Abstract
Importance
Although vitamin E and memantine have been shown to have beneficial effects in moderately severe Alzheimer disease (AD), evidence is limited in mild to moderate AD.Objective
To determine if vitamin E (alpha tocopherol), memantine, or both slow progression of mild to moderate AD in patients taking an acetylcholinesterase inhibitor.Design, setting, and participants
Double-blind, placebo-controlled, parallel-group, randomized clinical trial involving 613 patients with mild to moderate AD initiated in August 2007 and concluded in September 2012 at 14 Veterans Affairs medical centers.Interventions
Participants received either 2000 IU/d of alpha tocopherol (n = 152), 20 mg/d of memantine (n = 155), the combination (n = 154), or placebo (n = 152).Main outcomes and measures
Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS-ADL) Inventory score (range, 0-78). Secondary outcomes included cognitive, neuropsychiatric, functional, and caregiver measures.Results
Data from 561 participants were analyzed (alpha tocopherol = 140, memantine = 142, combination = 139, placebo = 140), with 52 excluded because of a lack of any follow-up data. Over the mean (SD) follow-up of 2.27 (1.22) years, ADCS-ADL Inventory scores declined by 3.15 units (95% CI, 0.92 to 5.39; adjusted P = .03) less in the alpha tocopherol group compared with the placebo group. In the memantine group, these scores declined 1.98 units less (95% CI, -0.24 to 4.20; adjusted P = .40) than the placebo group's decline. This change in the alpha tocopherol group translates into a delay in clinical progression of 19% per year compared with placebo or a delay of approximately 6.2 months over the follow-up period. Caregiver time increased least in the alpha tocopherol group. All-cause mortality and safety analyses showed a difference only on the serious adverse event of "infections or infestations," with greater frequencies in the memantine (31 events in 23 participants) and combination groups (44 events in 31 participants) compared with placebo (13 events in 11 participants).Conclusions and relevance
Among patients with mild to moderate AD, 2000 IU/d of alpha tocopherol compared with placebo resulted in slower functional decline. There were no significant differences in the groups receiving memantine alone or memantine plus alpha tocopherol. These findings suggest benefit of alpha tocopherol in mild to moderate AD by slowing functional decline and decreasing caregiver burden.Trial registration
clinicaltrials.gov Identifier: NCT00235716.Free full text

Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease
Abstract
Importance
Although vitamin E and memantine have been shown to have beneficial effects in moderately severe Alzheimer disease (AD), evidence is limited in mild to moderate AD.
Objective
To determine if vitamin E (alpha tocopherol), memantine, or both slow progression of mild to moderate AD in patients taking an acetylcholinesterase inhibitor.
Design, Setting, and Participants
Double-blind, placebo-controlled, parallel-group, randomized clinical trial involving 613 patients with mild to moderate AD initiated in August 2007 and concluded in September 2012 at 14 Veterans Affairs medical centers.
Interventions
Participants received either 2000 IU/d of alpha tocopherol (n = 152), 20 mg/d of memantine (n = 155), the combination (n = 154), or placebo (n = 152).
Main Outcomes and Measures
Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS-ADL) Inventory score (range, 0-78). Secondary outcomes included cognitive, neuropsychiatric, functional, and caregiver measures.
Results
Over the mean (SD) follow-up of 2.27 (1.22) years, participants receiving alpha tocopherol had slower decline than those receiving placebo as measured by the ADCS-ADL. The change translates into a delay in clinical progression of 19% per year compared with placebo (approximately 6.2 months over the follow-up period). Caregiver time increased least in the alpha tocopherol group. All-cause mortality and safety analyses showed a difference only on the serious adverse event of “infections or infestations” with greater frequencies in the memantine (31 events in 23 participants) and combination groups (44 events in 31 participants) compared with placebo (13 events in 11 participants).
| ADCS-ADL Inventory | Vitamin E (n = 140) | Memantine (n = 142) | Vitamin E + Memantine (n = 139) | Placebo (n = 140) |
| Baseline score, mean (SD) | 57.20 (14.38) | 57.77 (13.78) | 57.16 (13.59) | 56.93 (13.61) |
| Least squares mean (SE) change from baseline | −13.81 (1.11) | −14.98 (1.10) | −15.20 (1.11) | −16.96 (1.11) |
| Mean change difference compared with placebo (95% CI) | 3.15 (0.92 to 5.39) | 1.98 (−0.24 to 4.20) | 1.76 (−0.48 to 4.00) |
Conclusions and Relevance
Among patients with mild to moderate AD, 2000 IU/d of alpha tocopherol compared with placebo resulted in slower functional decline. There were no significant differences in the groups receiving memantine alone or memantine plus alpha tocopherol. These findings suggest benefit of alpha tocopherol in mild to moderate AD by slowing functional decline and decreasing caregiver burden.
Trial Registration
clinicaltrials.gov Identifier: NCT00235716
Alpha tocopherol, a fat-soluble vitamin and antioxidant, has been studied in patients with moderately severe Alzheimer disease (AD)1 and in participants with mild cognitive impairment (MCI)2 but has not been studied in patients with mild to moderate AD. In patients with moderately severe AD,1 alpha tocopherol (2000 IU/d) was shown to be effective in slowing clinical progression. In participants with MCI,2 however, alpha tocopherol (2000 IU/d) had no benefit compared with placebo in reducing the rate of conversion to AD.
Memantine, a moderate-affinity NMDA antagonist, was shown to be effective in 2 randomized clinical trials (RCTs),3,4 both of which were in patients with AD and moderately severe dementia. Three RCTs of memantine in AD patients with mild to moderate dementia have been published5-7and reviewed in a meta-analysis.8 There were no significant differences between memantine and placebo in patients with mild AD, either within any of the trials or when data were combined. For patients with moderate AD, there were small improvements in cognitive but not functional measures. Because the duration of each of these trials was only 6 months, these studies do not assess the long-term efficacy of memantine in AD patients with mild to moderate dementia.
