Abstract
Background
Community-acquired pneumonia (CAP) is the third-leading infectious cause of death worldwide. The standard treatment of CAP has not changed for the past fifty years and its mortality and morbidity remain high despite adequate antimicrobial treatment. Systemic corticosteroids have anti-inflammatory effects and are therefore discussed as adjunct treatment for CAP. Available studies show controversial results, and the question about benefits and harms of adjunct corticosteroid therapy has not been conclusively resolved, particularly in the non-critical care setting.Methods/design
This randomized multicenter study compares a treatment with 7 days of prednisone 50 mg with placebo in adult patients hospitalized with CAP independent of severity. Patients are screened and enrolled within the first 36 hours of presentation after written informed consent is obtained. The primary endpoint will be time to clinical stability, which is assessed every 12 hours during hospitalization. Secondary endpoints will be, among others, all-cause mortality within 30 and 180 days, ICU stay, duration of antibiotic treatment, disease activity scores, side effects and complications, value of adrenal function testing and prognostic hormonal and inflammatory biomarkers to predict outcome and treatment response to corticosteroids. Eight hundred included patients will provide an 85% power for the intention-to-treat analysis of the primary endpoint.Discussion
This largest to date double-blind placebo-controlled multicenter trial investigates the effect of adjunct glucocorticoids in 800 patients with CAP requiring hospitalization. It aims to give conclusive answers about benefits and risks of corticosteroid treatment in CAP. The inclusion of less severe CAP patients will be expected to lead to a relatively low mortality rate and survival benefit might not be shown. However, our study has adequate power for the clinically relevant endpoint of clinical stability. Due to discontinuing glucocorticoids without tapering after seven days, we limit duration of glucocorticoid exposition, which may reduce possible side effects.Trial registration
7 September 2009 on ClinicalTrials.gov: NCT00973154.Free full text

Corticosteroid treatment for community-acquired pneumonia - the STEP trial: study protocol for a randomized controlled trial
Abstract
Background
Community-acquired pneumonia (CAP) is the third-leading infectious cause of death worldwide. The standard treatment of CAP has not changed for the past fifty years and its mortality and morbidity remain high despite adequate antimicrobial treatment. Systemic corticosteroids have anti-inflammatory effects and are therefore discussed as adjunct treatment for CAP. Available studies show controversial results, and the question about benefits and harms of adjunct corticosteroid therapy has not been conclusively resolved, particularly in the non-critical care setting.
Methods/Design
This randomized multicenter study compares a treatment with 7 days of prednisone 50 mg with placebo in adult patients hospitalized with CAP independent of severity. Patients are screened and enrolled within the first 36 hours of presentation after written informed consent is obtained. The primary endpoint will be time to clinical stability, which is assessed every 12 hours during hospitalization. Secondary endpoints will be, among others, all-cause mortality within 30 and 180 days, ICU stay, duration of antibiotic treatment, disease activity scores, side effects and complications, value of adrenal function testing and prognostic hormonal and inflammatory biomarkers to predict outcome and treatment response to corticosteroids. Eight hundred included patients will provide an 85% power for the intention-to-treat analysis of the primary endpoint.
Discussion
This largest to date double-blind placebo-controlled multicenter trial investigates the effect of adjunct glucocorticoids in 800 patients with CAP requiring hospitalization. It aims to give conclusive answers about benefits and risks of corticosteroid treatment in CAP. The inclusion of less severe CAP patients will be expected to lead to a relatively low mortality rate and survival benefit might not be shown. However, our study has adequate power for the clinically relevant endpoint of clinical stability. Due to discontinuing glucocorticoids without tapering after seven days, we limit duration of glucocorticoid exposition, which may reduce possible side effects.
Trial registration
7 September 2009 on ClinicalTrials.gov: NCT00973154.
Background
Respiratory infections, consisting mainly of pneumonia, are the third leading infectious cause of death worldwide [1]. Community-acquired pneumonia (CAP) is the main cause for sepsis and septic shock, both of which carry a high risk of long-term morbidity and mortality [2,3].
The standard treatment of CAP has not changed for the past 50 years, and its mortality and morbidity has remained high at 5 to 15% despite the availability of broad-spectrum antibiotics [4]. Systemic corticosteroids have anti-inflammatory effects [5,6] which, in CAP, attenuate the systemic inflammatory process which may have a detrimental impact [7] on the lung [8]. Therefore, adjunct treatment with corticosteroids has been discussed since the 1950s [9]. As early as 1955, favorable effects of corticosteroids were reported in patients with pneumococcal pneumonia [9]. A small-sized multicenter randomized trial showed a significant reduction in hospital mortality in severe CAP with a seven-day continuous infusion of hydrocortisone (240 mg/day), although this study was not powered for mortality [10]. Another large, but retrospective, single center study evaluated more than 300 patients and demonstrated in multivariable analysis that use of corticosteroids was associated with a lower mortality [11]. More recently, two randomized placebo-controlled trials involving 200 to 300 patients showed controversial results, one study showing no benefit of adjunct prednisolone treatment and one study showing a significant reduction in duration of hospital stay using dexamethasone [6,12]. Three systematic reviews [13-15] and one meta-analysis [16] concluded that the available evidence suggests that administration of corticosteroids in patients with CAP might be beneficial. However, a large and adequately powered randomized trial is warranted to support or refute this beneficial effect before recommendations can be made with regard to use of corticosteroids in the treatment of CAP. In addition, as the potential harmful effects of corticosteroids in high-dose and long-term treatment are well-known [17,18], special attention has to be given to the side effects of corticosteroid treatment.
Methods
Objective and design
The objective of this trial is to evaluate whether treatment with prednisone for seven days in patients with CAP as compared to placebo reduces time to clinical stability.
This is a multicenter randomized placebo-controlled trial. The local ethics committees: Ethikkommission beider Basel EKBB (Reference Number 370/08), Ethikkommission der Kantone Aargau und Solothurn (Reference Number 2011/031), commission d’ethique Lausanne (Reference Number 116/10) and Ethikkommission Bern (Reference Number 193/11), as well as the Swiss Agency for Therapeutic Products SWISSMEDIC (Reference Number 2009DR3227) approved the study protocol after meeting imposed conditions. This trial adheres to the declaration of Helsinki and was registered at clinicaltrials.gov as NCT00973154 on 7 September 2009. The protocol follows the recommendations of the SPIRIT initiative [19], and the trial results will be reported according to the latest version of the CONSORT statement [20].
