Abstract
Objectives
Latent tuberculosis infection and tuberculosis disease are prevalent worldwide. However, antimycobacterial rifamycins have drug interactions with many antiretroviral drugs. We evaluated the effect of rifapentine on the pharmacokinetic properties of raltegravir.Methods
In this open-label, fixed-sequence, three-period study, 21 healthy volunteers were given: raltegravir alone (400 mg every 12 h for 4 days) on days 1-4 of Period 1; rifapentine (900 mg once weekly for 3 weeks) on days 1, 8 and 15 of Period 2 and raltegravir (400 mg every 12 h for 4 days) on days 12-15 of Period 2; and rifapentine (600 mg once daily for 10 scheduled doses) on days 1, 4-8 and 11-14 of Period 3 and raltegravir (400 mg every 12 h for 4 days) on days 11-14 of Period 3. Plasma raltegravir concentrations were measured. ClinicalTrials.gov database: NCT00809718.Results
In 16 subjects who completed the study, coadministration of raltegravir with rifapentine (900 mg once weekly; Period 2) compared with raltegravir alone resulted in the geometric mean of the raltegravir AUC from 0 to 12 h (AUC0-12) being increased by 71%; the peak concentration increased by 89% and the trough concentration decreased by 12%. Coadministration of raltegravir with rifapentine in Period 3 did not change the geometric mean of the raltegravir AUC0-12 or the peak concentration, but it decreased the trough concentration by 41%. Raltegravir coadministered with rifapentine was generally well tolerated.Conclusions
The increased raltegravir exposure observed with once-weekly rifapentine was safe and tolerable. Once-weekly rifapentine can be used with raltegravir to treat latent tuberculosis infection in patients who are infected with HIV.Free full text

Pharmacokinetic interaction of rifapentine and raltegravir in healthy volunteers
Abstract
Objectives
Latent tuberculosis infection and tuberculosis disease are prevalent worldwide. However, antimycobacterial rifamycins have drug interactions with many antiretroviral drugs. We evaluated the effect of rifapentine on the pharmacokinetic properties of raltegravir.
Methods
In this open-label, fixed-sequence, three-period study, 21 healthy volunteers were given: raltegravir alone (400 mg every 12 h for 4 days) on days 1–4 of Period 1; rifapentine (900 mg once weekly for 3 weeks) on days 1, 8 and 15 of Period 2 and raltegravir (400 mg every 12 h for 4 days) on days 12–15 of Period 2; and rifapentine (600 mg once daily for 10 scheduled doses) on days 1, 4–8 and 11–14 of Period 3 and raltegravir (400 mg every 12 h for 4 days) on days 11–14 of Period 3. Plasma raltegravir concentrations were measured. ClinicalTrials.gov database: NCT00809718.
Results
In 16 subjects who completed the study, coadministration of raltegravir with rifapentine (900 mg once weekly; Period 2) compared with raltegravir alone resulted in the geometric mean of the raltegravir AUC from 0 to 12 h (AUC0–12) being increased by 71%; the peak concentration increased by 89% and the trough concentration decreased by 12%. Coadministration of raltegravir with rifapentine in Period 3 did not change the geometric mean of the raltegravir AUC0–12 or the peak concentration, but it decreased the trough concentration by 41%. Raltegravir coadministered with rifapentine was generally well tolerated.
Conclusions
The increased raltegravir exposure observed with once-weekly rifapentine was safe and tolerable. Once-weekly rifapentine can be used with raltegravir to treat latent tuberculosis infection in patients who are infected with HIV.
