Abstract
Aim
We evaluated the capability of "microRNA sponges" in sequestering and inhibiting the over-expressed miR-221 in HCC cell lines.Background
Advanced hepatocellular carcinoma (HCC) is a serious public health problem, with no effective cure at present. It has been demonstrated that the deregulation of microRNAs expression contributes to tumorigenesis. In HCC, miR-221 was shown to be up-regulated in more than 70% of the cases and was associated with higher tumor stage, metastasis and a shorter time to recurrence after surgery, suggesting an important pathogenic role. A tumor promoting function of miR-221 was proved in a transgenic mouse model, which was predisposed to the development of liver cancers. These findings suggested that miR-221 could represent a potential target for anti-tumor approaches.Material and methods
Novel adeno and adeno-associated viral vectors (AAVs) were developed: they were genetically modified to drive the expression of multiple binding sites for miR-221, the "miR-221 sponge", which was designed to sequester miR-221 cellular molecules.Results
Analysis of viral vectors activity in HCC cells revealed their capability to reduce miR-221 endogenous levels, which was accompanied by the increase in CDKN1B/ p27 protein, a known target of miR-221. An increase in apoptosis was also measured in Hep3B cells after infection with any of the two viral vectors in comparison with control vectors, with stronger effects induced by adenovirus compared to AAV vectors.Conclusion
The depletion of oncogenic microRNAs represents a potential anti-cancer approach that needs to be tested for safety and efficacy. Here, we describe the development of novel "miR-221 sponge" vectors, which can reduce miR-221 activity in vitro and may be used for in vivo delivery.Free full text

Inhibiting the oncogenic mir-221 by microRNA sponge: toward microRNA-based therapeutics for hepatocellular carcinoma
Abstract
Aim
We evaluated the capability of “microRNA sponges” in sequestering and inhibiting the over-expressed miR-221 in HCC cell lines.
Background
Advanced hepatocellular carcinoma (HCC) is a serious public health problem, with no effective cure at present. It has been demonstrated that the deregulation of microRNAs expression contributes to tumorigenesis. In HCC, miR-221 was shown to be up-regulated in more than 70% of the cases and was associated with higher tumor stage, metastasis and a shorter time to recurrence after surgery, suggesting an important pathogenic role. A tumor promoting function of miR-221 was proved in a transgenic mouse model, which was predisposed to the development of liver cancers. These findings suggested that miR-221 could represent a potential target for anti-tumor approaches.
Material and Methods
Novel adeno and adeno-associated viral vectors (AAVs) were developed: they were genetically modified to drive the expression of multiple binding sites for miR-221, the “miR-221 sponge”, which was designed to sequester miR-221 cellular molecules.
Results
Analysis of viral vectors activity in HCC cells revealed their capability to reduce miR-221 endogenous levels, which was accompanied by the increase in CDKN1B/ p27 protein, a known target of miR-221. An increase in apoptosis was also measured in Hep3B cells after infection with any of the two viral vectors in comparison with control vectors, with stronger effects induced by adenovirus compared to AAV vectors.
Conclusion
The depletion of oncogenic microRNAs represents a potential anti-cancer approach that needs to be tested for safety and efficacy. Here, we describe the development of novel “miR-221 sponge” vectors, which can reduce miR-221 activity in vitro and may be used for in vivo delivery.
Introduction
Primary liver cancers include hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and hepatic angiosarcoma (1). HCC accounts for 85-90% of primary liver cancers; it is the fifth most common malignancy worldwide and the third most common cause of cancer-related mortality (2). Development and progression of HCC is a multistage process that includes the deregulation of critical genes involved in cell cycle control, apoptosis and cell migration. MicroRNAs (miRNAs) are among the molecules that regulate these events (3). miRNAs are a highly conserved group of endogenous small non-coding RNAs, which can be expressed in a tissue specific manner (4). Many experimental and clinical studies revealed the aberrant expression of miRNAs is associated with initiation, progression and metastasis of cancers (5, 6). Numerous reports have demonstrated alterations in intracellular miRNAs expression in liver diseases (7–9). Among miRNAs deregulated in HCC, miR-221 is of particular interest (6, 10–12). miR-221 over-expression inhibits apoptosis of hepatocytes and delays fulminant liver failure (13); miR-221 can modulate the proliferation of HCC cells by regulating the expression of cyclin-dependent kinase inhibitor 1B (CDKN1B/p27) and 1C (CDKN1C/p57) (8). It has also been shown that miR-221 accelerates hepatocyte proliferation during liver regeneration (14). miR-221 stimulates the onset of tumors and promotes tumor progression, significantly shortening the median time to death in a mouse liver cancer model (10). Over-expression of miR-221 in a transgenic mouse model induced a strong predisposition to the development of liver cancers, whose number and size could be significantly reduced by the delivery of anti-miR-221 oligonucleotides (15). Available evidences indicate miR-221 is a potential target for non-conventional treatments against HCC (16). For miRNA inhibition, miRNA antisense oligonucleotides and miRNA sponges have been described (17). Antisense oligonucleotides provide a transient inhibition of microRNA. Vector-encoded miRNA sponges are instead expected to inhibit miRNA activity over a longer period of time. MiRNA sponges are competitive inhibitors that contain multiple miRNA antisense binding sites (MBS) able to sequester miRNAs and saturate the cellular pool of microRNA (17–19). Ebert et al. proved the expression of a miRNA sponge at high levels could inhibit the activity of a family of miRNAs sharing a common seed (20). Viral vectors could enforce miRNA sponge-based therapy by forcing a high prolonged expression of the sponge and by providing an efficient way for in vivo delivery (3, 16). This approach has been successfully used to inhibit miR-223 using a lentiviral vector (21). Therefore, we considered this approach to sequester and inhibit miR-221. For achieving prolonged inhibition of miR-221, we developed two different recombinant viral vectors, one based on adenovirus (rAd) and another based on adeno-associated virus (rAAV) and tested their ability to repress miR-221 in HCC cells.
