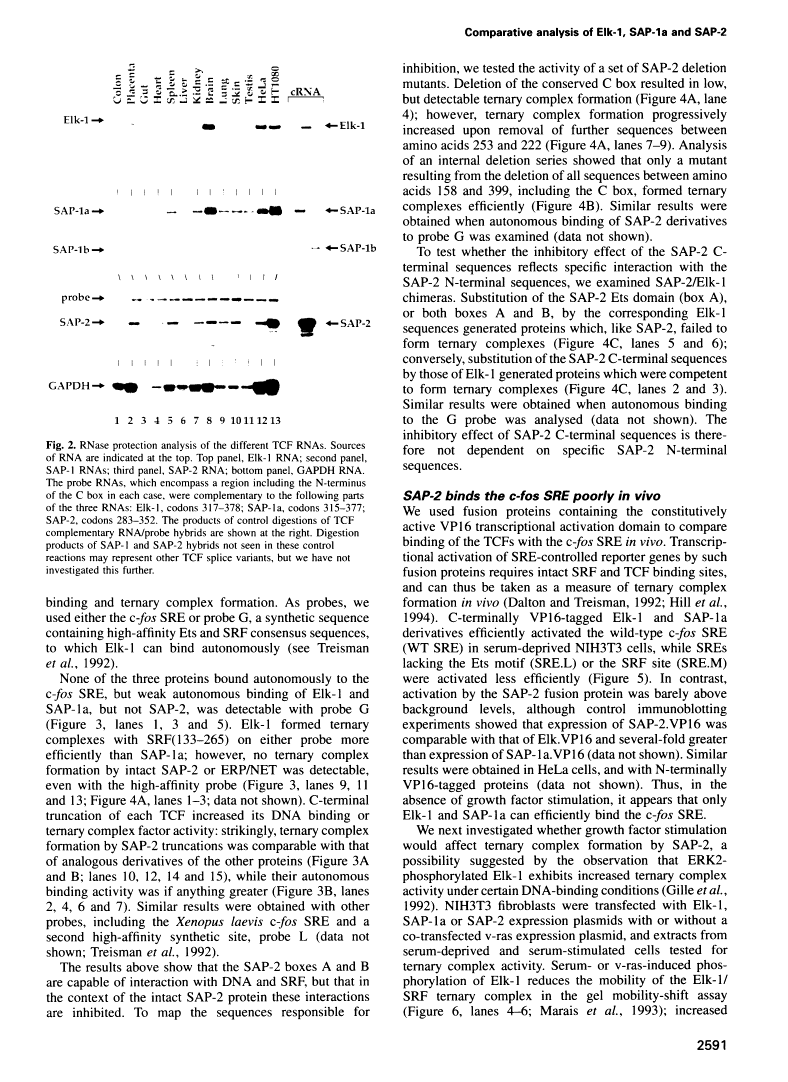
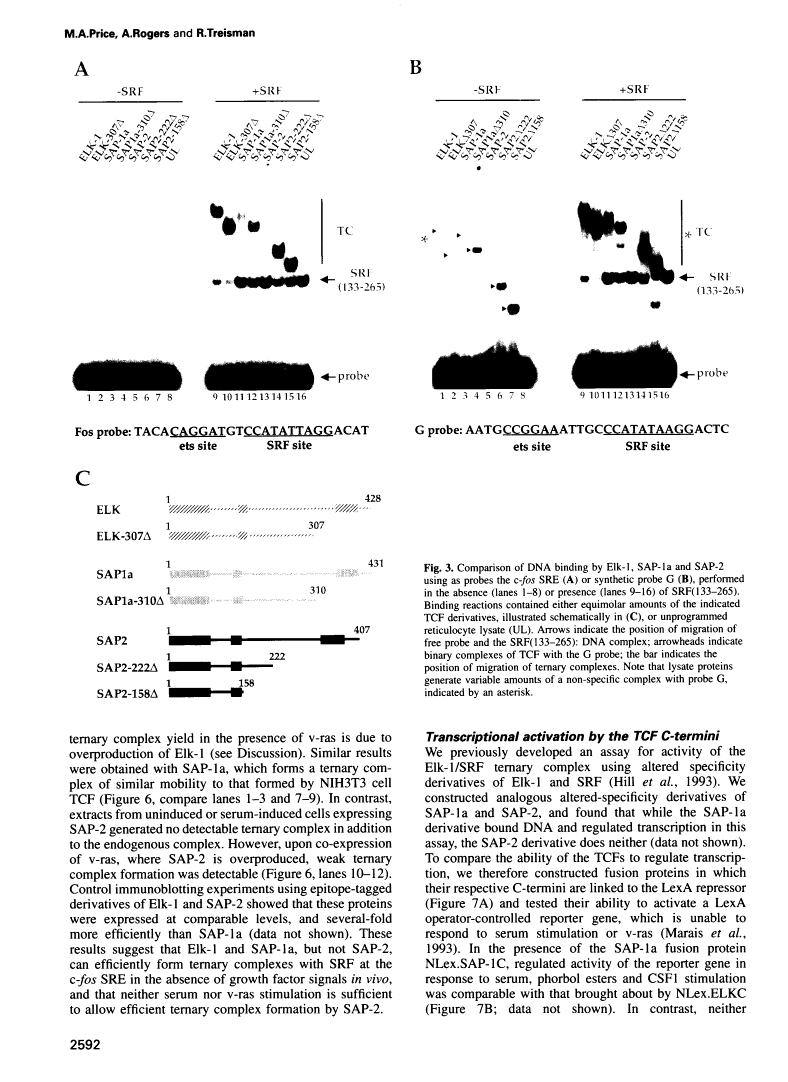
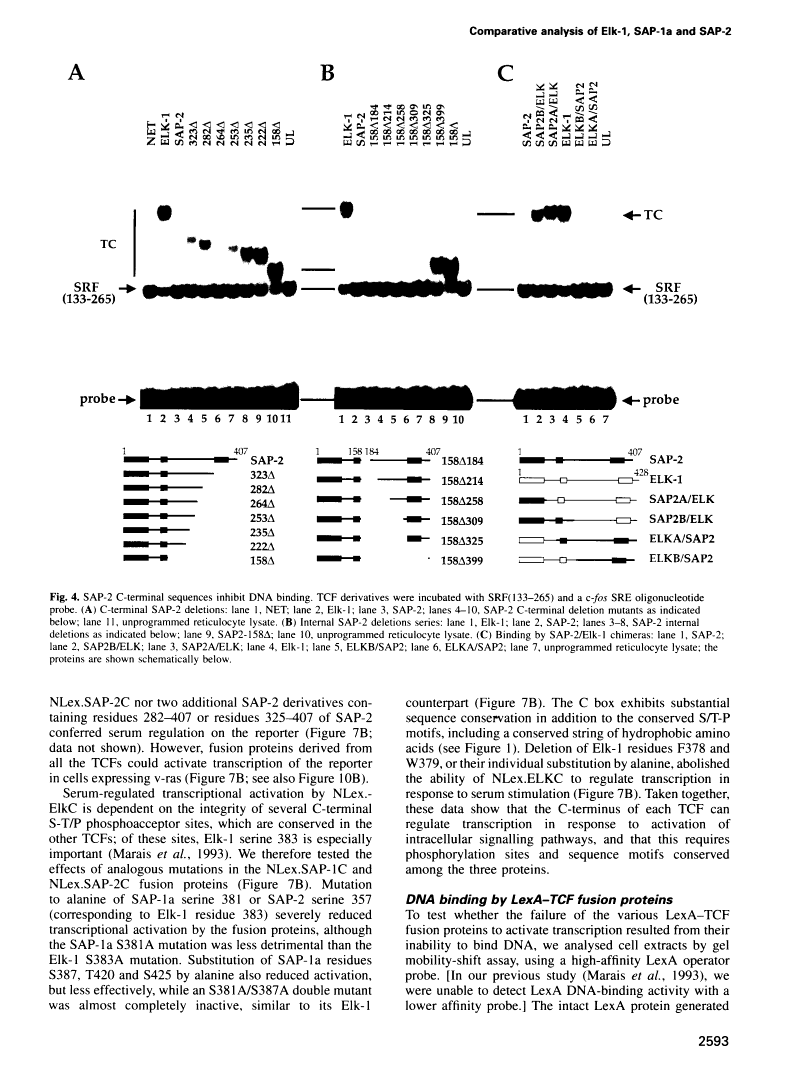
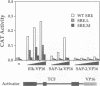
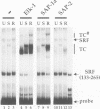
A transcription factor ternary complex composed of Serum Response Factor (SRF) and Ternary Complex Factor (TCF) mediates the response of the c-fos Serum Response Element (SRE) to growth factors and mitogens. Three Ets domain proteins, Elk-1, SAP-1 and ERP/NET, have been reported to have the properties of TCF. Here we compare Elk-1 and SAP-1a with the human ERP/NET homologue SAP-2. All three TCF RNAs are ubiquitously expressed at similar relative levels. All three proteins contain conserved regions that interact with SRF and the c-fos SRE with comparable efficiency, but in vitro complex formation by SAP-2 is strongly inhibited by its C-terminal sequences. Similarly, only Elk-1 and SAP-1a efficiently bind the c-fos SRE in vivo; ternary complex formation by SAP-2 is weak and is substantially unaffected by serum stimulation or v-ras co-expression. All three TCFs contain C-terminal transcriptional activation domains that are phosphorylated following growth factor stimulation. Activation requires conserved S/T-P motifs found in all the TCF family members. Each TCF activation domain can be phosphorylated in vitro by partially purified ERK2, and ERK activation in vivo is sufficient to potentiate transcriptional activation.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams PD, Parker PJ. TPA-induced activation of MAP kinase. FEBS Lett. 1991 Sep 23;290(1-2):77–82. [Abstract] [Google Scholar]
- Bardwell VJ, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994 Jul 15;8(14):1664–1677. [Abstract] [Google Scholar]
- Bott CM, Thorneycroft SG, Marshall CJ. The sevenmaker gain-of-function mutation in p42 MAP kinase leads to enhanced signalling and reduced sensitivity to dual specificity phosphatase action. FEBS Lett. 1994 Sep 26;352(2):201–205. [Abstract] [Google Scholar]
- Brunner D, Oellers N, Szabad J, Biggs WH, 3rd, Zipursky SL, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994 Mar 11;76(5):875–888. [Abstract] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. [Abstract] [Google Scholar]
- Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. [Abstract] [Google Scholar]
- Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994 Sep 8;371(6493):171–175. [Abstract] [Google Scholar]
- Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. [Abstract] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995 Mar 1;14(5):951–962. [Europe PMC free article] [Abstract] [Google Scholar]