The Trial of Vitamin E and Memantine in Alzheimer's Disease (TEAM-AD) examined the effectiveness and safety of alpha tocopherol (vitamin E), memantine (Namenda), and the combination for treatment of functional decline in patients with mild to moderate AD who were taking a background acetylcholinesterase inhibitor (AChEI).
Methods
The Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) designed the TEAM-AD trial (CSP No. 546) as a double-blind, placebo-controlled, parallel-group RCT to assess the effectiveness of 2000 IU/d of alpha tocopherol, 20 mg/d of memantine, and the combination in delaying clinical progression in patients with AD currently taking an AChEI. The duration of treatment ranged from 6 months to 4 years. Details regarding the study design and baseline characteristics of the participants have been previously published.9
The study was approved by the institutional review board at each participating medical center and by the human rights committee at the West Haven CSP Coordinating Center. An independent data monitoring committee oversaw the conduct, safety, and efficacy of the trial. (The organizational structure and participating investigators are listed at the end of the article.)
Participants
Veterans with a diagnosis of possible or probable AD10 of mild to moderate severity, defined as a Mini-Mental State Examination (MMSE) total score between 12 and 26 inclusive,11 who were currently taking an AChEI were recruited from 14 VA medical centers between August 2007 and March 2012. Potential participants were initially screened by medical record review, and only those who appeared to be eligible were approached for consent. After formal screening, all participants or their surrogates provided written informed consent. Written consent for collection of caregiver data was also obtained from the participants' designated caregivers.
Interventions
Eligible participants were randomized centrally by the coordinating center to 1 of the 4 treatment groups stratified by site using a random permuted block design with randomly varying block sizes. The treatment allocation ratio was 1:1:1:1. Patients, caregivers, and site investigators were blinded to treatment assignment. Eligible patients were randomly assigned to receive either alpha tocopherol plus a matching placebo for memantine, memantine plus a matching placebo for alpha tocopherol, alpha tocopherol plus memantine, or matching placebos for both memantine and alpha tocopherol. Alpha tocopherol (or matching placebo) was given as an oral dose of 1000 IU twice a day. The form of alpha tocopherol used in this study was DL-alpha-tocopheryl acetate (“synthetic” vitamin E) formulated as hard-gelatin, liquid-filled capsules. Memantine (or matching placebo) was titrated over 4 weeks to a maintenance dosage of 10 mg twice a day. Site investigators were directed to reduce the dosage of memantine (or matching placebo) to 5 mg twice a day for individuals whose estimated creatinine clearance at entry or during follow-up was less than 30 mL/min. Other dosage adjustments for memantine and alpha tocopherol were permitted based on participant tolerability.
Outcome Measures
The primary outcome of the study was the Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS-ADL) Inventory.12 The ADCS-ADL Inventory is designed to assess functional abilities to perform activities of daily living in Alzheimer patients with a broad range of dementia severity. The total score ranges from 0 to 78 with lower scores indicating worse function. Although there is not a consensus on a minimally clinically important difference for the ADCS-ADL Inventory, some clinicians, patients, or caregivers would consider a difference of 2 points as meaningful because it potentially represents, for example, a loss of dressing or bathing independently.
The ADCS-ADL Inventory was selected as the primary outcome measure for a number of reasons. First, it is an established outcome measure that is sensitive to multiple levels of functioning in AD. Second, it has substantial clinical relevance and can be more meaningful as an outcome measure than cognition; additionally, a patient's inability to perform ADLs may be more apparent to a caregiver than cognitive loss. Third, unlike a global measure such as the Clinical Global Impression of Change13 that relies on subjective judgment and has inconsistent interrater reliability, the ADCS-ADL Inventory is based on scoring of specific questions posed to an informant and has demonstrated excellent interrater reliability. Fourth, data on estimated rate of change based on a large cohort of patients with mild to moderate Alzheimer disease allowed for an efficiently designed study. Fifth, the ADCS-ADL Inventory can be administered by a telephone interview with the caregiver.
Secondary outcome measures included the MMSE,11 the most widely used instrument to assess cognitive function and dementia severity; the Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-cog),14,15 a commonly used measure to assess memory, language, and praxis functions in AD; the 12-item Neuropsychiatric Inventory (NPI),16 an assessment of the severity and frequency of psychological and behavioral problems in patients with dementia; the Caregiver Activity Survey (CAS),17 a measurement of the time caregivers spend assisting Alzheimer patients in 6 major areas of daily activities; and the Dependence Scale,18 which assesses 6 levels of functional dependence.
All adverse events (AEs) and serious adverse events (SAEs) were recorded. Serious AEs were coded according to the Medical Dictionary for Regulatory Activities. Caregivers and patients were queried about adverse experiences at each contact and specifically for patient falls, syncope, and congestive heart failure because of concerns from previous studies of high-dose alpha tocopherol treatment.1,19 Annual assessments also included a physical examination, a review of concomitant medications, and a blood draw for alpha tocopherol and memantine serum concentrations. In addition to mean changes in serum concentrations, cut points of 1.3-fold or greater increases in alpha tocopherol and 6 μg/dL or greater absolute increases in memantine compared with baseline were monitored because these increases are associated with a reasonable level of medication adherence. All randomized participants were scheduled for assessments every 6 months for a minimum of 6 months to a maximum of 4 years.
Statistical Analysis
The original sample size of 840 participants was designed to provide 90% power to detect a 4-point mean treatment difference with a mean of 2.5 years of follow-up (0.8 units per 6 months) between either treatment given alone vs placebo in the ADCS-ADL Inventory, assuming a type I error of 0.0083 to control for 6 treatment comparisons and adjusted for 2.5% losses per 6-month follow-up. A difference of 4 points was chosen based on previous studies of vitamin E and memantine in populations with more severe AD.1,3,4 This difference was also estimated as an approximate 20% reduction in the annual rate of decline, equivalent to slowing the rate of progression of the disease by nearly 6 months over the follow-up period. In January 2011, and prior to the scheduled end of recruitment, sample size reestimation was conducted per protocol based on the nuisance parameters and not the observed treatment effect that included the observed overall primary outcome variance (12.1), the correlation of the repeated measures (0.57), the loss rate (7.8% per 6 months), and the original protocol hypothesized slope of the treatment effect of 0.8 units per 6 months. Based on these data, the data monitoring committee recommended an extension of the enrollment period from 3 to 4.5 years and an increase in median follow-up from 2.5 to an estimated 3 years (minimum follow-up, 0.5 years; maximum, 4.0 years) to allow for a reduction in sample size to maintain approximately 90% power.