The aim of this study is construction of miR-221 decoy targets based on sponge technology. Our optimized sponges provided precious tools for studying the function of miRNA in vivo as well as for addressing the needs for novel and unconventional therapeutic approaches against HCC.
Material and Methods
Designing of miR-221sponge
The sponge oligonucleotide and the reverse complement were synthesized at IDT (Coralville, Iowa, USA). The sequences were as following: (1): 5′-CTA GAc ccG AAA CCC AGC ATT GGA TGT AGC Tcc cGA AAC CCA GCA ATT AAT GTA GCT ccc GAA ACC CAG CAG AGA ATG TAG CTc ccG AAA CCC AGC AGT TCA TGT AGC Tcc cT-3′ and (2): 5′-CTA Gag ggA GCT ACA TGA ACT GCT GGG TTT CGg gAG CTA CAT TCT CTG CTG GGT TTC ggg AGC TAC ATT AAT TGC TGG GTT TCg ggA GCT ACA TCC AAT GCT GGG TTT Cgg gT-3′ for miR-221 target and complementary oligos, respectively. The two oligonucleotides were annealed to make double stranded DNA fragment with XbaI overhangs. Polynucleotide Kinase (PNK) (Roche Applied Science, Indianapolis, USA) was used to add a phosphate to the 5’ end of a DNA fragment. We cloned it into pGL3-control vector (Promega, Madison, WI, USA) using XbaI site immediately downstream of the firefly luciferase reporter gene.
Development of recombinant adenoviruses
To produce the adenoviral vector, we employed the adenoviral backbone from the replication-competent adenovirus vector Ad-199T (22). We sub-cloned a 2,181bp fragment containing the SV40-Luciferase-miR-221sponge fragment originated from the engineered pGL3-control vector, described above into pAd-199T. pAd-Control vector without miR-221sponge sequence was also developed. The viral progeny were packaged with the use of 293FT cells and purified using PEG-it Virus Precipitation Solution (System Biosciences, Mountain View, CA), according to manufacturer's instructions. To determine the titer of rAd-199T-miR-221sponge and rAd-199T control viruses, 7.5×104 HepG2 cells were seeded in a 24 well plate; after 24 hours cells were infected with 1 microliter of either viruses. One day after infection, cells were harvested and total genomic DNA purified by QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. 50ng of genomic DNA was analyzed by quantitative Real Time PCR, using EVA green (Biotium.Inc, Hayward, CA, USA) and primers specific for wild type Ad5 sequences: wtAd5Fwd, 5′- CGC ATA CGA GCA GAC GGT GAA C-3′; wtAd5Rev, 5′-GCA CTA TAA GGA ACA GCT GCG CC-3′. PCR was performed by initial denaturation at 95°C for 15min followed by 40 cycles of 95°C 30 sec, 58°C 30 sec and 72°C 30 sec. A standard curve was generated by using serial dilutions of the plasmid pAd-Control DNA (from 10ng to 100fg) spiked in 50ng of HepG2 non infected genomic DNA. The number of viral infectious units (I.U.) was determined comparing the threshold cycle (Ct) values of each sample with those of standard samples. Fluorescence was measured by using a Bio-Rad light Cycler Chromo 4 thermal cycler.
Assessment of adenoviral replication
Genomic DNA from infected cells was extracted with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. Real Time PCR using EVA green (Biotium.Inc., Hayward, CA, USA) and primers wtAd5Fwd and wtAd5Rev specific for wild type Ad5 were utilized to assess viral replication in DNA content. Fluorescence was measured by using a Biorad-Chromo4 thermal cycler real time PCR instrument. Human β-actin gene was used to normalize data. Primers were as follows: Hβ-actinDNA2810Fwd, 5′-AGC TGT CAC ATC CAG GGT CC-3′; Hβ-actinDNA2960Rev, 5′-TCA TAC TCC TGC TTG CTG ATC C-3′.
Development of recombinant adeno-associated virus
The AAV-DJ Helper Free Bicistronic Expression System kit, which include the pAAV-IRES-GFP expression vector, the pAAV-DJ vector carrying the replication and capsid formation genes and the pHelper vector harboring adenovirus genes needed for production of AAV viral particle were purchased from Cell Biolabs, Inc. (San Diego, CA, 92126, USA). The cassette of miR-221sponge was cloned upstream of IRES-GFP sequence into pAAV-IRES-GFP expression vector using XbaI sites. The infectious rAAV was generated by a 1:1:1 (molar ratio) triple-transfection of pAAV-miR-221sponge-IRES-GFP with helper plasmids (pAAV-DJ and pHelper) into 293FT cells a commonly used AAV production cells, contains the E1A/E1B genes. A total of 30µg of plasmid DNA was considered per each 175 cm2 cell culture flask. Following incubation for 72 hours, the 293FT cells were harvested and subjected for four rounds of freezing thawing by placing it alternately in a dry ice/ethanol and a water bath of 37°C to prepare rAAV crude lysates. The lysates were centrifuged at 10,000 g for 10 min to remove 293FT cell debris. rAAV was purified using ViraBind™ AAV purification kits (Cell Biolabs, San Diego, CA, 92126, USA). Titration of genomic particles of virus were carried out in Bio-Rad light cycler chromo 4 by absolute quantitative real-time PCR approach using EVA green (Biotium,Inc, Hayward, CA, USA). The virus crude was supplemented with 10U rDNase1 and nuclease reaction buffer (Promega, Madison, WI, USA), followed by an inactivation step according to manufacturer's instruction. 1µl of virus stock and 10 to 1000 serial diluted samples were analyzed, using primers specific for the GFP gene: GFP_ 1318_Fwd, 5’-ACG TAA ACG GCC ACA AGT TC-3’; and GFP_1584_ Rev, 5’-AAG TCG TGC TGC TTC ATG TG-3’. PCR was performed by initial denaturation at 95°C for 15 min followed by 35 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec. A standard curve was generated using serial dilutions of the pAAV-IRES-GFP (from 10ng to 100fg). Genomic particles per ml were calculated as: [a*b/c]*1000 in which a: number of molecule b: medium of concentration of DNA (pg) for technical triplicate and c: molecular weight of single strand viral genome (pg).