- Giovane A, Pintzas A, Maira SM, Sobieszczuk P, Wasylyk B. Net, a new ets transcription factor that is activated by Ras. Genes Dev. 1994 Jul 1;8(13):1502–1513. [Abstract] [Google Scholar]
- Golemis EA, Brent R. Fused protein domains inhibit DNA binding by LexA. Mol Cell Biol. 1992 Jul;12(7):3006–3014. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham R, Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. [Abstract] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. [Abstract] [Google Scholar]
- Herrera RE, Shaw PE, Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. [Abstract] [Google Scholar]
- Hill CS, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. [Abstract] [Google Scholar]
- Hill CS, Wynne J, Treisman R. Serum-regulated transcription by serum response factor (SRF): a novel role for the DNA binding domain. EMBO J. 1994 Nov 15;13(22):5421–5432. [Europe PMC free article] [Abstract] [Google Scholar]
- Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. [Abstract] [Google Scholar]
- Hipskind RA, Büscher D, Nordheim A, Baccarini M. Ras/MAP kinase-dependent and -independent signaling pathways target distinct ternary complex factors. Genes Dev. 1994 Aug 1;8(15):1803–1816. [Abstract] [Google Scholar]
- Howe LR, Leevers SJ, Gómez N, Nakielny S, Cohen P, Marshall CJ. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. [Abstract] [Google Scholar]
- Janknecht R, Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992 Jul 11;20(13):3317–3324. [Europe PMC free article] [Abstract] [Google Scholar]
- Janknecht R, Ernst WH, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. [Europe PMC free article] [Abstract] [Google Scholar]
- Johansen FE, Prywes R. Two pathways for serum regulation of the c-fos serum response element require specific sequence elements and a minimal domain of serum response factor. Mol Cell Biol. 1994 Sep;14(9):5920–5928. [Europe PMC free article] [Abstract] [Google Scholar]
- König H, Ponta H, Rahmsdorf U, Büscher M, Schönthal A, Rahmsdorf HJ, Herrlich P. Autoregulation of fos: the dyad symmetry element as the major target of repression. EMBO J. 1989 Sep;8(9):2559–2566. [Europe PMC free article] [Abstract] [Google Scholar]
- Kortenjann M, Thomae O, Shaw PE. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994 Jul;14(7):4815–4824. [Europe PMC free article] [Abstract] [Google Scholar]
- Lange-Carter CA, Pleiman CM, Gardner AM, Blumer KJ, Johnson GL. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. [Abstract] [Google Scholar]