The effect of treatment on the primary end point was analyzed by a longitudinal repeated-measures mixed-effects model assuming missing at random, adjusted for medical center as a random effect and for the baseline ADCS-ADL Inventory score using all available data. In building the model, we first determined the best mean structure of the outcome (eg, time as linear or categorical and treatment × time interactions) and then determined the best-fitting and most parsimonious covariance structure for the data. Additional sensitivity analyses were conducted to examine the missing-at-random assumption (eTable 5 in the Supplement). Results are presented as least squares (LS) means differences from baseline with standard errors and 95% confidence intervals. P values are presented as unadjusted and adjusted for the 6 treatment comparisons using an overall type I error of 5% (2-sided).20 The model estimated LS means are presented as a delay in clinical progression over the average follow-up period by estimating the time it would take for a treatment group to decline to the level of the placebo group based on the annual rate of decline. Delays in clinical progression at annual time points are also presented using the same method. The ratio of the annual rates of decline are calculated to estimate an annual rate of delay in progression. The same analytic approach was used for the secondary outcomes except for the Dependence Scale, which was analyzed by a time-to-event (increase in dependence) interval-censored model assuming a Weibull distribution because the time of the event was known only at the end of a discrete interval of time (every 6 months).21,22 All fitted models included time as categorical with an unstructured covariance matrix. The test for a treatment × time interaction was not significant for any of the outcomes. The P values for SAE and mortality were not adjusted for multiple comparisons. All analyses were performed according to randomized treatment assignment using SAS version 9.2 (SAS Institute).
Results
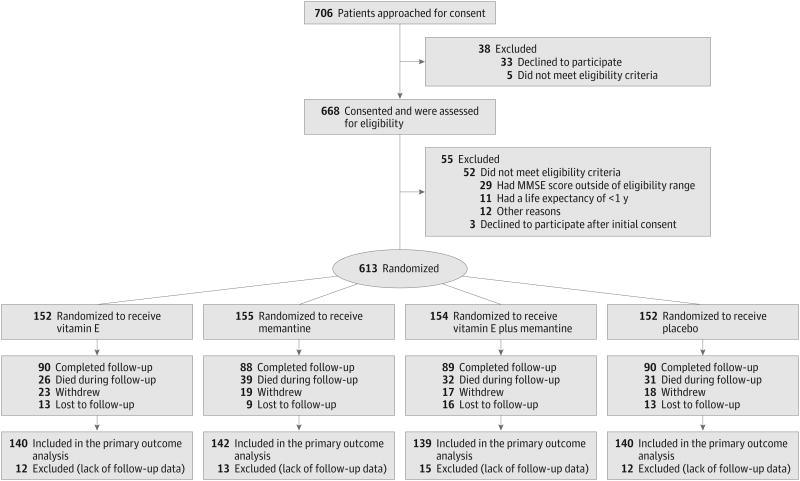
From August 7, 2007, to March 31, 2012, 706 veterans were approached for consent after initial medical record screening, 668 consented and were formally screened, and 613 were randomized: 152 (24.8%) to receive alpha tocopherol alone, 155 (25.3%) to receive memantine alone, 154 (25.1%) to receive alpha tocopherol plus memantine, and 152 (24.8%) to receive placebo (Figure 1). The most common reason for exclusion was an MMSE score outside the inclusion range of 12 to 26 (53% of those excluded). Participant follow-up concluded in September 2012 with an overall mean (SD) follow-up time of 2.27 (1.22) years with 2.35 (1.21) years for alpha tocopherol alone, 2.23 (1.22) years for memantine alone, 2.31 (1.24) years for alpha tocopherol plus memantine, and 2.18 (1.22) years for the placebo group. A total of 256 participants (42%) did not complete the trial (Figure 1). The most common reasons were death, 128 (50%), and withdrawal of consent, 77 (30%); 3 (1%) withdrew because of an adverse event possibly related to the study medication. Withdrawal rates were comparable across treatment groups.
Baseline Characteristics of the Patients
The baseline characteristics of the treatment groups were similar (Table 1 and eTable 1 in the Supplement). The mean (SD) age at enrollment was 78.8 (7.1) years; 97% of the participants were men; 86% were white, 13% were black, and 11% were Hispanic. The mean (SD) Charlson Risk Index score23 at entry was 2.5 (1.7), and the majority of participants (54%) had 2 or more comorbidity domains on the Comorbidity Disease Index.24,25 A total of 612 participants (99.8%) were taking an AChEI at baseline. The 2 most commonly prescribed AChEIs were donepezil (65%) and galantamine (32%). The mean (SD) ADCS-ADL Inventory and MMSE scores at baseline were 56.8 (14.2) and 21.0 (3.6), respectively. The apolipoprotein E (APOE) ε2, ε3, and ε4 allele frequencies for the entire group were 0.04, 0.66, and 0.30, respectively, with 50% of participants having at least 1 ε4 allele. The allele frequencies, genotype frequencies, and number of ε4 alleles did not differ between treatment groups (Table 1 and eTables 2, 3, and 4 in the Supplement).