Cell culture, transfection and virus infection
The hepatocellular carcinoma cell lines HepG2 (ATCC HB-8065) and Hep3B (ATCC HB-8064) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The human embryonic kidney cell, 293FT was obtained from Invitrogen (Carlsbad, CA, USA). Stable clone of HepG2 expressing miR199a (22) was gifted. Cell lines were cultured in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 10% fetal bovine serum (FBS), 0.1% gentamycin and 1% L-glutamine (Sigma, St Louis, MO). Cells were maintained in a humidified incubator at 37°C and 5% CO2. Lipofectamine 2000 and lipofectamine LTX (Invitrogen, Carlsbad, CA, USA) were utilized in transient transfection and rAAV production steps respectively, according to the manufacturer's instructions. Cell lines were transduced with, rAd-V5 or rAAV-DJ by applying the viral particles into the culture media.
Dual Luciferase Reporter Assay
293FT cells were seeded at density of 7.5×104 cells/well in 24 well plate. 200ng of pAAV.miR-221 sponge vector or pAAV.control, co-trasfected along with 200ng of firefly luciferase construct: pGL3-3'UTRp27 (8). The pRL-TK (Promega, Madison, WI, USA) was also transfected as a normalization control. Cells were collected 24 hours after transfection and luciferase activity was measured using a Dual Luciferase Reporter Assay kit (Promega, Madison, WI, USA) following the manufacturer′s protocol. Data were represented as mean values from three replicate and shown as percentage activity by setting the firefly luciferase of control transfected sample to 100%.
Quantitative Real Time PCR (qPCR) of microRNA
The mature miRNA was quantified by quantitative PCR (qPCR) using the TaqMan microRNA Assays (Applied Biosystems, Grand Island, NY, USA). Total RNA was purified using TRIzol®Reagent (Ambion®) (Invitrogen, Carlsbad, CA, USA). Reverse transcription reaction was done starting from 5ng of total RNA. qPCR (10mL reaction) was carried out in triplicate using a 1:50 dilution of cDNA using the standard TaqMan MicroRNA Assay protocol. The reference gene was U6 snRNA (RNU6B, Applied Biosystems, Grand Island, NY, USA). The relative expression levels of miRNAs were calculated using the comparative ΔΔCt method. The fold changes in miRNAs were calculated by the equation 2-ΔΔCt. The custom primers and probes were supplied in TaqMan® MicroRNA Assays kit (Applied Biosystems, Grand Island, NY, USA) and used according to manufacturer's protocol.
Western Blot analysis
7.5×104 HepG2 cells were infected by rAd-199T-miR-221 sponge or corresponding control virus (MOI:10) and rAAV-miR-221 sponge or rAAV-control (MOI:100) in 24 well plate. Cells harvested at 72 h post infection and lysed by using RIPA lysis buffer (150mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) (Sigma, St Louis, MO) with complete protease inhibitor cocktails (Sigma, St Louis, MO). Homogenates were then centrifuged at 13000 rpm for 15 min at 4°C and supernatants were collected and analyzed by western blotting. Protein concentration was measured using Bradford reagent and the BSA protein. 30 micrograms of total extracted protein were loaded on a 4-15% Mini-PROTEAN® TGX™ precast gels (Bio-Rad Laboratories, Hercules, CA94547). Blotting was performed for CDKN1B/ p27 (BD Transduction Laboratories™, 610242, dilution 1:1500). Beta-actin (Sigma Aldrich, A4700, dilution 1:1000) was used for normalization purposes. For recognition of the primary antibodies, anti-mouse IgG (whole molecule)–peroxidase antibody produced in rabbit (Sigma Aldrich, A9044 dilution 1:10000) were used. Protein bands were visualized using the ChemiDocTMMP Imaging System and Quatified using image lab 4.0 software (Bio-Rad Laboratories,Hercules,CA94547).
Analysis of Cell Viability and Apoptosis
Hep3B cells (7.5×104) were further selected to confirm the effect of arresting of miR-221 by sponge on apoptosis and viability, using Muse Annexin V and Dead Cell Assay Kit (EMD Millipore, Billerica, MA, USA) in Muse® Cell Analyzer: Mini, Affordable Flow Cytometry. The procedure was performed according to the manufacturer's manual. This assay allows to identify percentage of viable cells [Annexin V-PE (–) and Dead Cell Marker (–)] early apoptotic cells [Annexin V-PE (+) and Dead Cell Marker (–)], late apoptosis [Annexin V-PE (+) and Dead Cell Marker (+)] and already dead [Annexin V-PE (–) and Dead Cell Marker (+)]. The experiment was done in triplicate for each infected well.
Statistical analysis
Significance was determined with the two-tailed student's t-test. A p-value threshold <0.05 was considered significant. Results were representative of three independent experiments. Values were presented as the mean ± standard deviation (SD).