Introduction
The WHO estimates that one-third of the world's population has latent tuberculosis infection (LTBI).1–3 Rifapentine, a potent antimycobacterial rifamycin antibiotic, may be 2–4 times as active as rifampicin against Mycobacterium tuberculosis in vitro and in animal models.4,5The US CDC has recommended a short-course alternative treatment for LTBI in adults who are at high risk of developing active tuberculosis.6–8 In the PREVENT TB Phase 3, randomized treatment trial of 8053 patients who had LTBI, a 12-dose, once-weekly regimen of rifapentine and isoniazid had similar efficacy and tolerability compared with daily isoniazid for 9 months.6 Clinical dose-ranging studies of daily rifapentine in intensive-phase therapy have been performed in patients with tuberculosis because in vivo models of active tuberculosis have shown that the bactericidal and sterilizing activity of rifapentine is concentration dependent, increasing at higher mg/kg doses.4,5,9
Raltegravir10 is an integrase inhibitor and potent antiretroviral agent, and clinical trials have established the clinical efficacy, safety and tolerability of raltegravir. In a Phase 3, double-blind, non-inferiority study, regimens containing raltegravir or efavirenz were similar in reducing the viral load of HIV-1 to undetectable levels (<50 copies/mL) at 240 weeks.11 The immunological response was greater in the regimen containing raltegravir (CD4 cell count 295 cells/mm3) than efavirenz (CD4 cell count 236 cells/mm3).11 Raltegravir, combined with emtricitabine and tenofovir, is currently recommended as one of four preferred regimens for patients who have not had previous treatment for HIV. Furthermore, raltegravir may be substituted for efavirenz in pregnant women, and there are fewer drug interactions with raltegravir than with the other preferred regimens. Therefore, it is important to understand the interactions of raltegravir with each of the rifamycins.12
Rifamycins may decrease plasma concentrations of antiretroviral integrase inhibitors, protease inhibitors and non-nucleoside reverse transcriptase inhibitors.13 The primary metabolic pathway for the inactivation of raltegravir is through the cytosol enzyme uridine diphosphate glucuronosyltransferase 1A1, and the activity of glucuronosyltransferase is up-regulated by rifampicin.14–17 Compared with raltegravir alone, the coadministration of rifampicin (600 mg once daily) with raltegravir (a 400 mg single oral dose) caused a decrease in the geometric mean raltegravir trough concentration 12 h after administration (C12) by 61%, the AUC from 0 to 12 h (AUC0–12) by 40% and the peak concentration (Cmax) by 38%.15 Compared with raltegravir alone, coadministration of rifabutin (300 mg once daily) with raltegravir (a 400 mg single oral dose) caused a decrease in the geometric mean raltegravir C12 by 20%, but the AUC0–12 increased by 19% and the Cmax increased by 39%.16 The effect of a third rifamycin, rifapentine, on raltegravir or other antiretroviral agents has not been evaluated.
Pharmacokinetic drug interactions between rifapentine and raltegravir may determine the potential utility of once-weekly rifapentine in treating LTBI and daily rifapentine for active tuberculosis in patients who are infected with HIV. The purpose of the present study was to evaluate the effect of rifapentine, at doses that are used to treat LTBI and tuberculosis, on the pharmacokinetic properties of raltegravir.
Methods
Subjects
This was a single-centre, open-label, fixed-sequence, three-period pharmacokinetic study (Figure 1) (ClinicalTrials.gov database: NCT00809718). There were 21 healthy adults [age >18 years; median age 30 years (IQR 25–39 years)] (12 men and 9 women) who were recruited for the study from the University of Texas Health Science Center, the South Texas Veterans Administration Medical Center and ClinicalTrials.gov. Inclusion criteria were normal haematological, renal and liver function tests and a Karnofsky score ≥90. Subjects were excluded because of HIV infection, illicit drug use, prior gastrointestinal surgery, known intolerance to rifamycin drugs or raltegravir, prior use of these drugs in the previous 30 days, a regular use of other drugs or current pregnancy or breastfeeding. Women of childbearing potential agreed to practise birth control by a barrier method or abstinence during the study.
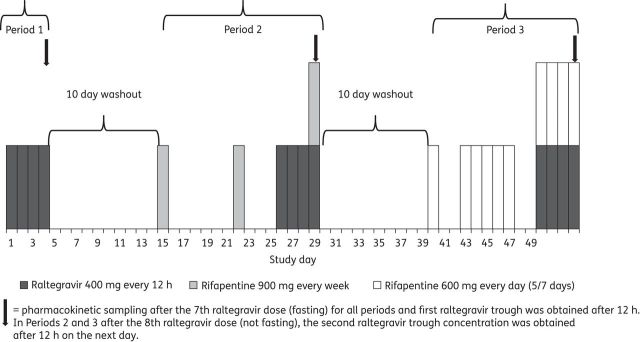
Study design to evaluate the effect of rifapentine on the pharmacokinetic properties of raltegravir.