Table 1
| Characteristic | Vitamin E (n = 152) | Memantine (n = 155) | Vitamin E + Memantine (n = 154) | Placebo (n = 152) |
|---|---|---|---|---|
| Age, mean (SD) [range], y | 78.6 (7.2) [55-93] | 78.8 (7.2) [53-92] | 78.3 (7.0) [54-94] | 79.4 (7.0) [61-96] |
| Male sex, No. (%) | 146 (96) | 149 (96) | 150 (97) | 149 (98) |
| Race/ethnicity, No. (%)a | ||||
 White White | 131 (86) | 132 (85) | 136 (88) | 131 (86) |
 Black Black | 21 (14) | 21 (14) | 18 (12) | 20 (13) |
 Other Other | 1 (1) | 2 (1) | 0 | 1 (1) |
 Hispanic Hispanic | 17 (11) | 15 (10) | 15 (10) | 19 (13) |
| Education, No. (%) | ||||
 <High school graduation <High school graduation | 41 (27) | 41 (26) | 26 (17) | 29 (19) |
 High school graduation High school graduation | 46 (30) | 48 (31) | 57 (37) | 56 (37) |
 Some college Some college | 27 (18) | 38 (25) | 37 (24) | 33 (22) |
 College graduation or advanced degree College graduation or advanced degree | 38 (25) | 28 (18) | 34 (22) | 34 (22) |
| APOE ε4 status, No. (%) | ||||
 Noncarriers Noncarriers | 47 (49.0) | 48 (45.7) | 59 (53.2) | 55 (53.4) |
 One ε4 allele One ε4 allele | 39 (40.6) | 44 (41.9) | 44 (39.6) | 38 (36.9) |
 Two ε4 alleles Two ε4 alleles | 10 (10.4) | 13 (12.4) | 8 (7.2) | 10 (9.7) |
| Charlson Risk Index score, mean (SD)b | 2.4 (1.7) | 2.4 (1.6) | 2.4 (1.8) | 2.7 (1.8) |
| Comorbidity Disease Index domains, No. (%)c | ||||
 ≤1 ≤1 | 64 (42) | 73 (47) | 75 (49) | 71 (47) |
 2 2 | 39 (26) | 43 (28) | 40 (26) | 36 (24) |
 ≥3 ≥3 | 49 (32) | 39 (25) | 39 (25) | 45 (30) |
| AChEI, No. (%)d | ||||
 Donepezil Donepezil | 104 (68) | 100 (65) | 100 (65) | 96 (63) |
 Galantamine Galantamine | 43 (28) | 47 (30) | 49 (32) | 55 (36) |
 Rivastigmine Rivastigmine | 5 (3) | 8 (5) | 4 (3) | 1 (1) |
 Time from AChEI start to randomization Time from AChEI start to randomization | ||||
  ≤12 wk ≤12 wk | 46 (31) | 36 (23) | 49 (32) | 37 (24) |
  >12 wk >12 wk | 105 (69) | 119 (77) | 104 (68) | 115 (76) |
| ADCS-ADL, mean (SD) [range]e | 56.6 (14.9) [13-78] | 57.3 (14.2) [11-78] | 56.4 (14.0) [15-78] | 56.8 (13.7) [8-78] |
| MMSE, mean (SD) [range]f | 21.3 (3.3) [12-26] | 20.8 (3.8) [12-26] | 21.3 (3.4) [12-26] | 20.8 (3.8) [12-26] |
| ADAS-cog score, mean (SD) [range]g | 18.5 (8.8) [5.0-51.0] | 19.5 (7.9) [2.3-50.0] | 18.0 (8.4) [4.0-56.0] | 19.1 (8.4) [3.0-46.3] |
| NPI score, median (range)]h | 7.5 (0-61) | 8.0 (0-95) | 8.0 (0-81) | 8.0 (0-62) |
| CAS time, median (range), hi | 2.7 (0-144) | 3.2 (0-49) | 3.2 (0-97) | 3.0 (0-59) |
| Dependence Scale level, No. (%)j | ||||
 0 0 | 5 (3) | 6 (4) | 8 (5) | 3 (2) |
 1 1 | 8 (5) | 6 (4) | 8 (5) | 5 (3) |
 2 2 | 85 (56) | 91 (59) | 80 (52) | 79 (52) |
 3 3 | 31 (20) | 35 (23) | 31 (20) | 37 (24) |
 4 4 | 4 (3) | 7 (5) | 10 (6) | 8 (5) |
 5 5 | 19 (13) | 10 (6) | 17 (11) | 20 (13) |
Abbreviations: AChEI; acetylcholinesterase inhibitor; ADAS-cog, Alzheimer Disease Assessment Scale–Cognitive Subscale; ADCS-ADL, Alzheimer's Disease Cooperative Study/Activities of Daily Living; APOE, apolipoprotein E; CAS, Caregiver Activity Survey; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory.
Primary Outcome
The results from the prespecified longitudinal analyses are presented in Figure 2, Figure 3, Figure 4, and Table 2. Data from 561 participants were analyzed (alpha tocopherol = 140, memantine = 142, combination = 139, placebo = 140) with 52 excluded because of a lack of any follow-up data. Over the mean follow-up time of 2.27 years, participants receiving alpha tocopherol had significantly slower decline than those receiving placebo as measured by the ADCS-ADL Inventory. The LS mean change (decline) from baseline in the ADCS-ADL Inventory for the alpha tocopherol treatment group was 3.15 units less (95% CI, 0.92-5.39; adjusted P = .03) than the decline in the placebo group (Table 2). Results from missing data sensitivity analyses were similar (eTable 5 in the Supplement).
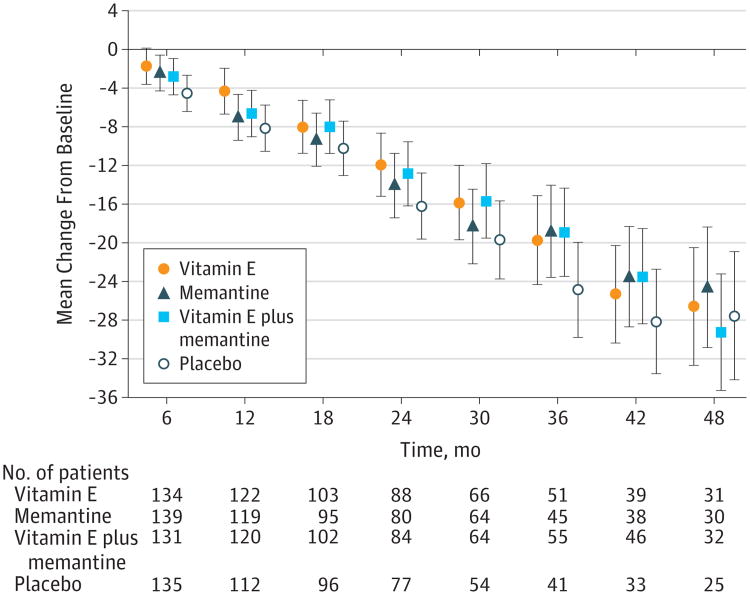
In this between-group comparison, lower scores indicate worse functioning. Data are least squares means at each time point. Values have been adjusted for baseline scores as a fixed effect and the study site as a random effect. ADCS-ADL indicates Alzheimer's Disease Cooperative Study/Activities of Daily Living; error bars, 95% CIs.