Results
Designing and Construction of miR-221 sponge
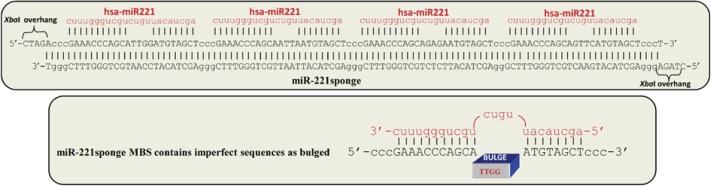
For this study the sequence of mature miR-221 (hsa-miR-221 MIMAT0000278) was considered for designing an anti-miR-221. A sponge oligonucleotide contained 4 copies of the miR-221 binding sites and 4 nucleotide spacers. Each MBS included a bulged site (Figure 1), as imperfect miRNA “bulged sponges” were reported to be more effective for the sequestration of miRNAs than sponges with perfect antisense sequence (23). The number of binding sites is also important for the effectiveness of sponge. More binding sites increase the likelihood of reaching maximal miRNA sequestration, but it may also increase the chance of sponge transcript degradation. Typical sponge constructs contain 4 to 10 microRNA binding sites separated by a few nucleotides each (23). The synthetic sponge oligos were assembled to make double-stranded DNA duplexes. The annealed sponge was 113 bp long. The sequence of endogenous miR-221 and designed sponge is depicted in Figure 1.
Functionality of miR-221 sponge
To evaluate the suppressive activity of the sponge construct in vitro, we asked whether the sequestration of miR-221 by the sponge products could disrupt the binding of miRNA to target sites in 3’-UTR of a target mRNA. Thus, we performed a dual luciferase assay using the luciferase reporter pGL3-3'UTRp27, a vector in which the 3’-UTR of CDKN1B/p27 mRNA, a known target of miR-221, was cloned downstream of the firefly luciferase gene (8). The renilla luciferase reporter vector pRL-TK was used as normalizer. These plasmids were co-transfected along with vector harboring miR-221sponge and the control vector into 293FT cells. A scheme of the experiment is depicted in Figure 2A. We assumed that binding of the sponge to endogenous miR-221 could rescue the target site in 3'UTR of pGL3-3'UTRp27 vector and lead to an increased level of firefly luciferase activity. Firefly and renilla luciferase expression were measured 24 hours post-transfection and their ratio was used to calculate the relative luciferase activity. The ratio of control vector without sponge sequence was set at 100%. Results showed that sponge construct were highly effective and revealed an increase of relative Luc expression by more than 2 times (Figure 2B).
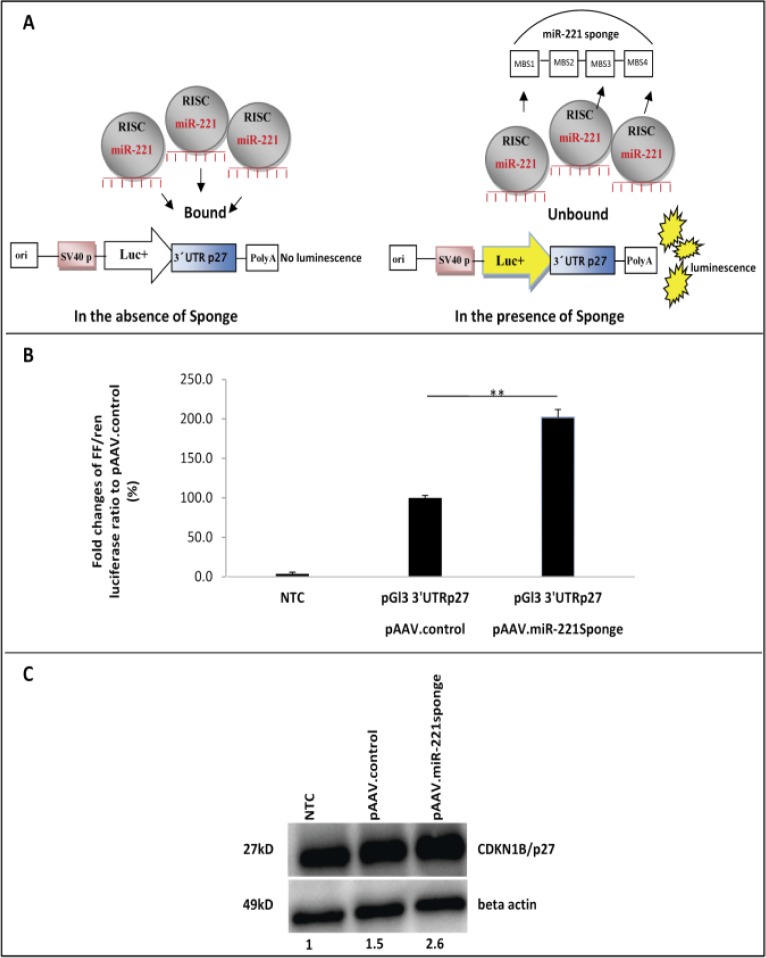
Inhibition of endogenous miR-221 by sponge construct in vitro. (A). A scheme of luciferase reporter assay that shows function of miR-221sponge. In the lack of sponge inhibitor, miRNA such as miR-221, loaded in RNA induced silencing complexes (RISCs), will bind to their endogenous targets and silence target genes and subsequently luciferase gene. However, in the presence of sponge transcripts, miR-221 will bind to the exogenous binding sites and be unavailable for silencing endogenous targets (3'UTR27), resulted in raising the luciferase expression. (B) Duel luciferase reporter assay. The control-GFP construct lack of sponge sequence was used as a control means 100% FF/renilla luc expression in the case of co-transfection with pGl3-3'UTRp27. miR-221sponges construct was able to relieve 3'UTRp27 via blocking by endogenous miR-221, as detected by increased luciferase activity around 200%. n=3, **p=0.001. (C) Western blot analysis of p27 target protein and beta actin. Transfection of 293FT cells by sponge expressing vector (pAAV.miR-221sponge) revealed 2.6 and 1.73 fold increase in p27 protein level compared to untransfected control (NTC) and control construct without sponge (pAAV.control), respectively.