Study setting and participants
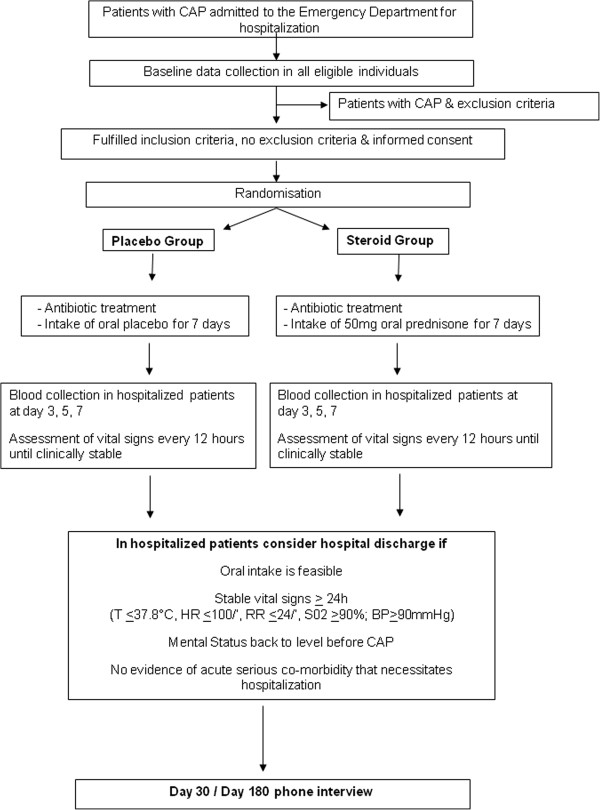
All adult patients with CAP are screened and enrolled at emergency departments or medical wards in seven tertiary care hospitals of Switzerland within 24 hours; on weekends within 36 hours of hospitalization. Eligible patients are asked for their informed consent and are then centrally randomized to either 50 mg prednisone or to placebo, taken orally for 7 days in a ratio of 1:1. If the patient is not able to give informed consent at the time of hospitalization due to severity of CAP, informed consent from next of kin and an independent physician is obtained. In these cases, informed consent from the patient himself has to be obtained as soon as possible. Patient recruitment started in December 2009 and is planned to be completed in April 2014.
Inclusion criteria are age of 18 years or older and hospitalization with CAP defined by a new infiltrate on chest radiograph and the presence of one or several of the following acute respiratory signs or symptoms: cough, sputum production, dyspnea, core body temperature ≥
≥ 38.0°C, auscultatory findings of abnormal breath sounds and rales and leukocyte count
38.0°C, auscultatory findings of abnormal breath sounds and rales and leukocyte count >
> 10 or
10 or <
< 4 G/L [21].
4 G/L [21].
Exclusion criteria are permanent inability for informed consent, active intravenous drug abuse, acute burn injury, gastrointestinal bleeding within the past three months, known adrenal insufficiency, a condition requiring more than 0.5 mg/kg/day prednisone equivalent, pregnancy or breast feeding, and severe immunosuppression defined as previously known infection with HIV and a CD4 cell count below 350 G/L, immunosuppressive therapy after solid organ transplantation, neutropenia <
< 500 G/L or a neutrophil count of 500 to 1,000 G/L with an expected decrease to values
500 G/L or a neutrophil count of 500 to 1,000 G/L with an expected decrease to values <
< 500 G/L in patients under ongoing chemotherapy, cystic fibrosis and active tuberculosis.
500 G/L in patients under ongoing chemotherapy, cystic fibrosis and active tuberculosis.
Randomization and blinding
Allocation of patients to either intervention in this randomized placebo-controlled trial is concealed due to a pre-specified computer-generated randomization list which is kept centrally at the pharmacy of the main study center. Randomization between the two arms are 1:1 with variable block sizes of 4 to 6 and are stratified at the time of study entry by study center.
Generator and executor of randomization are separated. Patients are randomly assigned to receive a prepared set of study medication that contains 7 tablets of 50 mg prednisone or placebo. The drugs are prepared prior to the initiation of the study and packed by the Pharmacology Department, University Hospital, Basel, according to the randomization list. The numbered study medications are then delivered to the study team. By this method, study team including data analysts, patients and treating physicians will be blinded to group allocation. Unblinding will be performed only upon request of a treating physician or of the data safety and monitoring board (DSMB).
Study protocol
The study flow chart is shown in Figure 1. After informed consent is obtained, baseline blood is drawn and a low-dose adrenocorticotropic hormone (ACTH) test is performed (measurement of basal cortisol followed by cortisol after 0.001 mg of tetracosactid (Synacthen®)). Study medication is started after the ACTH test, and timing in relation to start of antibiotics will be monitored. Patients are evaluated for the primary endpoint of clinical stability every 12 hours during the hospitalization. All patients are being treated according to current CAP guidelines [22,23]. Antibiotic therapy guided by an established procalcitonin algorithm [24] is encouraged. The time of discharge is not determined by the study investigators, but based on the decision of the treating physician team.
Laboratory assessment
The blood samples drawn on days 1, 3, 5, 7 and at discharge are immediately centrifuged and stored at -70°C until further processing. All laboratories of the participating centers are internationally validated and standardized. Cortisol is measured immediately. Routine laboratory tests in both groups include procalcitonin, C-reactive protein (CRP), white blood cell count, sputum and blood cultures, nasal swab for virus multiplex PCR, Legionella and pneumococcal antigen in urine. Other laboratory markers as well as genetic markers will be measured in batch analysis from stored serum or plasma samples, respectively, after the study is terminated.
Data collection
Baseline data are collected by electronic case report forms (eCRF) compliant to good clinical practice (GCP) and contain date and time of randomization, birth date, sex, medical history items (dyspnea at rest or after having taken a flight of stairs, cough, smoking history (pack-years) and status (pack per day), relevant co-morbidities, degree of autonomy at home, clinical items of pneumonia, pneumonia severity index (PSI), extent of pneumonia, underlying chronic obstructive pulmonary disease (COPD) as assessed by patient history and medical documents.