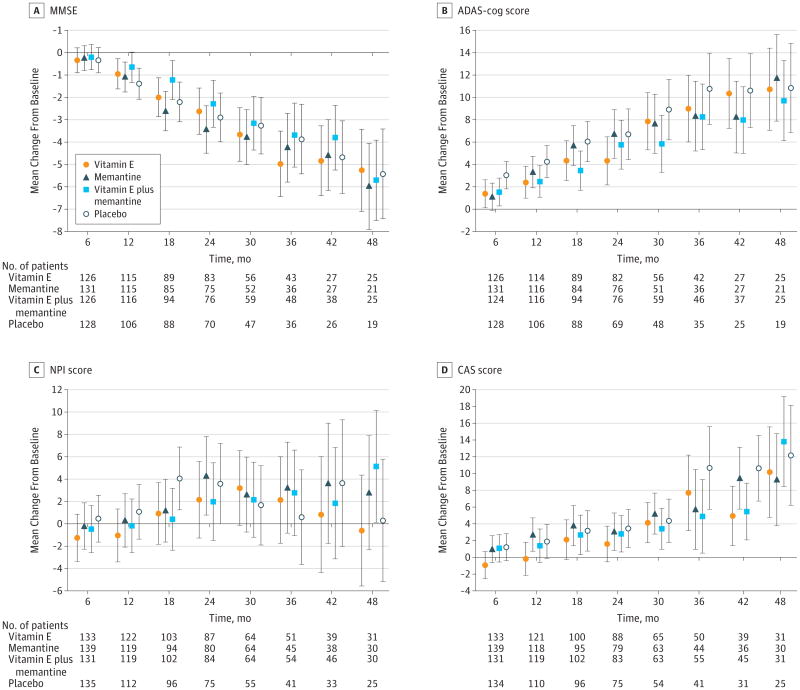
Data are least squares means at each time point. Values have been adjusted for baseline scores as a fixed effect and the study site as a random effect. For between-group comparisons of scores on the the Mini-Mental State Examination (MMSE) (A), lower scores indicate worse functioning. For comparisons of the Alzheimer Disease Assessment Scale–Cognitive portion (ADAS-cog) (B), Neuropsychiatric Inventory (C), and the Caregiver Activity Survey (D), higher scores indicate worse functioning. Error bars indicate 95% CIs.
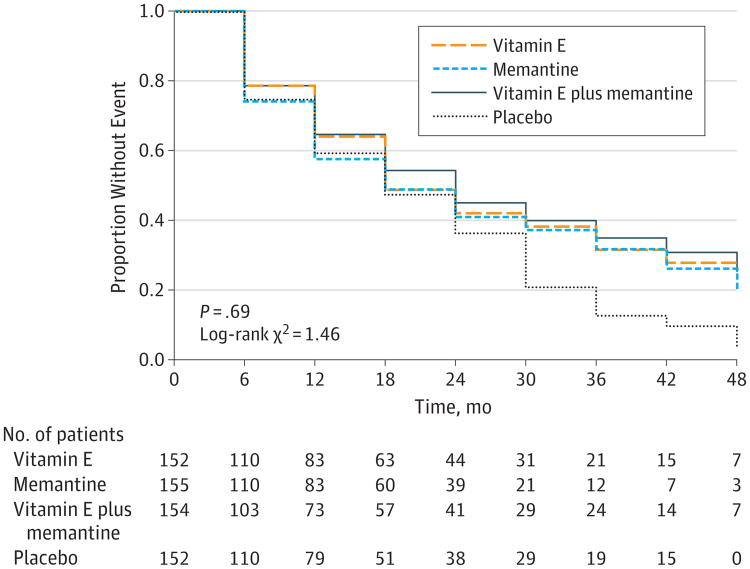
The Dependence Scale assesses 6 levels of functional dependence. Time to event is the time to loss of 1 dependence level (increase in dependence). We used an interval-censored model assuming a Weibull distribution because the time of the event was known only at the end of a discrete interval of time (every 6 months).