Next we used western blot analysis to determine protein levels of a validated miR-221 target gene upon its inhibition by sponge. We transfected 293FT cells with pAAV. miR-221sponge or with pAAV.control. We observed 2.6 and 1.73 fold increase in CDKN1B/p27 protein level in comparison with non-transfected control and pAAV.control transfected cells, respectively (Figure 2C). In summary, these results indicated that miR-221sponge could be used to sequester miRNA activity on target genes.
Recombinant Adenovirus Express Functional miR-221 sponge
We hypothesized the incorporation of miR-221 sponge into Ad-199T (22) could provide the capability of inhibiting miR-221 specifically in HCC cells. Details of vectors construction are described in materials and methods section of this paper and depicted in Figure 3A. To verify whether engineered adenovirus was able to express a functional miR-221 sponge, we infected HepG2 cells with rAd-199T-miR-221 sponge and rAd-199T control at MOI=10. Real Time TaqMan assay revealed that sponge decrease the level of miR-221 up to 75.5% and 55% relative to uninfected and control virus infected samples, respectively (Figure 3B). To assess if the miR-221 sponge expressed by rAd could modulate protein level of miR-221 target, we assayed CDKN1B/ p27 levels by western blot analysis. Following 72 hours of infection, increasing in the CDKN1B/p27 protein levels by ~8.5 fold was detected compared to uninfected and rAd control infected samples (Figure 3C).
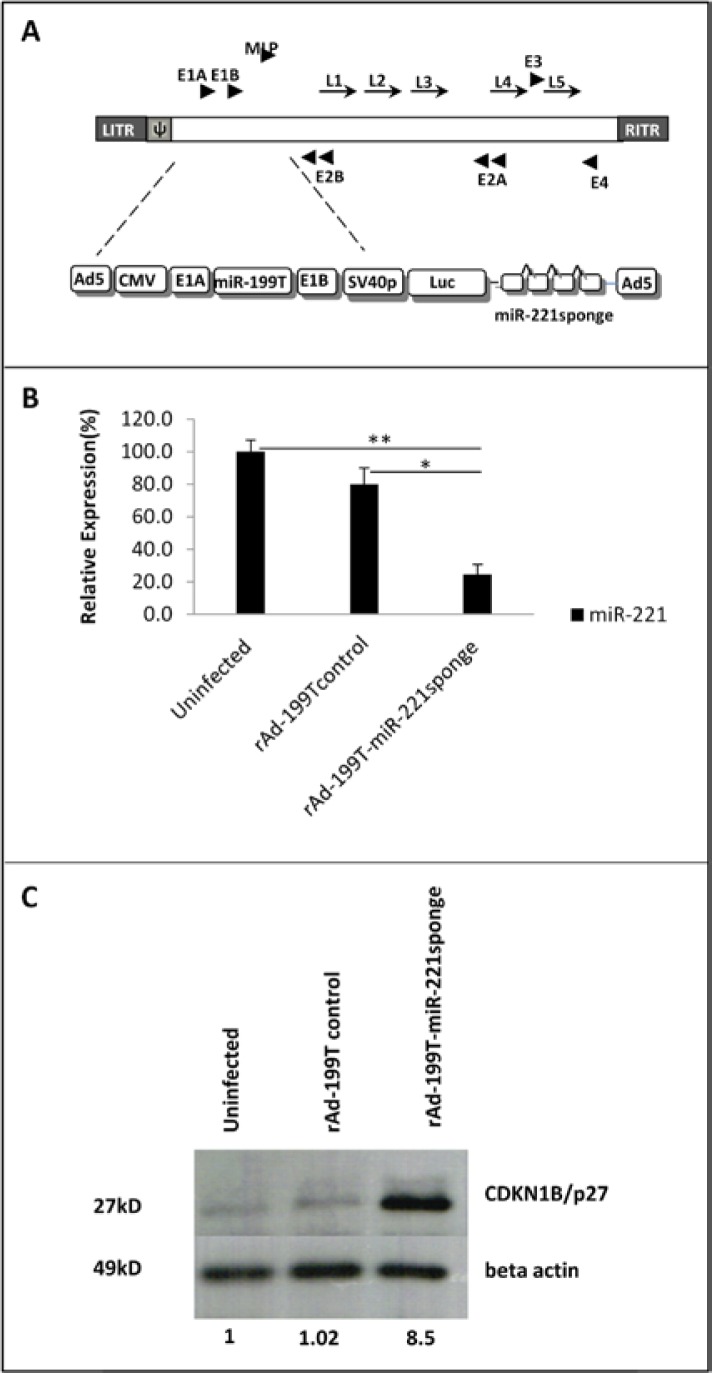
Functionality of oncolytic recombinant adenovirus expressed microRNA sponge. (A) Scheme of the Ad-199T-miR-221 sponge adenoviral vector, showing the location of the critical elements in the context of the adenoviral vector genome: CMV= CMV promoter; E1A= adenoviral E1A gene; miR-199T= target site for miR-199a; E1B= adenoviral E1B gene; SV40p= SV40 early promoter; Luc= firefly luciferase gene; miR-221sponge. (B) Quantitative RT-PCR detection of miR-221 at 24 hours following infection of HepG2 cells with rAd-199T-miR-221sponge or rAd-199T-control, at MOI=10. Data are represented as mean values ± SD from three replicates. The results showed that miR-221 expression was inhibited 75.5% and 55% compared to uninfected (**p=0.002) or control virus infected (*p =0.001) samples, respectively. (C) Western blot analysis of CDKN1B/p27 protein, confirmed an increase in target protein around 8.5 fold in comparison with uninfected or rAd-199T control virus in HepG2 cells. Beta actin protein was employed as reference normalizes.