If the subject needed to take non-study drugs such as non-narcotic pain relievers, investigators determined whether the drug was anticipated to alter concentrations of raltegravir or rifapentine. Non-study drugs taken during study periods included ibuprofen (200 mg once after a minor toe injury in one subject during Period 2); acetaminophen (one or two doses for upper respiratory symptoms in one subject during Period 2 and one subject during Period 3); aciclovir (400 mg, three times daily for 5 days for a localized herpetic rash in one subject 1 day after completion of Period 2); topical ophthalmic drops (to one eye after traumatic corneal abrasion in one subject during Period 3); and ondansetron (4 mg tablet, three tablets, for nausea in one subject during a washout interval after Period 2 of the study).
The Institutional Review Boards of the US CDC and University of Texas Health Science Center at San Antonio approved the study. Written informed consent was obtained from all participants. Adverse events were classified for severity using a graded toxicity scale adapted from the National Cancer Institute (Grade 1, mild; Grade 2, moderate; Grade 3, severe; Grade 4, life-threatening).18
Drug dosage and pharmacokinetic sampling
Subjects were given: raltegravir alone (400 mg every 12 h for 4 days) on days 1–4 of Period 1; rifapentine (900 mg once weekly for 3 weeks) on days 1, 8 and 15 of Period 2 and raltegravir (400 mg every 12 h for 4 days) on days 12–15 of Period 2; and rifapentine (600 mg once daily for 10 scheduled doses) on days 1, 4–8 and 11–14 of Period 3 and raltegravir (400 mg every 12 h for 4 days) on days 11–14 of Period 3. The timing of pharmacokinetic sampling for each period is shown in Figure 1. The dosing periods were separated by 10 day washout intervals with no study drugs. Drug administration diaries were kept by the subjects. On the morning of pharmacokinetic sampling in each period, the subjects were not allowed any food (from 8 h before to 2 h after the administration of the study drugs), and raltegravir (with or without rifapentine) was administered with direct observation. All other doses of raltegravir and rifapentine were taken without regard to food. Blood samples for the pharmacokinetic measurements of raltegravir in each study period and for rifapentine in Periods 2 and 3 were collected just before the morning study drugs were given and at 1, 2, 3, 4, 5, 6, 8 and 12 h after the study drugs had been given. Additional blood samples in Periods 2 and 3 to measure raltegravir concentration were obtained 12 h after the eighth dose of raltegravir (24 h after the seventh raltegravir dose).
Raltegravir concentrations were below the level of quantification in one subject at baseline pharmacokinetic sampling (before the administration of the study drugs) in Periods 2 and 3. These two baseline samples were not included in the pharmacokinetic analyses.
Drug determinations
The measurement of raltegravir concentration in human plasma was performed at a commercial laboratory (Bioanalytical Systems, Inc., McMinnville, OR, USA) using an automated sample preparation process followed by reversed-phase HPLC and tandem mass spectrometry modified from a procedure previously described.19 Internal standard solution (13C6-raltegravir, 1000 ng/mL, 20 μL) was added to plasma (200 μL) and acidified with ammonium acetate (200 mM, 150 μL, pH 4). The samples were transferred to 96-well plates and extracted into hexane and methylene chloride (50 :
: 50 solution, 1.2 mL). The organic layer was transferred to a clean plate, evaporated to dryness and reconstituted in methanol and 0.1 mM EDTA with 0.1% formic acid (55
50 solution, 1.2 mL). The organic layer was transferred to a clean plate, evaporated to dryness and reconstituted in methanol and 0.1 mM EDTA with 0.1% formic acid (55 :
: 45 solution, 350 μL). Samples (5 μL) were injected onto an analytic column (3.0
45 solution, 350 μL). Samples (5 μL) were injected onto an analytic column (3.0 ×
× 50 mm, 3 μm, Ace C18 column, Part number ACE-111-0503; Mac-Mod Analytical, Chadds Ford, PA, USA) with titanium frits at ambient temperature. Mobile phase A was 0.1% formic acid in 0.1 mM EDTA and mobile phase B was methanol. The flow rate was 0.5 mL/min with an isocratic run at 57.5% mobile phase B. Detection by tandem mass spectrometry incorporated an atmospheric pressure chemical ionization interface in positive ion mode. The range of the validated assay for raltegravir was 0.002–1.000 μg/mL. The accuracy of undiluted control samples between days was 101.5%–102.9% and the coefficient of variation was 2.1%–3.6%. Plasma concentrations of rifapentine were determined with a validated HPLC assay.20
50 mm, 3 μm, Ace C18 column, Part number ACE-111-0503; Mac-Mod Analytical, Chadds Ford, PA, USA) with titanium frits at ambient temperature. Mobile phase A was 0.1% formic acid in 0.1 mM EDTA and mobile phase B was methanol. The flow rate was 0.5 mL/min with an isocratic run at 57.5% mobile phase B. Detection by tandem mass spectrometry incorporated an atmospheric pressure chemical ionization interface in positive ion mode. The range of the validated assay for raltegravir was 0.002–1.000 μg/mL. The accuracy of undiluted control samples between days was 101.5%–102.9% and the coefficient of variation was 2.1%–3.6%. Plasma concentrations of rifapentine were determined with a validated HPLC assay.20
Statistical and pharmacokinetic analysis
Pharmacokinetic parameters were calculated with non-compartmental analyses (WinNonlin, Version 5.3; Pharsight Corporation, Mountain View, CA, USA). Statistical analysis of pharmacokinetic parameters transformed to natural logarithm was performed using mixed model analysis of variance with repeated measures, a general variance–covariance matrix and adjustments for period and subject (SAS, version 9.2; SAS Institute Inc., Cary, NC, USA), and the data were transformed back to the original linear scale. Pharmacokinetic values were reported as the geometric mean adjusted for period and subject, the ratios of geometric means between different study periods, 90% CIs and coefficients of variation (%).