Table 2
| Vitamin E | Memantine | Vitamin E + Memantine | Placebo | |
|---|---|---|---|---|
| ADCS-ADL | (n = 140) | (n = 142) | (n = 139) | (n = 140) |
 Mean (SD) baseline scoreb Mean (SD) baseline scoreb | 57.20 (14.38) | 57.77 (13.78) | 57.16 (13.59) | 56.93 (13.61) |
 Least squares mean follow-up score (95% CI) Least squares mean follow-up score (95% CI) | 44.15 (41.98 to 46.32) | 42.98 (40.82 to 45.14) | 42.76 (40.58 to 44.94) | 50.00 (38.82 to 43.17) |
 Mean annual rate of functional decline (95% CI)c Mean annual rate of functional decline (95% CI)c | −6.08 (−7.04 to −5.13) | −6.60 (−7.55 to −5.65) | −6.70 (−7.66 to −5.74) | −7.47 (−8.43 to −6.52) |
 Least squares means change (SE) Least squares means change (SE) | −13.81 (1.11) | −14.98 (1.10) | −15.20 (1.11) | −16.96 (1.11) |
 Mean difference compared with placebo (95% CI) Mean difference compared with placebo (95% CI) | 3.15 (0.92 to 5.39) | 1.98 (−0.24 to 4.20) | 1.76 (−0.48 to 4.00) | |
 Unadjusted P valued Unadjusted P valued | .006 | .08 | .12 | |
 Adjusted P value Adjusted P value | .03 | .40 | .49 | |
| MMSE | (n = 136) | (n = 140) | (n = 136) | (n = 137) |
 Mean annual rate of cognitive declinec Mean annual rate of cognitive declinec | −1.37 (−1.66 to −1.07) | −1.41 (−1.70 to −1.11) | −1.29 (−1.59 to −0.99) | −1.46 (−1.76 to −1.16) |
 Least squares means change (SE) Least squares means change (SE) | −2.97 (0.33) | −3.05 (0.33) | −2.80 (0.33) | −3.16 (0.33) |
 Mean difference compared with placebo (95% CI) Mean difference compared with placebo (95% CI) | 0.19 (−0.54 to 0.92) | 0.12 (−0.61 to 0.84) | 0.37 (−0.36 to 1.10) | |
 Unadjusted P valued Unadjusted P valued | .60 | .75 | .32 | |
 Adjusted P value Adjusted P value | .84 | .84 | .84 | |
| ADAS-cog | (n = 135) | (n = 140) | (n = 136) | (n = 137) |
 Mean annual rate of cognitive declinec Mean annual rate of cognitive declinec | 2.75 (2.12 to 3.39) | 2.94 (2.31 to 3.57) | 2.82 (2.18 to 3.46) | 3.59 (2.95 to 4.22) |
 Least squares means change (SE) Least squares means change (SE) | 5.97 (0.70) | 6.38 (0.70) | 6.13 (0.71) | 7.78 (0.70) |
 Mean difference compared with placebo (95% CI) Mean difference compared with placebo (95% CI) | −1.80 (−3.28 to −0.33) | −1.39 (−2.85 to 0.07) | −1.65 (−3.12 to −0.17) | |
 Unadjusted P valued Unadjusted P valued | .02 | .06 | .03 | |
 Adjusted P value Adjusted P value | .10 | .25 | .14 | |
| NPI | (n = 140) | (n = 142) | (n = 139) | (n = 140) |
 Mean annual rate of increased symptomsc Mean annual rate of increased symptomsc | 0.35 (−0.51 to 1.21) | 0.82 (−0.04 to 1.69) | 0.79 (−0.08 to 1.66) | 1.00 (0.12 to 1.87) |
 Least squares means change (SE) Least squares means change (SE) | 0.79 (1.00) | 1.87 (1.00) | 1.79 (1.00) | 2.26 (1.01) |
 Mean difference compared with placebo (95% CI) Mean difference compared with placebo (95% CI) | −1.46 (−3.55 to 0.63) | −0.39 (−2.47 to 1.70) | −0.47 (−2.57 to 1.63) | |
 Unadjusted P valued Unadjusted P valued | .17 | .72 | .66 | |
 Adjusted P value Adjusted P value | .94 | .94 | .94 | |
| CAS | (n = 140) | (n = 142) | (n = 139) | (n = 140) |
 Mean annual rate of increased caregiver time, hc Mean annual rate of increased caregiver time, hc | 1.48 (0.80 to 2.15) | 2.43 (1.76 to 3.11) | 2.20 (1.52 to 2.88) | 2.26 (1.58 to 2.94) |
 Least squares means change (SE), h Least squares means change (SE), h | 3.35 (0.78) | 5.52 (0.78) | 5.00 (0.78) | 5.14 (0.79) |
 Mean difference compared with placebo (95% CI) Mean difference compared with placebo (95% CI) | −1.79 (−3.35 to −0.23) | 0.38 (−1.18 to 1.94) | −0.14 (−1.70 to 1.42) | |
 Unadjusted P valued Unadjusted P valued | .02 | .63 | .86 | |
 Adjusted P value Adjusted P value | .12 | .86 | .86 |
Abbreviations: AChEI, acetylcholinesterase inhibitor; ADAS-cog, Alzheimer Disease Assessment Scale–Cognitive Subscale; ADCS-ADL, Alzheimer's Disease Cooperative Study/Activities of Daily Living; APOE, apolipoprotein E; CAS, Caregiver Activity Survey; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory.
The annual rate of decline in ADLs was reduced by 19% with alpha tocopherol (−6.08) compared with placebo (−7.47) (Table 2). The mean treatment effect of 3.15 units also translates into a clinically meaningful delay in progression in the alpha tocopherol group of 6.2 months (95% CI, 5.4-7.4) compared with the placebo group. A delay in progression was sustained throughout most of the 4-year trial with delays at 1, 2, 3, and 4 years of 10.6, 8.7, 9.3, and 1.8 months, respectively. The LS mean decline for the placebo group was also greater than the decline for the memantine and the alpha tocopherol plus memantine groups, but the differences were not statistically significant. A significant negative treatment interaction between alpha tocopherol and memantine was detected on the primary outcome (P = .03).
The rate of decline was greater in those participants who were more severe and there was a larger treatment effect in the more severe group (eTable 6 in the Supplement); however, the interaction test of treatment and severity level was not significant (P = .38). The interaction test of treatment and medical center was also nonsignificant (P = .35).
Adherence
The mean percentage of days taking alpha tocopherol reported by care-givers was 65% in the alpha tocopherol group and 68% in the alpha tocopherol plus memantine group. The mean percentage of days taking memantine reported by caregivers was 68% in the memantine group and 66% in the alpha tocopherol plus memantine group. Of the 435 participants (71%) who had blood drawn for serum concentration assays at baseline and year 1, alpha tocopherol levels increased at year 1 by 1.3 times from baseline levels in 82% of the alpha tocopherol group (mean [SD] increase, 1.6 [1.4] mg/dL), 74% of the alpha tocopherol plus memantine group (mean [SD] increase, 1.5 [1.7] mg/dL), and in 7% of the 2 alpha tocopherol placebo groups (mean [SD] increase, 0.0 [0.2] mg/dL). Year 1 serum levels of memantine were 6 μg/dL or greater in 70% of the memantine group (mean [SD] increase, 9.9 [6.0] μg/dL) and in 70% of the alpha tocopherol plus memantine group (mean [SD] increase, 10.1 [10.1] μg/dL), and in 6% of the 2 memantine placebo groups (mean [SD] increase, 0.9 [3.6] μg/dL).