In both groups, hospitalized patients are reassessed clinically every 12 hours (including vital signs) and blood sampling on days 3, 5 and 7 including 4 measurements of glucose levels on days 3, 5 and 7, respectively. Details on dosing of all prescribed antimicrobials during the study period are recorded. In hospitalized patients, hospital discharge is recommended if oral intake is feasible, vital signs are stable ≥
≥ 24 hours, and no evidence of acute serious co-morbidity that necessitates hospitalization is found. A final blood collection is foreseen on the day of discharge.
24 hours, and no evidence of acute serious co-morbidity that necessitates hospitalization is found. A final blood collection is foreseen on the day of discharge.
On days 1 and 5, the patient has to complete a short questionnaire on respiratory symptoms.
Any additional diagnostic testing in patients in both groups is at the full discretion of the treating physician.
Follow-up
Structured follow-up telephone interviews for secondary outcomes after discharge are performed on days 30 and 180. In case the patient cannot be contacted or cannot give sufficient information, the primary care physician is interviewed.
Follow-up items include assessment of adverse events, such as infections, recurrent pneumonia, re-hospitalization, new diabetes or insulin dependence, new hypertension, and the standardized questionnaire on respiratory symptoms that was used on days 1 and 5.
Endpoints
Primary endpoint
Primary endpoint is time to clinical stability, which is defined as follows: time (days) until stable vital signs for ≥
≥ 24 hours [21]:
24 hours [21]:
Temperature ≤
≤ 37.8°C without antipyretic agents, heart rate/minute
37.8°C without antipyretic agents, heart rate/minute ≤
≤ 100, spontaneous respiratory rate
100, spontaneous respiratory rate ≤
≤ 24 per minute, systolic blood pressure
24 per minute, systolic blood pressure ≥
≥ 90 mmHg (≥100 mmHg for patients diagnosed with hypertension) without vasopressor support, mental status back to level before CAP, adequate oxygenation on room air of oxygen therapy (PaO2
90 mmHg (≥100 mmHg for patients diagnosed with hypertension) without vasopressor support, mental status back to level before CAP, adequate oxygenation on room air of oxygen therapy (PaO2 ≥
≥ 60 mmHg or pulse oximetry
60 mmHg or pulse oximetry ≥
≥ 90%). For patients with chronic hypoxemia or chronic oxygen therapy, PaO2 or pulse oximetry measurement must be back to baseline. Nurses on the wards unaware of treatment allocation assess clinical stability during hospitalization every 12 hours.
90%). For patients with chronic hypoxemia or chronic oxygen therapy, PaO2 or pulse oximetry measurement must be back to baseline. Nurses on the wards unaware of treatment allocation assess clinical stability during hospitalization every 12 hours.
Secondary endpoints
We will consider the following secondary endpoints:
• All-cause mortality within 30 and 180 days
• ICU stay and time to transfer to ICU
• in ICU patients: time to discharge from ICU; duration of vasopressor treatment; duration of mechanical ventilation
• Time to effective hospital discharge (days)
• Duration of intravenous and total antibiotic treatment (days)
• Disease activity scores. This will be evaluated by a CAP specific disease activity score [25]. Number of days with restriction from CAP, and daily function and health state will be evaluated, all assessed at baseline, at days 5, 30 and 180
• Side effects and adverse effects of corticosteroid treatment (which are rate of hyperglycemia, hypertension, weight gain, delirium, nosocomial infections) as assessed by a validated questionnaire [26] as well as by blood glucose, weight and blood pressure monitoring
• Incidence of CAP complications until days 30 and 180 (which are persistence of pneumonia, acute respiratory distress syndrome (ARDS), empyema)
• Microbiological, clinical and/or radiological recurrence within 30 and 180 days
• Time to earliest possible hospital discharge (days) based on objective criteria (possible oral intake of food, liquids and drugs, stable vital signs >
> 24 hours, recovery from CAP-related worsening of mental status, no evidence of acute serious CAP-related co-morbidity that necessitates hospitalization. This endpoint will minimize a potential bias due to extended hospitalization for non-disease specific reasons (request from patients or their relatives, lack of adequate home care support, or possibility for transferral to nursing home, lack of assurance about compliance with treatment)
24 hours, recovery from CAP-related worsening of mental status, no evidence of acute serious CAP-related co-morbidity that necessitates hospitalization. This endpoint will minimize a potential bias due to extended hospitalization for non-disease specific reasons (request from patients or their relatives, lack of adequate home care support, or possibility for transferral to nursing home, lack of assurance about compliance with treatment)
• Late failure (that is recurrence of signs and symptoms of pneumonia after 72 hours of randomization after an initially beneficial response to treatment) [12]
Subgroup analyses
We plan to explore treatment effects according to the following parameters:
• Above versus below the median initial CRP and maximal CRP (hypothesis: patients with higher CRP benefit more from adjunct corticosteroid therapy)
• Age <
< 70 years versus
70 years versus >
> 70 years (hypothesis: patients above 70 years benefit more, as older-aged patients present with more severe CAP and have a higher mortality [27]. So far, there is no age-related data on adjunct glucocorticoid therapy, but in severe CAP there is more evidence for benefit of glucocorticoid therapy [16])
70 years (hypothesis: patients above 70 years benefit more, as older-aged patients present with more severe CAP and have a higher mortality [27]. So far, there is no age-related data on adjunct glucocorticoid therapy, but in severe CAP there is more evidence for benefit of glucocorticoid therapy [16])
• Severity of CAP (low-risk group: PSI I to III versus high-risk group: PSI IV to V (hypothesis: patients with more severe CAP and supposedly more severe inflammation benefit more)
• Microbiological subgroups with versus without bacteremia (hypothesis: patients with bacteremia benefit more)
• With versus without underlying COPD according to the Global initiative for chronic Obstructive Lung Disease (GOLD) classification (hypothesis: patients with COPD benefit more)
• COPD patients with GOLD category I to II versus III to IV (hypothesis: patients with GOLD category III to IV benefit more)
Sample size considerations
This study is designed to show superiority of the steroid group compared to placebo treatment. Our primary hypothesis is that adjunct corticosteroid treatment in patients with CAP will result in a reduced time to clinical stability compared to placebo treatment. To estimate the frequency of our primary endpoint, we used data of CAP patients from our completed studies [24,28,29] which were performed in the same study centers. Based on these previous trials, we assumed a mortality rate of 10% in the placebo group and 7.5% in the corticosteroid group over 14 days of follow-up with a proportion of 75% survivors being clinically stable after 7 days in the corticosteroid group. Estimating a decrease in the risk of non-stability after 1 week among survivors by 25% through adjunct corticosteroids, a sample size of 800 patients followed for at least 14 days after randomization will be needed to achieve a statistical power of 85% and a sample size of 700 patients to achieve a statistical power of 80% (2-sided type 1 error of 5% in both scenarios). We intend to recruit 800 study patients from December 2009 to April 2014.