Drug concentrations were examined for outlier status by the Extreme Studentized Deviate test.21 All raltegravir concentrations were used in the primary analyses with one exception: an outlier value of C12 in one sample from Period 1 was 52-fold greater than the median, 3.7 SD away from the mean and identified as an outlier by statistical testing (Extreme Studentized Deviate test; P ≤
≤ 0.05). When the outlier was included in the analysis of variance, a similar result was obtained.
0.05). When the outlier was included in the analysis of variance, a similar result was obtained.
Results
Effects of rifapentine on raltegravir
Of the 21 subjects who enrolled and started the study, 16 subjects (76%) completed the 10 week study; five subjects did not complete the study during Period 2 or soon thereafter (two subjects had mild or moderate Grade 2 constitutional or gastrointestinal symptoms, one subject relocated to another city, one withdrew due to the duration of the study and one subject was withdrawn due to a rash to a non-study drug, ondansetron, during the washout after Period 2). Subjects self-reported race/ethnicity as non-Hispanic white in 11 subjects, Hispanic white in 7 subjects, African-American in 2 subjects and Asian in 1 subject. The median body weight of all the subjects was 75 kg (IQR 64–80 kg). The median dosage of raltegravir (all periods) was 5.3 mg/kg (IQR 5.0–6.2 mg/kg) every 12 h. The median dosage of rifapentine in Period 2 was 12.0 mg/kg (IQR 11.3–14.0 mg/kg) once weekly and in Period 3 was 8.0 mg/kg (IQR 7.5–9.3 mg/kg) once daily on 5 of 7 days per week.
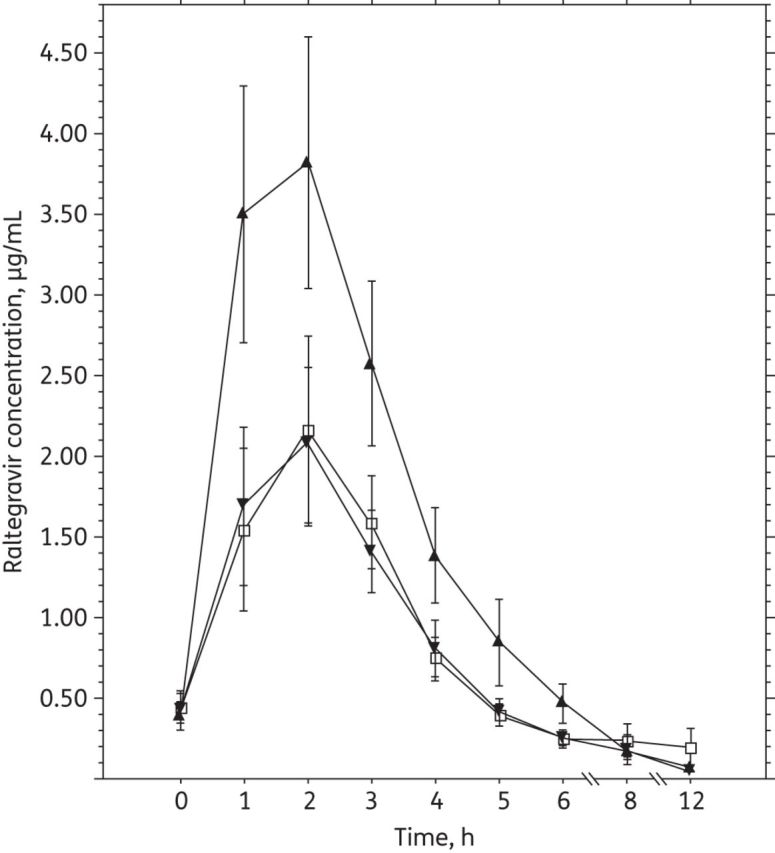
In all three study periods, the time of peak concentration of raltegravir was 2 h (Figure 2). Coadministration of raltegravir with rifapentine (900 mg once weekly; Period 2) compared with raltegravir alone resulted in the geometric mean of the raltegravir AUC0–12 being increased by 71%; Cmax increased by 89% and C12 decreased by 12% (Table 1). Coadministration of raltegravir with rifapentine (600 mg once daily for 5 of 7 days per week; Period 3) did not change the geometric mean of the raltegravir AUC0–12 or Cmax, but the geometric mean of raltegravir C12 decreased by 41% (Table 1). The raltegravir dose (not fasting and not observed) ~12 prior to the baseline plasma raltegravir concentration was taken without concomitant rifapentine. The baseline, pre-dose raltegravir concentrations [geometric mean (90% CI)] in sequential periods were 0.221 (0.123–0.396), 0.212 (0.113–0.399) and 0.233 (0.141–0.385) μg/mL. These baseline, pre-dose concentrations were significantly different from the first C12 raltegravir concentrations (all comparisons P <