Secondary Outcomes
None of the treatment differences was significant for any of the secondary outcomes based on adjusted P values except for the comparison of alpha tocopherol vs memantine on the CAS (P = .03). The LS mean difference in the change (increase in hours per day) from baseline over the follow-up period on the CAS was 2.17 hours less (95% CI, 0.63-3.71) in the alpha tocopherol group (7.3 to 10.6) compared with the memantine group (6.7 to 12.2). Alpha tocopherol vs placebo treatment differences before adjustment for multiple comparisons favored alpha tocopherol on both the ADAS-cog and the CAS, but these differences were not statistically significant after adjustment for multiple comparisons (Table 2).
Safety
There were no significant differences between treatment groups on total AEs or SAEs (Table 3), including heart failure, falls, syncope, or bleeding events. Serious AEs occurring in 5% or more of participants in an active treatment group that were also greater than the frequency in the placebo group included falls (P = .89), bleeding (P = .78), pneumonia (P = .12), urinary tract infection (P = .66), and any infections or infestations (P = .01) (Table 3). When frequencies for the SAE category of infections or infestations were compared between treatment groups and placebo, the frequencies in the memantine alone group (31 events in 23 participants) and the combination group (44 events in 31 participants) were significantly greater than in the placebo group (13 events in 11 participants); however, alpha tocopherol alone vs placebo was not significant.
Table 3
| Event | Vitamin E (n = 152) | Memantine (n = 155) | Vitamin E + Memantine (n = 154) | Placebo (n = 152) |
|---|---|---|---|---|
| Adverse events | ||||
 Patients with event, No. (%) Patients with event, No. (%) | 91 (60) | 97 (63) | 90 (58) | 89 (59) |
 Adverse event, No. of events Adverse event, No. of events | 198 | 196 | 211 | 202 |
 Event attributed to study medications, No. of eventsa Event attributed to study medications, No. of eventsa | 35 | 29 | 33 | 24 |
| Adverse event >5% and ≥placebo, No. of events (% of patients with event)b | ||||
 Falls Falls | 58 (20) | 38 (17) | 64 (23) | 48 (20) |
 Bleedingc Bleedingc | 6 (4) | 5 (3) | 11 (6) | 5 (3) |
| Serious adverse events | ||||
 Patients with event, No. (%) Patients with event, No. (%) | 82 (54) | 84 (54) | 83 (54) | 95 (63) |
 Serious adverse event, No. of events Serious adverse event, No. of events | 180 | 187 | 179 | 170 |
 Event attributed to study medications, No. of eventsa Event attributed to study medications, No. of eventsa | 19 | 18 | 13 | 19 |
| Type of event, No. of eventsd | ||||
 Hospitalization Hospitalization | 152 | 143 | 146 | 128 |
 Other Other | 14 | 20 | 41 | 28 |
 Death Death | 26 | 39 | 32 | 31 |
| Annual mortality rate, %e | 7.3 | 11.3 | 9.0 | 9.4 |
| Cause of deathf | ||||
 Cardiac disorders Cardiac disorders | 5 | 10 | 8 | 10 |
 Nervous system disorders Nervous system disorders | 4 | 6 | 6 | 5 |
 Infections or infestations Infections or infestations | 4 | 4 | 3 | 2 |
 Neoplasms Neoplasms | 4 | 5 | 3 | 1 |
 Respiratory disorders Respiratory disorders | 2 | 4 | 5 | 3 |
 Other Other | 5 | 6 | 6 | 8 |
 Unknown Unknown | 2 | 4 | 1 | 2 |
| Serious adverse event >5% and ≥placebo, No. of events (% of patients with an event)b | ||||
 Falls Falls | 16 (10) | 16 (10) | 14 (8) | 18 (10) |
 Bleedingc Bleedingc | 7 (5) | 10 (5) | 7 (5) | 10 (5) |
 Pneumonia Pneumonia | 11 (5) | 9 (5) | 15 (10) | 5 (3) |
 Urinary tract infection Urinary tract infection | 8 (5) | 8 (5) | 13 (5) | 5 (3) |
 All infections or infestationsg All infections or infestationsg | 29 (13) | 31 (15) | 44 (20) | 13 (7) |
The annual mortality rate was 7.3% for the alpha tocopherol group, 11.3% for memantine, 9.0% for alpha tocopherol plus memantine, and 9.4% for placebo. The hazard ratios for alpha tocopherol, memantine, and alpha tocopherol plus memantine vs placebo were 0.87 (95% CI, 0.67-1.13), 1.06 (95% CI, 0.91-1.24), and 0.94 (95% CI, 0.57-1.54), respectively. The mortality hazard ratio for the main effect of alpha tocopherol vs no alpha tocopherol (the 2 alpha tocopherol groups vs the alpha tocopherol placebo groups) was 0.78 (95% CI, 0.55-1.10) and for memantine vs no memantine it was 1.21 (95% CI, 0.86-1.72). Kaplan-Meier survival curves are presented in the eFigure in the Supplement.
Discussion
The current study is one of the largest and longest treatment trials in patients with mild to moderate AD.26 It is the first large-scale clinical trial to assess not only the effectiveness of alpha tocopherol in patients with mild to moderate AD, but also the combination of alpha tocopherol and memantine. In addition, the study provides information on reported safety issues of alpha tocopherol27 that have resulted in decreased prescribing of alpha tocopherol for patients with AD.28 The allele and genotype frequencies for our multiethnic population were comparable with other AD cohorts.29,30
We found that when compared with placebo, 2000 IU/d of alpha tocopherol significantly delayed clinical progression in ADLs in patients with mild to moderate AD who were taking an AChEI. This effect was not seen in the memantine and the memantine plus alpha tocopherol groups. The observed alpha tocopherol scores on each secondary outcome declined less that the placebo group; however, none of the differences were significant. In addition, caregiver time increased least in the alpha tocopherol group compared with the other 3 groups; however, only the comparison between the alpha tocopherol group and the memantine group was significant after adjustment for multiple comparisons.