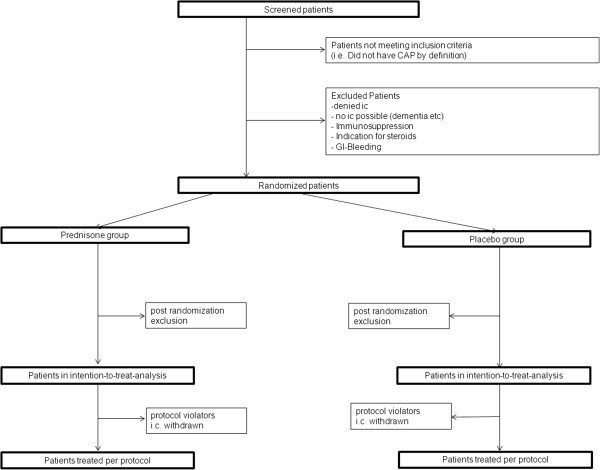
Intention-to-treat and per-protocol population
Following the intention-to-treat principle, all patients receiving at least one dose of study medication will be included in the analysis with group allocation as randomized; patients violating inclusion criteria or meeting exclusion criteria due to information that was not available at study entry will be excluded post randomization in a blinded manner [30]. Criteria for exclusion of patients from the per-protocol analysis are, in addition, withdrawal of informed consent or non-compliance with study medication or the concomitant procedures. Therefore, patients stopping or pausing the study medication at any time point, be it due to corticosteroid contraindications or due to active indication for glucocorticoid treatment, will not be considered in the per-protocol analysis. A patient will not be followed up if he withdraws informed consent for all study measures; in all other cases, follow-up will be performed as planned.
Statistical analysis
We will enter all relevant clinical and laboratory data obtained by interview, clinical tests, and reviewing of the medical records into an online database. Statistical Analysis System (SAS® Institute, Cary, NC, USA) and R for Windows (R Foundation for Statistical Computing, http://www.r-project.org) and STATA 9.2 (Stata Corp, College Station, TX, USA) will be used for data analysis.
The primary analysis population is the full analysis set which includes the intention-to-treat population. Every effort will be made to minimize the number of losses to follow-up by recording phone numbers before discharge, thereafter by repetitive follow-up calls by home or cell phone contact, or alternatively by contacting the primary care physician. For the primary outcome of time to clinical stability, we consider losses to follow-up in this sample of hospitalized patients as unlikely. If losses to follow-up do occur, patients will be censored at time of last assessment for clinical stability. A secondary analysis population, the per-protocol population, will exclude patients not treated according to the protocol (that is protocol violators). For the primary endpoint, we will calculate an unadjusted hazard ratio and 95% confidence interval by means of a Cox proportional hazards model. The primary objective is to show superiority of the corticosteroid group at a 2-sided 5% alpha-level with 85% power following the intention-to-treat principle. As a sensitivity analysis, the primary analysis will be repeated on the per-protocol patient population. As a further sensitivity analysis, a multivariable Cox proportional hazards model will be fitted with treatment group, patient age, and PSI scores as independent variables. This model will adjust the treatment effect for the potentially important confounders age and PSI score. Moreover, we plan to investigate whether the treatment effect varies among different degrees of severity of CAP (low-risk group: PSI I to III, and high-risk group: PSI IV to V), between patients with and without underlying COPD, and between bacteremic versus non-bacteremic patients. COPD will be defined by post-bronchodilator spirometric criteria, according to the GOLD guidelines [31], as a FEV1/FVC ratio below 70% and the severity categorized according to the GOLD criteria. In patients with a clinical history of COPD or smoking, lung function at the time of inclusion will not be mandatory. We will conduct the pre-specified subgroup analyses by including appropriate interaction terms in the above described multivariable Cox proportional hazards model.Complete case analyses will be used for secondary endpoints. For all secondary endpoints, we will calculate unadjusted and adjusted estimates of the effect size and corresponding 95% confidence intervals using linear, logistic, or Cox proportional hazards regression (as appropriate). Figure 2 shows the CONSORT diagram of the trial.
Study organization
All adverse events of category III or more according to Common Terminology Criteria for Adverse Events v4.0 [32] are recorded. Adverse events will be monitored for every subject participating in the study and attributed to the study medication and reported to the respective authorities according to law if necessary.
The trial is supervised by an independent and blinded DSMB that is not involved in the design and conduct of the trial, and has no affiliation with the sponsor. The DSMB is comprised of experts from different disciplines and supervises the recruitment of patients, patient flow and patient interviews. The board has access to the randomization code in case of need. In case of serious problems or adverse events that may occur during the trial, the board will review the preplanned interim analysis and inform the principle investigators whether the trial may be continued or has to be stopped early or it may require modifications of the protocol.
A blinded safety interim analysis was performed after 50% of patients had completed the 180 day follow-up. The DSMB recommended continuation of the study without changes to the protocol.
In accordance with applicable regulations and GCP, a study monitor is periodically controlling study procedures.