< 0.005) but not significantly different from the second C12 raltegravir concentrations (Period 1, P
0.005) but not significantly different from the second C12 raltegravir concentrations (Period 1, P =
= 1.00; Period 2, P
1.00; Period 2, P =
= 0.92; Period 3, P
0.92; Period 3, P =
= 0.14) (Table 1).
0.14) (Table 1).
Table 1.
Pharmacokinetic parameters of raltegravir in healthy subjects in three study periodsa
| Parameter | Period 1 | Period 2 | Ratio of geometric means for Periods 2 : : 1 1 | Period 3 | Ratio of geometric means for Periods 3 : : 1 1 |
|---|---|---|---|---|---|
| Dosage | |||||
 Raltegravir Raltegravir | 400 mg every 12 h | 400 mg every 12 h | — | 400 mg every 12 h | — |
 Rifapentine Rifapentine | none | 900 mg once weekly | — | 600 mg daily (5 of 7 days a week) | — |
| AUC0–12 (μg·h/mL) | 5.32 (3.71, 7.62) [79] | 9.07 (6.21, 13.24) [61] | 1.71 (1.07, 2.71) [86] | 5.04 (3.24, 7.84) [72] | 0.95 (0.59, 1.53) [101] |
| Cmax (μg/mL) | 1.59 (1.00, 2.52) [105] | 3.00 (1.90, 4.75) [72] | 1.89 (1.04, 3.44) [120] | 1.62 (1.00, 2.62) [77] | 1.02 (0.60, 1.72) [103] |
| C12 (first) (μg/mL)b | 0.057 (0.037, 0.089)d [136] | 0.050 (0.038, 0.066)c [105] | 0.88 (0.62, 1.25)d [46] | 0.033 (0.254, 0.044)e [62] | 0.59 (0.34, 1.02)d [100] |
| C12 (second) (μg/mL)f | 0.222 (0.127, 0.386)c [96] | 0.137 (0.074, 0.251)e [118] | |||
AUC0–12, Cmax and C12 are all for raltegravir.
an =
= 16 subjects. Data are reported as the geometric mean (90% CI) [coefficient of variation, %].
16 subjects. Data are reported as the geometric mean (90% CI) [coefficient of variation, %].
bThe first value of C12 was determined 12 h after the seventh dose (fasting) of raltegravir, which was taken with rifapentine.
cPeriod 2: comparison of morning and evening raltegravir C12 values: P ≤
≤ 0.001.
0.001.
dn =
= 15 subjects.
15 subjects.
ePeriod 3: comparison of morning and evening raltegravir C12 values: P ≤
≤ 0.001.
0.001.
fThe second value of C12 was determined 12 h after the eighth dose (non-fasting) of raltegravir (24 h after the seventh dose of raltegravir) in Periods 2 and 3. Data for a baseline C12 obtained just prior to the administration of the seventh dose of raltegravir are described in the text.