- Leevers SJ, Marshall CJ. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. [Europe PMC free article] [Abstract] [Google Scholar]
- Licht JD, Grossel MJ, Figge J, Hansen UM. Drosophila Krüppel protein is a transcriptional repressor. Nature. 1990 Jul 5;346(6279):76–79. [Abstract] [Google Scholar]
- Lopez M, Oettgen P, Akbarali Y, Dendorfer U, Libermann TA. ERP, a new member of the ets transcription factor/oncoprotein family: cloning, characterization, and differential expression during B-lymphocyte development. Mol Cell Biol. 1994 May;14(5):3292–3309. [Europe PMC free article] [Abstract] [Google Scholar]
- Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. [Abstract] [Google Scholar]
- Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994 Feb;4(1):82–89. [Abstract] [Google Scholar]
- Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994 Dec 9;266(5191):1719–1723. [Abstract] [Google Scholar]
- Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. [Europe PMC free article] [Abstract] [Google Scholar]
- Nebreda AR, Hill C, Gomez N, Cohen P, Hunt T. The protein kinase mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 1993 Oct 25;333(1-2):183–187. [Abstract] [Google Scholar]
- Norman C, Runswick M, Pollock R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. [Abstract] [Google Scholar]
- Pingoud V, Zinck R, Hipskind RA, Janknecht R, Nordheim A. Heterogeneity of ternary complex factors in HeLa cell nuclear extracts. J Biol Chem. 1994 Sep 16;269(37):23310–23317. [Abstract] [Google Scholar]
- Rao VN, Huebner K, Isobe M, ar-Rushdi A, Croce CM, Reddy ES. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. [Abstract] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994 Sep 23;78(6):1027–1037. [Abstract] [Google Scholar]
- Sánchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994 Dec 22;372(6508):794–798. [Abstract] [Google Scholar]
- Shaw PE, Schröter H, Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. [Abstract] [Google Scholar]
- Shore P, Sharrocks AD. The transcription factors Elk-1 and serum response factor interact by direct protein-protein contacts mediated by a short region of Elk-1. Mol Cell Biol. 1994 May;14(5):3283–3291. [Europe PMC free article] [Abstract] [Google Scholar]
- Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994 Feb;4(1):96–101. [Abstract] [Google Scholar]
- Treisman R, Ammerer G. The SRF and MCM1 transcription factors. Curr Opin Genet Dev. 1992 Apr;2(2):221–226. [Abstract] [Google Scholar]