As designed (22), this adenoviral vector is expected to replicate only in the absence of miR-199, like in HCC cells. To verify that miR-199 could indeed regulate viral replication of rAd-199T-miR-221 sponge, the viral vector was used to infect cells of two different cell lines: HepG2, which do not express miR-199; HepG2/199, a derivative of HepG2 cells engineered to constitutively express miR-199a (22). To this purpose, 7.5×104 cells of each cell line were seeded and infected with rAd-199T-miR-221 sponge or control virus at MOI=10. The cells were harvested at various time points. The results shown in supplementary Figure 1 established that viral replication of rAd-199T-miR-221sponge was indeed miR-199-dependent.
Development of Recombinant Adeno-Associated Virus for Expression and Delivery of miR-221 sponge
The development of self-complementary AAV (scAAV) vectors and the availability of multiple AAV serotypes for improved transduction of specific target tissues has expanded the capabilities of this virus for gene delivery in therapeutic applications (24). We chose the DJ serotype of AAV with higher infection efficiency particularly into hepatocytes in comparison with any other wild type serotype (25). The pAAV-miR-221 sponge-IRES-GFP and pAAV-IRES-GFP vectors were packaged according to the helper-free method. Figure 4A showed the final genome packaged as rAAV-miR-221 sponge. We removed unpackaged and defective packaged genome through DNase I treatment. As illustrated by green fluorescent protein detection, the produced rAAV particles could efficiently infect HeLa cells in vitro (Supplementary Figure 2). Then, the ability of rAAV-miR-221 sponge to knockdown endogenous miR-221 was assessed in HepG2 cells. For in vitro assay rAAVs were used to transduce cells at MOI:100. Uninfected and rAAV-control infected cells were used as controls. A TaqMan microRNA assay was employed to quantify miR-221 inhibition. As shown in Figure 4B, sponge against miR-221 reduced expression of endogenous miR-221 by 45% and 42% in comparison with uninfected and control virus infected cells respectively.
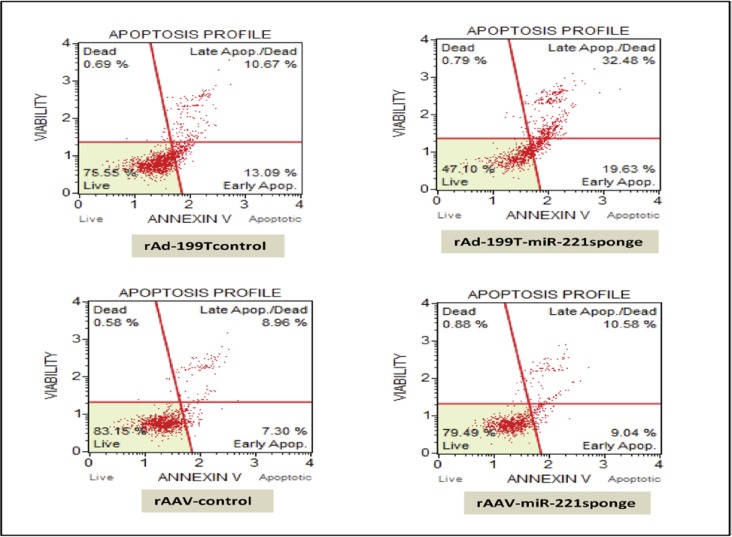
Effect of miR-221 sponge on apoptosis of HCC HepB3 cells by mini, affordable flow cytometry. HepB3 cells were infected by rAd-199T-miR-221 sponge at MOI = 10 and collected after 24 hrs, or rAAV-miR-221 sponge, at MOI = 100 and collected after 72 hrs. Control viruses were employed for comparison. Harvested cells were assayed for Annexin V and death markers to reveal the percentage of cells in early and late apoptotic as well as viable cells. A strong apoptotic effect was produced by rAd-199T-miR-221 sponge in comparison with rAd-199T control and a little increase in apoptosis was also induced by rAAV-miR-221sponge in comparison with rAAV control.
Furthermore, the protein level of CDKN1B/p27 exhibited a 2.2 fold up-regulation respect to uninfected cells. Also 1.46 fold increase was detected compared to rAAV-control (Figure 4C). These data demonstrated rAAV provides an effective means to deliver a functional miR-221 sponge.
miR-221sponge induces cell apoptosis and reduce viability in HCC cells
To verify whether miR-221sponge is able to influence apoptosis and cell viability, we transduced hepatocellular carcinoma Hep3B cell line, which are characterized by high level of miR-221 expression with rAd-199T-miR-221 sponge at MOI:10 as well as rAAV-miR-221 sponge at MOI:100. Comparable control rAd and rAAV infected cells were considered. We investigated the effect of miR-221 inhibition with 7-Amino-actinomycin D (7-AAD)/PE Annexin V staining.
The results showed a highly increase in late apoptosis along with reducing the number of viable cells in rAd-199T-miR-221 sponge infected cells compared to control virus infection condition. On the other hand, a slightly increase in both early and late apoptosis consistent with decrease in number of viable cells were achieved for rAAV-miR-221 sponge respect to rAAV-control infected cells. In this case no increase in number of dead cells was detected (Figure 5).