Relation between raltegravir plasma concentration (arithmetic mean ±
± SEM) and time. Sampling was carried out after the seventh dose of raltegravir (400 mg every 12 h) in each period of the study and after the morning dose of rifapentine had been given (Periods 2 and 3). In Period 1 (open squares), raltegravir was given alone, without rifapentine. In Period 2 (filled triangles), raltegravir was given with rifapentine (900 mg once weekly). In Period 3 (filled inverted triangles), raltegravir was given with rifapentine (600 mg once daily for 5 of 7 days a week).
SEM) and time. Sampling was carried out after the seventh dose of raltegravir (400 mg every 12 h) in each period of the study and after the morning dose of rifapentine had been given (Periods 2 and 3). In Period 1 (open squares), raltegravir was given alone, without rifapentine. In Period 2 (filled triangles), raltegravir was given with rifapentine (900 mg once weekly). In Period 3 (filled inverted triangles), raltegravir was given with rifapentine (600 mg once daily for 5 of 7 days a week).
The coefficients of variation showed that there was marked variation of the raltegravir pharmacokinetic parameters AUC0–12, Cmax and C12 in each study period (Table 1). In Periods 2 and 3, the second raltegravir C12 (obtained non-fasting and without administration of rifapentine) was ≥4-fold greater than the first raltegravir C12 (fasting and with coadministration of rifapentine) (Table 1).
Rifapentine exposure (geometric mean AUC0–24) in Period 2 was 323 μg·h/mL (90% CI 277–377 μg·h/mL) and in Period 3 was 346 μg·h/mL (90% CI 292–411 μg·h/mL).
Tolerability of drugs
The administration of raltegravir with and without rifapentine was well tolerated, and no Grade 3 or 4 adverse events occurred. During the 10 week study, 16 of the 21 subjects (76%) who took the study drugs had symptoms or signs of mild or moderate adverse events (Grade 1 or 2). The Grade 1 or 2 adverse events that occurred in >1 subject were constitutional, respiratory, neurological, gastrointestinal, endocrine and dermatological (Table 2).
Table 2.
Adverse events in healthy subjects who received raltegravir and rifapentinea
| Category | Adverse event | No. of subjects |
|---|---|---|
| Constitutional | fatigue | 5 |
| fever | 2 | |
| diaphoresis | 2 | |
| generalized weakness | 2 | |
| Respiratory | rhinorrhoea | 6 |
| sneezing | 5 | |
| sore throat | 5 | |
| cough | 4 | |
| Neurological | headache (mild) | 6 |
| drowsiness | 5 | |
| vivid dreams | 2 | |
| insomnia | 2 | |
| Gastrointestinal | diarrhoea | 5 |
| nausea | 5 | |
| loss of appetite | 5 | |
| vomiting | 2 | |
| dyspepsia | 2 | |
| abdominal cramps | 2 | |
| Endocrine | hot flashes | 3 |
| Dermatological | pruritus | 3 |
| rashb | 2 |
an =
= 16 study subjects who had Grade 1 (mild) to Grade 2 (moderate) adverse events. There were no Grade 3 (severe) or Grade 4 (life-threatening) adverse events. Adverse events listed were those that occurred in >1 subject.
16 study subjects who had Grade 1 (mild) to Grade 2 (moderate) adverse events. There were no Grade 3 (severe) or Grade 4 (life-threatening) adverse events. Adverse events listed were those that occurred in >1 subject.
bIn one subject, the rash appeared herpetic.
In the five subjects who did not complete the study, three subjects had adverse events: two subjects had mild or moderate constitutional or gastrointestinal symptoms and one subject had a rash. The latter subject, who had the highest raltegravir AUC0–12 and Cmax of all subjects in Period 2, discontinued the study 9 days after taking raltegravir and rifapentine [raltegravir parameters in Periods 1 and 2: AUC0–12, 10.74 and 33.56 μg·h/mL; Cmax, 3.95 and 11.70 μg/mL; and C12 (first), 0.058 and 0.049 μg/mL]. At 2 days after completing Period 2 and during the subsequent washout period, this subject developed low-grade nausea, vomiting and diarrhoea soon after a meal at a restaurant. Although the vomiting and diarrhoea resolved within 12 h, the nausea persisted for 7 days and a physician prescribed ondansetron. Soon after the second dose of ondansetron, she developed pruritus and a macular rash that covered <50% of her body surface and resolved without sequelae.