Planned subprojects
We will use data collected during this trial for further analyses investigating the following topics:
• Diagnostic value of adrenal function testing (ACTH test)
• Prognostic value of hormonal and inflammatory biomarkers (for example, total and free cortisol levels, copeptin, dehydroepiandrosterone (DHEA) as well as the ratio of cortisol to DHEA [33]) in a further attempt to identify those patients who benefit most from corticosteroid treatment
• Effect of corticosteroid treatment on inflammatory markers
• Prognostic value of hormonal biomarkers for risk assessment in patients with CAP
• Microbiological etiology of pneumonia (bacterial testing in blood, sputum and urine, viral multiplex PCR from nasal swabs)
• Predictability of patients and doctors whether the study medication was prednisone or placebo
Discussion
Even with the prescription of newer and more potent antimicrobial agents, mortality due to CAP has remained relatively constant over time. Corticosteroids have been proved to block several arms of the inflammatory cascade. Available evidence so far, derived from a few, mainly small randomized controlled trials, suggests that corticosteroid treatment might be beneficial in terms of survival in patients with CAP [6,9,10,34-36].
Most studies showing a beneficial effect of corticosteroids in patients with CAP only included severe pneumonia, as defined with a PSI class IV and V [11,34,37,38]. In these patients, reviews and meta-analyses [13-16] pointed to a possible benefit of corticosteroids on either length of stay or mortality without evidence of more complications. However, insufficient data on non-severe CAP are available. Recently, two randomized double-blind controlled trials including 200 to 300 patients with all severities of CAP found controversial results: the first study using 40 mg of prednisolone for seven days showed no effect on time to clinical stability, with a higher rate of late failure in the corticosteroid group, defined by recurrence of signs and symptoms of pneumonia after 72 hours of admission after an initially beneficial response to treatment [12]. The second study using 4 days of 5 mg of dexamethasone intravenously showed a significantly shorter duration of hospital stay in patients treated with corticosteroids [6,12]. In view of these controversial findings, a large prospective and adequately powered trial should conclusively determine the risks and benefits of adding corticosteroids to the treatment of patients with CAP.
In the current trial, we will include all severity levels of CAP (PSI I to V) requiring hospitalization. This will lead to a lower mortality rate than observed in previous studies only including severe CAP patients, and our study will not be powered to show a survival benefit. We therefore chose as primary endpoint time to clinical stability, which is a less stringent but still clinically relevant endpoint. By reducing the time to clinical stability and consecutively also length of stay (LOS), nosocomial complications like infections, thrombembolic events, worsening of pre-existing frailty or delirium may be prevented. This leads to better allocation of resources at the hospital and to a marked reduction of costs [39]. While Meijvis et al. looked at LOS [6], our primary endpoint will be time to clinical stability, as defined by IDSA/ATS guidelines [22]. As we have previously observed, LOS itself in community-acquired pneumonia may be confounded and prolonged by medical or organizational problems unrelated to CAP [39-41]. We believe that by measuring time to clinical stability, we will be looking at a clinically relevant parameter, as patients are able to switch to oral antibiotics and ready for discharge once they reach stability.
Inclusion of all patients with CAP will increase the generalizability of the findings and allow retrospective evaluation in exploratory analyses as to which patients indeed profited most from corticosteroid treatment.
In our study, we administer an oral dose of 50 mg prednisone for 7 days without tapering. We opted for an oral formula, as the bioavailability of oral prednisone is excellent, it may be ground and is therefore applicable in patients not able to swallow, such as in patients on mechanical ventilation or older-aged patients. Furthermore, by choosing a uniform application formula, further bias, like the ability to blind and to secure provision of blinded study medication 24 hours on 7 days per week, was prevented.
The ideal choice and duration of corticosteroid treatment in critically ill patients and patients with CAP is currently disputed. Hydrocortisone is the natural corticosteroid and was used in some studies in critically ill patients, partly together with a mineralocorticoid [42]. Most patients in our study, however, will not suffer from critical illness and will be hospitalized outside the ICU on the wards where oral intake of treatment is preferred. Since hydrocortisone is only available in 10 mg tablets, patients would have to take 20 tablets a day, which does not appear feasible. Methylprednisolone and prednisolone have also been used in some studies including patients with CAP and ARDS [10,12,43,44], putting it forward as an alternative formulation for our study, as adequate oral formulations are available. Prednisolone is the pharmacologically active metabolite of prednisone, which was used in this study; both are used interchangeably except in patients with severe liver insufficiency, where prednisolone is preferred. Dexamethasone was not recommended by guidelines for use in septic shock and ARDS at the time of study design [37]. However, a recent study showed a reduced length of hospital stay in CAP patients treated with dexamethasone as compared to placebo [6], also putting it forward as a possible type of corticosteroid in patients with CAP. The additional use of fludrocortisone as in the study by Annane et al. [42] is currently considered optional [37]. Furthermore, the optimal duration of glucocorticoid treatment and mode of discontinuing (tapering or not) is not known and heavily disputed [45,46].
In high-dose glucocorticoid studies, which used as high as 1 to 3 g hydrocortisone per day, short-term complications, such as co- and re-infections, led to excess mortality [47,48]. In so-called low- or physiological-dose glucocorticoid studies with 100 to 300 mg hydrocortisone-equivalent per day, the main adverse effects included hyperglycemia [6] and rebound pneumonia [12]. Although confusion, co-infection and gastrointestinal side effects were reported in association with glucocorticoid treatment, the incidence was not significantly higher in the steroid group [16]. While some authors advise five days of glucocorticoid treatment in critically ill patients without tapering [49-51], others recommend tapering of glucocorticoids to avoid a rebound of inflammatory markers with consecutive rebound pneumonia [37,45,46,52]. However, it has been shown that glucocorticoids given >
> seven days lead to a worse clinical course measured by length of stay, clinical stability and mechanical ventilation, and more systemic complications when looking at shock and cardiac arrhythmia [53]. From COPD studies, there is evidence that stopping glucocorticoids without tapering is safe without an increased recurrence rate [54]. For these reasons, we decided against tapering of the glucocorticoid dose in order not to prolong glucocorticoid exposure.
seven days lead to a worse clinical course measured by length of stay, clinical stability and mechanical ventilation, and more systemic complications when looking at shock and cardiac arrhythmia [53]. From COPD studies, there is evidence that stopping glucocorticoids without tapering is safe without an increased recurrence rate [54]. For these reasons, we decided against tapering of the glucocorticoid dose in order not to prolong glucocorticoid exposure.