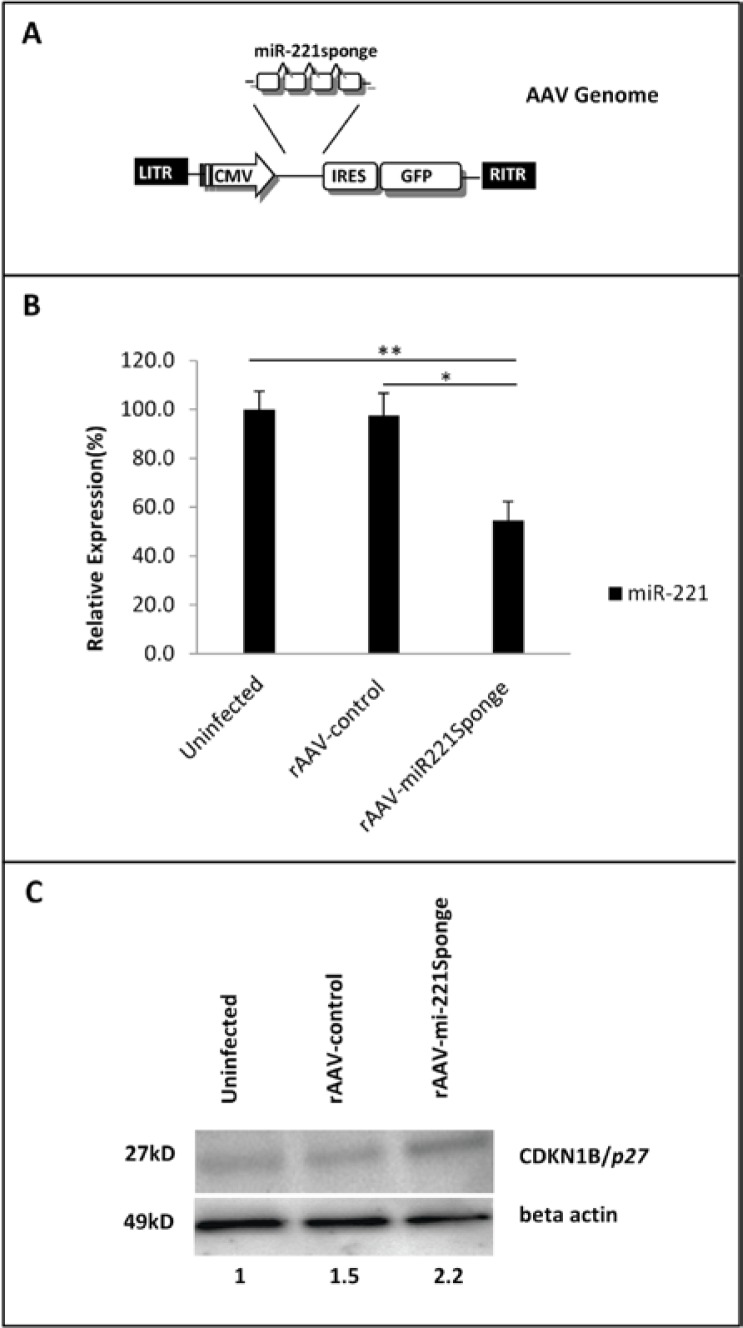
Molecular assay of rAAV-sponge221. (A) Scheme of the rAAV-miR-221sponge viralvector, showing the location of the critical elements in the context of the adeno-associated viral genome: the miR-221-sponge element was inserted upstream the IRES-GFP elements of the expression vector of AAV-DJ Bicistronic Expression System (IRES-GFP) (Cell Biolabs, San Diego, CA, USA). (B) Quantitative RT-PCR detection of miR-221 at 72 hours post infection of HepG2 cells with rAAV-miR-221sponge or rAAV-control, at MOI=100. Data are represented as mean values + SD from three replicates. rAAV-miR-221sponge induced a 45% and 42% reduction in miR-221 expression level in comparison with uninfected (**p= 0.01) or rAAV-control infected cells (*p=0.02), respectively. (C) Western blot analysis of CDKN1B/p27 protein in cells infected with rAAV-miR-221sponge vector caused a 2.2 and 1.46 fold raise in CDKN1B/p27 protein level in comparison with uninfected and rAAV-control, respectively. Beta actin protein was employed as reference normalizes.
Discussion
In the present study, we demonstrated the possibility to repress miR-221, one of the most consistently over-expressed miRNA in HCC, using a microRNA sponge approach. Therapeutic modulation of a single miRNA may simultaneously affect many pathways, hence targeting aberrantly expressed miRNAs represents a logical and attractive anti-cancer opportunity (18). Sponges may offer some advantages over chemically modified miRNA oligonucleotide inhibitors. First, the antisense oligonucleotides appear to be specific for one miRNA, while sponge have been shown to inhibit the activity of a family of miRNAs sharing a common seed (20). In addition, the risk of off-target gene silencing is likely to be lower with miRNA sponge than with synthetic oligonucleotide inhibitors (26). The viral vectors developed in the course of this study included tandemly arrayed binding sites for the mature miR-221, the “miR-221sponge”, that were inserted next to a reporter gene under the control of a strong promoter. The binding sites for miR-221 were designed to contain a bulge (i.e., nucleotide mismatches adjacent to the seed region, in order to prevent cleavage degradation of the sponge RNA) (20, 23) and strengthen the stable binding and sequestration of the targeted miRNAs (27). The results on miR-221sponge are promising, as they achieved a down-regulation of the levels of miR-221. Ebert et al. demonstrated the capability of sponges to de-repress endogenous targets in cultured cells, by reporting an up to 2.5-fold increase in a specific miR-20 target (E2F1) following transfection of the miR-20 sponge (18). Considering the highest effects required transfection of large amount of plasmids, it remained unclear whether comparable efficacies could be obtained using viral delivery vectors, especially those based on typical low-copy integrating vectors, such as lentiviruses. This issue has been addressed by Gentner et al., who demonstrated an intriguing novel application of lentiviral sponges (21). They engineered recombinant lentiviruses to express sponges against miR-223, a highly abundant miRNA in U937 monocytes. They found de-repression of endogenous targets requires high vector copy numbers, which are, however, hard to achieve and maintain with lentiviruses. Moreover, raising the number of genomic integrations may concurrently increase the risks of adverse insertional mutagenesis. Thus, the general usefulness of lentiviruses to express miRNA sponges remained unclear, especially in an in vivo setting. Other efficient non-integrating viral vectors, such as adenovirus or AAV might ultimately represent a better choice for miRNA sponge applications, particularly in vivo, as reviewed by Grimm et al. (27). Hence, we selected our sponge viral vectors either as adeno (Ad) or adeno-associated virus (AAV).