Discussion
The present study showed a substantial drug interaction between rifapentine and raltegravir. Rifapentine given once weekly, as currently recommended for the treatment of LTBI, did not affect the C12 of raltegravir but increased the AUC0–12 and Cmax (Table 1). Therefore, when administered with once-weekly rifapentine, raltegravir reached adequate concentrations, and the moderate increase in concentrations appeared to be safe and well tolerated. Rifapentine exposure was similar to that of those patients treated for LTBI. These pharmacokinetic findings suggest that once-weekly rifapentine can be used with raltegravir without dose adjustment for the treatment of LTBI in patients who are infected with HIV.
Rifapentine given for 5 of 7 days per week as treatment for active tuberculosis disease resulted in a 41% geometric mean decrease in the C12 of raltegravir without a significant change in the AUC0–12 or Cmax (Table 1). The proper dosing strategy of daily rifapentine for the treatment of active tuberculosis is still under clinical investigation.
The enzyme uridine diphosphate glucuronosyltransferase 1A1, which metabolizes raltegravir, is likely to be up-regulated by rifapentine, analogous to its up-regulation by rifampicin.14,15 The different effects observed with the coadministration of raltegravir with once-weekly or daily rifapentine in the present study are similar to those of atorvastatin with single-dose and daily rifampicin, which lead to increased and decreased atorvastatin AUCs, respectively.22–28
Although the clinical efficacy, safety and tolerability of raltegravir were established in previous clinical trials, the pharmacodynamic properties of raltegravir were not well characterized. The mean concentration of raltegravir necessary to in vitro inhibit wild-type HIV-1 (IC95) in the presence of 50% normal human serum is 31 nM, but no in vivo threshold has been established.10,14,16,29 In the present study, the C12 of raltegravir with fasting drug administration was substantially lower than the non-fasting C12, consistent with other reports of increased C12 of raltegravir after administration with food.30 Of note, two raltegravir C12 concentrations (baseline pre-dose C12 and the second C12) resulting from raltegravir doses taken under similar conditions (a non-fasting state in the evening and without concomitant rifapentine administration) were not significantly different. Diurnal variation also may affect C12, with morning concentrations >5-fold higher than the C12 in the evening.31
Although a fixed-sequence of periods was used in this pharmacokinetic study, it was not a design limitation. Rifapentine exposures in Period 3 were not affected by prior rifapentine use because rifapentine autoinduction with the regimen in Period 2 was not demonstrated in another pharmacokinetic study of 157 adults and children treated for LTBI who had weekly rifapentine exposures similar to those found in Period 2 of this study.32 Rifapentine has not been evaluated with regard to the number of days needed for rifapentine to reach a maximal induction effect on raltegravir. However, the closely related drug rifampicin has potent induction effects for many transporters and phase 2 enzymes. After 1 week of rifampicin administration, full induction with probe drug is generally reached and the induction effect resolves by 2 weeks after discontinuing rifampicin.33,34 Therefore, in this study, no effect on raltegravir pharmacokinetics in Period 3 sampling would be anticipated 24 days after Period 2 (because of a 10 day washout period and 10 daily doses of rifapentine given over 14 days).
The C12 concentration typically associated with a virological response for other antiretroviral drugs, such as protease inhibitors, may help to guide dosing with raltegravir,35,36 but the virological response data with raltegravir are unclear. In a Phase 3 clinical trial of raltegravir given once daily or twice daily, the shape of the plasma concentration–time curve was suggested as important for determining long-term efficacy outcomes. With twice-daily dosing, there was no significant pharmacokinetic or pharmacodynamic association of efficacy outcomes with the C12 and AUC0–12.29 The common occurrence of secondary peaks of raltegravir concentration, both in the present study and in previous studies, further complicates the relation between C12, Cmax and AUC0–12.29 A limitation in establishing pharmacokinetic and pharmacodynamic efficacy parameters associated with raltegravir may be the large variation in drug concentrations within and between individual subjects.37,38 The large variation in C12 between patients suggests that monitoring the AUC0–12 of raltegravir using a limited sampling strategy of three or four timepoints may be more useful than monitoring C12.39 A limitation of this study was that the participants were healthy volunteers. However, an evaluation of healthy volunteers is common practice for studies of drug interactions.