In conclusion, this current large and adequately powered randomized trial will determine the risks and benefits of adding 50 mg of prednisone for 7 days to the treatment of hospitalized patients with CAP. Our hypothesis is that corticosteroid treatment will result in a reduced time to clinical stability in CAP compared to placebo treatment.
Study limitations
The results of this trial may only be valid for the specific type, dose and duration of glucocorticoid studied.
This study is powered for clinical stability, but not for mortality; at least 4,000 patients would be needed to be powered to detect a clinically relevant difference in mortality.
Furthermore, we also included lower severity CAP patients, which might weaken the effect of glucocorticoids. A conclusion for ICU patients might not be possible as the number of these patients included will be low. There is an ongoing ICU multicenter study (clinicaltrials.gov number NCT01448109) aiming for the goal of 4,000 patients that will hopefully answer this question for ICU patients with CAP.
Abbreviations
ACTH: adrenocorticotropic hormone; ARDS: acute respiratory distress syndrome; CAP: community-acquired pneumonia; CONSORT: Consolidated Standards of Reporting Trials; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; DHEA: dehydroepiandrosterone; DSMB: Data Safety and Monitoring Board; eCRF: electronic case report form; GCP: good clinical practice; GOLD: The Global initiative for chronic Obstructive Lung Disease; PCR: polymerase chain reaction; PSI: Pneumonia Severity Index; STEP: ‘Corticosteroid treatment for community-acquired pneumonia’ trial.
Competing interests
The authors declare that they have no competing interests with regards to this study.
Authors’ contributions
CAB was involved in the study design and writing of the protocol, study coordination, recruited patients for the study, participated in statistical analysis and drafted the manuscript. MB participated in designing the study and writing the protocol, did the statistical analysis and helped draft the manuscript. IS was involved in the study design, study coordination and recruited patients for the study. NN was involved in study coordination and recruited patients for the study. BW was involved in study coordination and recruited patients for the study. RB was involved in study coordination and gave financial and staff support. WZ was involved in study coordination and gave financial and staff support. EU participated in study coordination and recruited patients for the study. HE participated in study coordination and recruited patients for the study. PT was involved in study coordination and gave staff support. SW was involved in study coordination and recruited patients for the study. RT was involved in study coordination and recruited patients for the study. EH was involved in study coordination and recruited patients for the study. NR was involved in study coordination, gave financial and staff support, and critically revised the manuscript. HD was involved in study coordination, gave staff support and recruited patients for the study. DB gave financial and staff support for performing the nasopharyngeal PCR. BM participated in designing the study and writing the protocol, in study coordination and gave financial and staff support. PS participated in designing the study and writing the protocol, did the statistical analysis and helped draft the manuscript. MCC designed the study, wrote the protocol, coordinated the study and gave financial and staff support. All authors read and approved the final protocol manuscript.
Acknowledgements
We gratefully thank the staff of the emergency departments and medical wards of all participating hospitals in supporting this study. Furthermore, we thank the many supporters, study and laboratory personnel at all participating centers, especially Cemile Bathelt, Maria Candela, Helga Schneider, Katharina Regez, Ursula Schild, Daniel Drozdov, Werner Albrich, Eva Grolimund, Alexander Kutz, Martina Bally, Birsen Arici, Deborah Steiner, Anna-Christina Rast, Ingeborg Schnyder, Katharina Timper, Julie Refardt, Sandrine Urwyler, Stefanie Meyer, Sonja Schwenne, Merih Guglielmetti, Kristina Schumacher, Nathalie Christa Schwab, Danielle Krebs, Cornelia Krismer, Manuel Blum, Christina Wirth, Christine Baumgartner for the coordination of patient management, and Patrick Simon, Clinical Trial Unit, University Hospital Basel, for data base support. We also thank the members of the DSMB for their valuable time, especially Prof. Marc Donath und Dr. Benjamin Kasenda for their expertise in clinical practice, trial methodology, and statistics.
Funding
Source(s) of funding for each author:
MCC, CAB: this study was supported by a grant by the Swiss National Foundation (PP0P3_123346) to MCC, the Nora van Meeuwen Häfliger Stiftung and the Gottfried Julia Bangerter-Rhyner Stiftung. Nasopharyngeal PCR is supported entirely by Viollier AG, 4002 Basel, Switzerland.
MB is supported by santésuisse and the Gottfried and Julia Bangerter-Rhyner Foundation.
NR: this study was supported by a grant from Inselspital, Bern University Hospital. Prof N Rodondi and his research team are supported by a grant from the Swiss National Science Foundation (SNSF 320030-138267).
BM, CAB and PS: research funds from the Department of Endocrinology, Diabetology and Metabolism, the Medical University Clinic and the ‘Argovia Professorship’ of the Medical Faculty of the University of Basel.