Among adenovirus vector, we selected an oncolytic adenovirus, with the property of replicating in and killing cancer cells. We selected a conditionally replicative adenoviruses (CRAds), whose replication was controlled by miR-199, which has been recently developed by Callegari, et al.; 2013 (22). miR-199 is highly expressed in normal liver, while it is strongly down-regulated in essentially 100% of HCCs, making cancer cells, but not normal hepatocytes permissive to viral expression and replication. We proposed that equipping the engineered adenovirus to sponge antisense miR-221 could arrest miR-221 mainly in HCC cells. The Ad-199T-miR-221 sponge was indeed able to replicate in HepG2 cells, but not in the HepG2-199 cells that express significant levels of miR-199. In the same experimental setting, we have shown that Ad-199T-miR-221 sponge produced a significant reduction of miR-221 levels and a concomitant increase of the CDKN1B/p27 gene, a miR-221 target, in comparison with control virus treatment. We also developed a miR-221 sponge viral vector based on rAAV. rAAV are well suited to mediate miRNA inhibitors because they can safely transduce a wide range of tissues and provide long term sustained gene expression. Here, we chose the DJ serotype because it shows a higher infection efficiency in comparison with any other wild type serotype, particularly into hepatocytes (25). This system is used to introduce genes into cells for gene therapy studies (24, 25) (28). Compared to retroviral delivery systems, rAAVs carry a substantially diminished risk of insertional mutagenesis since viral genomes persist primarily as episomes (29). Further, the availability of multiple AAV serotypes allows efficient targeting of many tissues of interest (24, 30). Finally, the general safety of AAV has been well documented, with clinical trials (31, 32). This study shows a miR-221 sponge delivered by AAV was effective at reducing endogenous miR-221 and altering the level of a miR-221 target protein. The biological effects of both miR-221 sponge viruses were assessed. Both were found to reduce viability and increase apoptosis of HCC cells. Adenovirus is more likely because of the high copy number that can achieve, which was more effective, but rAAV produced significant, though lower effects. rAAV effects could however persist for months and viral infections could be repeated in vivo without being inhibited by the immune response.
In summary, the viral vectors developed in this work may be ready for in vivo testing. These viruses can be produced at high titer; they can induce miR-221 inhibition and re-expression of gene targets. In HCC cells, these viruses may potentially induce measurable anti-cancer activity through the inhibition of miR-221, which has been shown to be a tumor promoting miRNA. Since miR-221 is increased in a number of other solid tumors including pancreas (5), non–small cell lung cancer (33), glioblastoma (12) and thyroid cancer (34), optimizing the delivery of antisense sponges might be a useful approach to target tumors besides HCC.
Notes
(Please cite as: Moshiri F, Callegari E, D’Abundo L, Corrà F, Lupini L, Sabbioni S, at al. Inhibiting the oncogenic mir-221 by microRNA sponge: toward microRNA-based therapeutics for HCC. Gastroenterol Hepatol Bed Bench 2014;7(1):43-54).
References
Articles from Gastroenterology and Hepatology From Bed to Bench are provided here courtesy of Shahid Beheshti University of Medical Sciences
Citations & impact
Impact metrics
Article citations
MicroRNAs in Hepatocellular Carcinoma Pathogenesis: Insights into Mechanisms and Therapeutic Opportunities.
Int J Mol Sci, 25(17):9393, 29 Aug 2024
Cited by: 0 articles | PMID: 39273339 | PMCID: PMC11395074
Review Free full text in Europe PMC
miRNA-Mediated Mechanisms in the Generation of Effective and Safe Oncolytic Viruses.
Pharmaceutics, 16(8):986, 25 Jul 2024
Cited by: 0 articles | PMID: 39204331 | PMCID: PMC11360794
Review Free full text in Europe PMC
Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma.
Virol J, 21(1):17, 12 Jan 2024
Cited by: 2 articles | PMID: 38216938 | PMCID: PMC10785434
Review Free full text in Europe PMC
miR-221/222 sponge abrogates tamoxifen resistance in ER-positive breast cancer cells through restoring the expression of ERα.
Mol Biomed, 2(1):20, 30 Jun 2021
Cited by: 13 articles | PMID: 35006452 | PMCID: PMC8607419
Oncolytic Viruses in Combination Therapeutic Approaches with Epigenetic Modulators: Past, Present, and Future Perspectives.
Cancers (Basel), 13(11):2761, 02 Jun 2021
Cited by: 18 articles | PMID: 34199429 | PMCID: PMC8199618
Review Free full text in Europe PMC
Go to all (29) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Long noncoding RNA MIR31HG inhibits hepatocellular carcinoma proliferation and metastasis by sponging microRNA-575 to modulate ST7L expression.
J Exp Clin Cancer Res, 37(1):214, 03 Sep 2018
Cited by: 59 articles | PMID: 30176933 | PMCID: PMC6122648
MicroRNA-101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity.
J Hepatol, 60(3):590-598, 06 Nov 2013
Cited by: 112 articles | PMID: 24211739
MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma.
Oncogene, 27(43):5651-5661, 02 Jun 2008
Cited by: 405 articles | PMID: 18521080
LncRNA DGCR5 represses the development of hepatocellular carcinoma by targeting the miR-346/KLF14 axis.
J Cell Physiol, 234(1):572-580, 01 Jan 2018
Cited by: 36 articles | PMID: 30216442

 2
2