The great utility of this pharmacokinetic study is the demonstration that the rifapentine regimen used in Period 2, effective in HIV-uninfected patients for the treatment of LTBI, may potentially also be used in HIV-infected patients taking raltegravir-based antiretroviral therapy. The rifapentine once-weekly doses in Period 2 were the same as recommended for LTBI in adults. Isoniazid is not reported to have an effect on the enzyme that metabolizes raltegravir.40 Finally, the 12 dose, once-weekly rifapentine and isoniazid regimen in 393 HIV-infected patients not receiving antiretroviral therapy was better tolerated and had a higher frequency of treatment completion than 9 months of isoniazid for treatment of LTBI.6
In summary, the present study showed a drug interaction between rifapentine and raltegravir that varied with the frequency of rifapentine dosing. Once-weekly rifapentine administration did not substantially affect the mean C12 of raltegravir but increased the AUC0–12 and Cmax of raltegravir. The increased raltegravir exposure observed with once-weekly rifapentine was safe and tolerable. Therefore, further clinical investigation is warranted to characterize the virological response to raltegravir-based antiretroviral therapy that includes rifapentine once weekly to treat patients infected with HIV and LTBI.
Funding
This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc. Support was also provided by the Veterans Administration, United States Centers for Disease Control and Prevention (Tuberculosis Trials Consortium), and National Center for Advancing Translational Sciences, through Grant 8UL1TR000149.
Disclaimer
The opinions expressed in this manuscript are those of the authors and do not reflect the official view of any of the supporting agencies.
Acknowledgements
We are grateful to Drs Andrew Vernon, Susan Dorman and Philip LoBue for reviewing the manuscript prior to submission and Dr Elly Trepman for medical editing.
References
Articles from Journal of Antimicrobial Chemotherapy are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/jac/dkt483
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jac/article-pdf/69/4/1079/2084030/dkt483.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/jac/dkt483
Article citations
Therapeutic drug monitoring in tuberculosis.
Eur J Clin Pharmacol, 80(11):1659-1684, 06 Sep 2024
Cited by: 0 articles | PMID: 39240337
Review
Pharmacokinetics, Safety, and Tolerability of Once-Daily Darunavir With Cobicistat and Weekly Isoniazid/Rifapentine.
J Acquir Immune Defic Syndr, 94(5):468-473, 01 Dec 2023
Cited by: 0 articles | PMID: 37955446
Pharmacokinetics of Antiretroviral Drugs in Older People Living with HIV: A Systematic Review.
Clin Pharmacokinet, 62(9):1219-1230, 10 Aug 2023
Cited by: 1 article | PMID: 37561283
Review
Evaluation of drug-drug interaction between rilpivirine and rifapentine using PBPK modelling.
Front Pharmacol, 13:1076266, 15 Dec 2022
Cited by: 0 articles | PMID: 36588698 | PMCID: PMC9797969
Care cascade of tuberculosis infection treatment for people living with HIV in the era of antiretroviral therapy scale-up.
Sci Rep, 12(1):16136, 27 Sep 2022
Cited by: 0 articles | PMID: 36167744 | PMCID: PMC9515204
Go to all (35) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT00809718
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of intermittent rifampicin on the pharmacokinetics and safety of raltegravir.
J Antimicrob Chemother, 70(2):550-554, 26 Sep 2014
Cited by: 4 articles | PMID: 25261424
Minimal pharmacokinetic interaction between the human immunodeficiency virus nonnucleoside reverse transcriptase inhibitor etravirine and the integrase inhibitor raltegravir in healthy subjects.
Antimicrob Agents Chemother, 52(12):4228-4232, 06 Oct 2008
Cited by: 58 articles | PMID: 18838586 | PMCID: PMC2592888
Pharmacokinetic Drug-Drug Interaction Study Between Raltegravir and Atorvastatin 20 mg in Healthy Volunteers.
J Acquir Immune Defic Syndr, 69(1):44-51, 01 May 2015
Cited by: 5 articles | PMID: 25942458
Rifampin vs. rifapentine: what is the preferred rifamycin for tuberculosis?
Expert Rev Clin Pharmacol, 10(10):1027-1036, 18 Aug 2017
Cited by: 27 articles | PMID: 28803492
Review
Funding
Funders who supported this work.
NCATS NIH HHS (4)
Grant ID: KL2 TR001118
Grant ID: 8UL1TR000149
Grant ID: UL1 TR000149
Grant ID: UL1 TR001120