References
- WHO. The Top 10 Causes of Death. 2013. http://www.who.int/mediacentre/factsheets/fs310/en/index.html.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. 10.1007/s00134-012-2769-8. [Abstract] [CrossRef] [Google Scholar]
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;168:165–172. 10.1164/rccm.2201087. [Abstract] [CrossRef] [Google Scholar]
- Bartlett JG, Dowell SF, Mandell LA, File TM Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31:347–382. 10.1086/313954. [Abstract] [CrossRef] [Google Scholar]
- Remmelts HH, Meijvis SC, Biesma DH, van Velzen-Blad H, Voorn GP, Grutters JC, Bos WJ, Rijkers GT. Dexamethasone downregulates the systemic cytokine response in patients with community-acquired pneumonia. Clin Vaccine Immunol. 2012;19:1532–1538. 10.1128/CVI.00423-12. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, Voorn GP, van de Garde EM, Endeman H, Grutters JC, Bos WJ, Biesma DH. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:2023–2030. 10.1016/S0140-6736(11)60607-7. [Abstract] [CrossRef] [Google Scholar]
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. 10.1016/S0140-6736(04)17667-8. [Abstract] [CrossRef] [Google Scholar]
- Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappaB- and glucocorticoid receptor alpha-mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation. 2005;12:321–338. 10.1159/000091126. [Abstract] [CrossRef] [Google Scholar]
- Wagner HN, Bennett IL, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bulletin of Johns Hopkins Hospital. 1955;98:197–215. [Abstract] [Google Scholar]
- Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, Narumoto O, Kichikawa Y, Kawai M, Tashimo H, Arai H, Horiuchi T, Sakamoto Y. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007;185:249–255. 10.1007/s00408-007-9020-3. [Abstract] [CrossRef] [Google Scholar]
- Garcia-Vidal C, Calbo E, Pascual V, Ferrer C, Quintana S, Garau J. Effects of systemic steroids in patients with severe community-acquired pneumonia. Eur Respir J. 2007;30:951–956. 10.1183/09031936.00027607. [Abstract] [CrossRef] [Google Scholar]
- Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–982. 10.1164/rccm.200905-0808OC. [Abstract] [CrossRef] [Google Scholar]
- Salluh JI, Bozza FA, Soares M, Verdeal JC, Castro-Faria-Neto HC, Lapa ESJR, Bozza PT. Adrenal response in severe community-acquired pneumonia: impact on outcomes and disease severity. Chest. 2008;134:947–954. 10.1378/chest.08-1382. [Abstract] [CrossRef] [Google Scholar]
- Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community-acquired pneumonia: a systematic review. J Antimicrob Chemother. 2008;62:661–668. 10.1093/jac/dkn283. [Abstract] [CrossRef] [Google Scholar]
- Shafiq M, Mansoor MS, Khan AA, Sohail MR, Murad MH. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68–75. 10.1002/jhm.1992. [Abstract] [CrossRef] [Google Scholar]
- Nie W, Zhang Y, Cheng J, Xiu Q. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926. 10.1371/journal.pone.0047926. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Myerson RM. Undesirable side effects associated with prednisone therapy. Del Med J. 1956;28:264–272. [Abstract] [Google Scholar]
- Saag KG. Short-term and long-term safety of glucocorticoids in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2012;70(Suppl 1):21–25. [Abstract] [Google Scholar]
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, Mann H, Dickersin K, Berlin JA, Dore CJ, Parulekar WR, Summerskill WS, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. 10.7326/0003-4819-158-3-201302050-00583. [Abstract] [CrossRef] [Google Scholar]
- CONSORT Statement. 2013. http://www.consort-statement.org.
- Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA, Martinez F, Marrie TJ, Plouffe JF, Ramirez J, Sarosi GA, Torres A, Wilson R, Yu VL. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. 10.1164/ajrccm.163.7.at1010. [Abstract] [CrossRef] [Google Scholar]
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. [Abstract] [Google Scholar]
- Moran GJ, Rothman RE, Volturo GA. Emergency management of community-acquired bacterial pneumonia: what is new since the 2007 Infectious Diseases Society of America/American Thoracic Society guidelines. Am J Emerg Med. 2007;2013(31):602–612. [Abstract] [Google Scholar]
- Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, Zimmerli W, Harbarth S, Tamm M, Muller B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. 10.1164/rccm.200512-1922OC. [Abstract] [CrossRef] [Google Scholar]
- El Moussaoui R, Opmeer BC, Bossuyt PM, Speelman P, de Borgie CA, Prins JM. Development and validation of a short questionnaire in community acquired pneumonia. Thorax. 2004;59:591–595. 10.1136/thx.2003.015107. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Arlt W, Rosenthal C, Hahner S, Allolio B. Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin Endocrinol (Oxf) 2006;64:384–389. [Abstract] [Google Scholar]
- Rello J, Rodriguez R, Jubert P, Alvarez B. Severe community-acquired pneumonia in the elderly: epidemiology and prognosis. Study Group for Severe Community-Acquired Pneumonia. Clin Infect Dis. 1996;23:723–728. 10.1093/clinids/23.4.723. [Abstract] [CrossRef] [Google Scholar]
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Muller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. 10.1016/S0140-6736(04)15591-8. [Abstract] [CrossRef] [Google Scholar]
- Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, Regez K, Schoenenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Mueller B. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. Jama. 2009;302:1059–1066. 10.1001/jama.2009.1297. [Abstract] [CrossRef] [Google Scholar]
- Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–654. 10.1136/bmj.325.7365.652. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [Abstract] [Google Scholar]
- Liu YJ, Zhu GP, Guan XY. Comparison of the NCI-CTCAE version 4.0 and version 3.0 in assessing chemoradiation-induced oral mucositis for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48:554–559. 10.1016/j.oraloncology.2012.01.004. [Abstract] [CrossRef] [Google Scholar]
- Arlt W, Hammer F, Sanning P, Butcher SK, Lord JM, Allolio B, Annane D, Stewart PM. Dissociation of serum dehydroepiandrosterone and dehydroepiandrosterone sulfate in septic shock. J Clin Endocrinol Metab. 2006;91:2548–2554. 10.1210/jc.2005-2258. [Abstract] [CrossRef] [Google Scholar]
- Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. 10.1164/rccm.200406-808OC. [Abstract] [CrossRef] [Google Scholar]
- Sabry NA, Omar EE-D. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm. 2011;2:73–81. 10.4236/pp.2011.22009. [CrossRef] [Google Scholar]
- Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest. 1993;104:389–392. 10.1378/chest.104.2.389. [Abstract] [CrossRef] [Google Scholar]