- Treisman R, Marais R, Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. [Europe PMC free article] [Abstract] [Google Scholar]
- Wotton D, Ways DK, Parker PJ, Owen MJ. Activity of both Raf and Ras is necessary for activation of transcription of the human T cell receptor beta gene by protein kinase C, Ras plays multiple roles. J Biol Chem. 1993 Aug 25;268(24):17975–17982. [Abstract] [Google Scholar]
- Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994 Dec 22;372(6508):798–800. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1995.tb07257.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc398373?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1995.tb07257.x
Article citations
Crosstalk between phytochemicals and inflammatory signaling pathways.
Inflammopharmacology, 31(3):1117-1147, 06 Apr 2023
Cited by: 3 articles | PMID: 37022574
Review
Antisense oligonucleotide is a promising intervention for liver diseases.
Front Pharmacol, 13:1061842, 09 Dec 2022
Cited by: 2 articles | PMID: 36569303 | PMCID: PMC9780395
Review Free full text in Europe PMC
Critical Protein-Protein Interactions Determine the Biological Activity of Elk-1, a Master Regulator of Stimulus-Induced Gene Transcription.
Molecules, 26(20):6125, 11 Oct 2021
Cited by: 5 articles | PMID: 34684708 | PMCID: PMC8541449
Review Free full text in Europe PMC
Serum Response Factor (SRF) Drives the Transcriptional Upregulation of the MDM4 Oncogene in HCC.
Cancers (Basel), 13(2):E199, 08 Jan 2021
Cited by: 4 articles | PMID: 33429878 | PMCID: PMC7829828
Screening and functional prediction of differentially expressed circRNAs in proliferative human aortic smooth muscle cells.
J Cell Mol Med, 24(8):4762-4772, 10 Mar 2020
Cited by: 11 articles | PMID: 32155686 | PMCID: PMC7176856
Go to all (170) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Integration of growth factor signals at the c-fos serum response element.
Philos Trans R Soc Lond B Biol Sci, 351(1339):551-559, 01 Apr 1996
Cited by: 29 articles | PMID: 8735278
Net (ERP/SAP2) one of the Ras-inducible TCFs, has a novel inhibitory domain with resemblance to the helix-loop-helix motif.
EMBO J, 15(21):5849-5865, 01 Nov 1996
Cited by: 61 articles | PMID: 8918463 | PMCID: PMC452333
Interaction of serum response factor (SRF) with the Elk-1 B box inhibits RhoA-actin signaling to SRF and potentiates transcriptional activation by Elk-1.
Mol Cell Biol, 22(20):7083-7092, 01 Oct 2002
Cited by: 38 articles | PMID: 12242287 | PMCID: PMC139817
Ets ternary complex transcription factors.
Gene, 324:1-14, 01 Jan 2004
Cited by: 254 articles | PMID: 14693367
Review