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. 10.1097/CCM.0b013e31817603ba. [Abstract] [CrossRef] [Google Scholar]
- Monton C, Ewig S, Torres A, El-Ebiary M, Filella X, Rano A, Xaubet A. Role of glucocorticoids on inflammatory response in nonimmunosuppressed patients with pneumonia: a pilot study. Eur Respir J. 1999;14:218–220. 10.1034/j.1399-3003.1999.14a37.x. [Abstract] [CrossRef] [Google Scholar]
- Albrich WC, Ruegger K, Dusemund F, Schuetz P, Arici B, Litke A, Blum CA, Bossart R, Regez K, Schild U, Guglielmetti M, Conca A, Schafer P, Schubert M, de Geest S, Reutlinger B, Irani S, Burgi U, Huber A, Muller B. Biomarker-enhanced triage in respiratory infections: a proof-of-concept feasibility trial. Eur Respir J. 2013;42:1064–1075. 10.1183/09031936.00113612. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Suter-Widmer I, Christ-Crain M, Zimmerli W, Albrich W, Mueller B, Schuetz P. Predictors for length of hospital stay in patients with community-acquired pneumonia: results from a Swiss multicenter study. BMC Pulm Med. 2012;12:21. 10.1186/1471-2466-12-21. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Baehni C, Meier S, Spreiter P, Schild U, Regez K, Bossart R, Thomann R, Falconnier C, Christ-Crain M, De Geest S, Muller B, Schuetz P. Which patients with lower respiratory tract infections need inpatient treatment? Perceptions of physicians, nurses, patients and relatives. BMC Pulm Med. 2010;10:12. 10.1186/1471-2466-10-12. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. Jama. 2002;288:862–871. 10.1001/jama.288.7.862. [Abstract] [CrossRef] [Google Scholar]
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. 10.1378/chest.06-2100. [Abstract] [CrossRef] [Google Scholar]
- Fernandez-Serrano S, Dorca J, Garcia-Vidal C, Fernandez-Sabe N, Carratala J, Fernandez-Aguera A, Corominas M, Padrones S, Gudiol F, Manresa F. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. 2011;15:R96. 10.1186/cc10103. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Confalonieri M, Kodric M, Santagiuliana M, Longo C, Biolo M, Cifaldi R, Torregiani C, Jevnikar M. To use or not to use corticosteroids for pneumonia? A clinician's perspective. Monaldi Arch Chest Dis. 2012;77:94–101. [Abstract] [Google Scholar]
- Meduri GU, Bell WA, Confalonieri M. Glucocorticoid treatment in community-acquired pneumonia without severe sepsis: no evidence of efficacy. Am J Respir Crit Care Med. 2010;181:880–882. 10.1164/rccm.201001-0034ED. [Abstract] [CrossRef] [Google Scholar]
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ Jr. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. 10.1097/00003246-199508000-00019. [Abstract] [CrossRef] [Google Scholar]
- Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. 10.1056/NEJM198709103171101. [Abstract] [CrossRef] [Google Scholar]
- Annane D. Corticosteroids for severe sepsis: an evidence-based guide for physicians. Ann Intensive Care. 2011;1:7. 10.1186/2110-5820-1-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–2375. 10.1001/jama.2009.815. [Abstract] [CrossRef] [Google Scholar]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database Syst Rev. 2004;1 CD002243. [Abstract] [Google Scholar]
- Nawab QU, Golden E, Confalonieri M, Umberger R, Meduri GU. Corticosteroid treatment in severe community-acquired pneumonia: duration of treatment affects control of systemic inflammation and clinical improvement. Intensive Care Med. 2011;37:1553–1554. 10.1007/s00134-011-2274-5. [Abstract] [CrossRef] [Google Scholar]
- Polverino E, Cilloniz C, Dambrava P, Gabarrus A, Ferrer M, Agusti C, Prina E, Montull B, Menendez R, Niederman MS, Torres A. Systemic corticosteroids for community-acquired pneumonia: reasons for use and lack of benefit on outcome. Respirology. 2013;18:263–271. 10.1111/resp.12013. [Abstract] [CrossRef] [Google Scholar]
- Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher T, Duerring U, Henzen C, Leibbrandt Y, Maier S, Miedinger D, Muller B, Scherr A, Schindler C, Stoeckli R, Viatte S, von Garnier C, Tamm M, Rutishauser J. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223–2231. 10.1001/jama.2013.5023. [Abstract] [CrossRef] [Google Scholar]
Articles from Trials are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1745-6215-15-257
Read article for free, from open access legal sources, via Unpaywall:
https://trialsjournal.biomedcentral.com/track/pdf/10.1186/1745-6215-15-257
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1186/1745-6215-15-257
Article citations
Adjunct prednisone in community-acquired pneumonia: 180-day outcome of a multicentre, double-blind, randomized, placebo-controlled trial.
BMC Pulm Med, 23(1):500, 11 Dec 2023
Cited by: 0 articles | PMID: 38082273 | PMCID: PMC10712075
Clinical Evaluation and Exploration of Mechanisms for Modified Xiebai Powder or Modified Xiebai Powder Combined with Western Medicine in the Treatment of Pneumonia.
Comput Math Methods Med, 2022:2287470, 11 Oct 2022
Cited by: 3 articles | PMID: 36276995 | PMCID: PMC9581678
No obesity paradox in patients with community-acquired pneumonia - secondary analysis of a randomized controlled trial.
Nutr Diabetes, 12(1):12, 23 Mar 2022
Cited by: 6 articles | PMID: 35322019 | PMCID: PMC8943130
Influence of Prednisone on Inflammatory Biomarkers in Community-Acquired Pneumonia: Secondary Analysis of a Randomized Trial.
J Clin Pharmacol, 61(11):1406-1414, 27 Jul 2021
Cited by: 7 articles | PMID: 34031890 | PMCID: PMC8242868
The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) Study. Rationale and Design.
Ann Am Thorac Soc, 17(7):879-891, 01 Jul 2020
Cited by: 189 articles | PMID: 32267771 | PMCID: PMC7328186
Go to all (12) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (2 citations) ClinicalTrials.gov - NCT00973154
- (1 citation) ClinicalTrials.gov - NCT01448109
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial.
Lancet, 385(9977):1511-1518, 19 Jan 2015
Cited by: 187 articles | PMID: 25608756
Benefit of adjunct corticosteroids for community-acquired pneumonia in diabetic patients.
Diabetologia, 59(12):2552-2560, 10 Sep 2016
Cited by: 11 articles | PMID: 27614658
Adjunct prednisone in community-acquired pneumonia: 180-day outcome of a multicentre, double-blind, randomized, placebo-controlled trial.
BMC Pulm Med, 23(1):500, 11 Dec 2023
Cited by: 0 articles | PMID: 38082273 | PMCID: PMC10712075
Adjunctive Systemic Corticosteroids for Hospitalized Community-Acquired Pneumonia: Systematic Review and Meta-Analysis 2015 Update.
Sci Rep, 5:14061, 16 Sep 2015
Cited by: 37 articles | PMID: 26374694 | PMCID: PMC4571641
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Swiss National Science Foundation (2)
Cardiovascular and neuropsychiatric outcomes associated with subclinical thyroid dysfunction: a prospective evaluation
Nicolas Rodondi, University of Berne
Grant ID: 138267
Grant ID: 320030

 1
1