A delay in the annual rate of clinical progression in the alpha tocopherol group of 19% or approximately 6.2 months over the follow-up period is a meaningful treatment effect and is comparable with positive AChEI Alzheimer clinical trials that used the ADCS-ADL Inventory.31,32 This delay along with a reduction of approximately 2 hours of caregiver time per day in the alpha tocopherol group could have a major effect on informal and direct medical care costs.33,34 The results of the current study are consistent with the findings of Sano et al,1 which showed a delay in clinical progression of approximately 7 months for moderately severe AD in patients who took 2000 IU/d of alpha tocopherol over a 2-year period.
Decline in functioning in AD is increasingly recognized as an important determinant of both patient quality of life and social and economic costs.35 Maintaining the ability to perform ADLs is very relevant to patients and to caregivers. The loss of the ability to perform ADLs is associated not only with increased caregiver burden but also with nursing home placement.36-38 In the current study, the placebo group lost approximately 3 units more on the ADCS-ADL Inventory than the alpha tocopherol group. A loss of this magnitude could translate into either the complete loss of being able to dress or bath independently, for example, or losing independence on any 3 different ADLs. Because vitamin E is inexpensive, it is likely these benefits are cost-effective as alpha tocopherol improves functional outcomes and decreases caregiver burden.23,39
The lack of effectiveness of memantine in the current study is consistent with the negative findings reported in previous studies of AD patients with mild dementia.5-8 Our findings reinforce current VA memantine treatment guidelines that restrict the use of memantine to patients with moderately severe AD.3-5 The absence of memantine related safety issues in the current study agrees with the findings in previously reported studies of memantine.5-8
When compared with placebo, the alpha tocopherol alone group showed significant benefit, but paradoxically, the combination of alpha tocopherol and memantine had less effect than either alpha tocopherol alone or memantine alone. Although it is possible that memantine could have interfered with the effectiveness of alpha tocopherol, it is difficult to postulate a plausible mechanism. To our knowledge, no studies have examined whether memantine interferes with alpha tocopherol's antioxidant effects. This study and previously published studies3,4 do not address whether a similar interaction exists for patients with AD and severe dementia for whom a combination of alpha tocopherol and memantine could be recommended.
In contrast to the conclusion drawn from a 2005 metaanalysis of vitamin E,27 which showed that high-dose vitamin E (≥400 IU/d) may increase the risk of all-cause mortality, we found no significant increase in mortality with vitamin E. The annual mortality rate was 7.3% in the alpha tocopherol group vs 9.4% for the placebo group. The observed hazard rate for mortality was reduced by 13% (95% CI, −33% to 13%) in the alpha tocopherol group compared with the placebo group and by 22% (95% CI, −45% to 10%) in the 2 alpha tocopherol groups compared with the 2 alpha tocopherol placebo groups. Of note, the meta-analysis included 1 Alzheimer study,1 and in that study the all-cause mortality rate was also reduced in the alpha tocopherol groups compared with the other treatment groups.
The original target sample size of 840 could not be achieved because of a lower than expected number of eligible patients, greater than anticipated staff workload to enroll and follow up participants, and higher than predicted refusal rates. The reestimated sample size with the extension of follow-up reduced the sample size to a feasible target (600). By the completion of the trial, the revised target sample size was exceeded (613); however, the mean follow-up was less than expected primarily because of a greater withdrawal and mortality rate than estimated, a non-uniform enrollment rate over time, and a larger number of missed final visits than expected. This reduced power and possibly contributed to some of the nonsignificant adjusted P values for the secondary outcomes that were significant prior to adjustment for multiple comparisons.
Based on our data, there was no evidence that any of the treatments were unsafe; however, the size of the study did not allow us to detect infrequent but potentially significant adverse events. Only 1 of the 21 system organ classes recorded for SAEs showed a significant difference in frequencies among treatment groups, namely infections or infestations. None of the 30 individual preferred terms that comprised the events in this system organ class, showed a significant difference among treatment groups. An increase in infections or infestations has not been reported in other studies on alpha tocopherol.1,2,27 Based on the number of different types of AEs examined, it is plausible that the significant difference detected for infections or infestations is due to chance alone. Although falls and syncope were more frequent in participants taking alpha tocopherol in the study by Sano et al,1 there were no statistically significant differences among the groups for these AEs. The incidences of falls and syncope in the current study did not differ among treatment groups for both AEs and SAEs.
One limitation of the study was the higher than anticipated rates of withdrawal, although the withdrawal rates were nearly equivalent across all treatment groups. Because the average length of treatment for participants was considerably longer than most published trials in patients with similar AD severity,9 the overall proportion of withdrawals in the current study was somewhat higher. The higher than anticipated withdrawal rates were mostly addressed with the sample size reestimation. A second limitation was the small percentage of women in the study; however, based on the results from previous studies, there is no evidence that the effectiveness of alpha tocopherol or memantine differs in males compared with females.1-10
Conclusions
We found that a dosage of 2000 IU/d of alpha tocopherol was effective in slowing the functional decline of mild to moderate AD and was also effective in reducing caregiver time in assisting patients. Neither memantine nor the combination of alpha tocopherol and memantine showed clinical benefit in patients with mild to moderate AD. These findings suggest that alpha tocopherol is beneficial in mild to moderate AD by slowing functional decline and decreasing caregiver burden.
Acknowledgments
Funding/Support: VA TEAM-AD was funded by the Veterans Affairs Cooperative Studies Program, Office of Research and Development, Clinical Science R&D. Forest Research Institute, a Division of Forest Laboratories, donated the memantine and matching placebo tablets. DSM Nutritional Products donated the DL-alpha-tocopheryl acetate oil and funding for the purchase of the soybean oil from Arista Industries.
Role of the Sponsors:The US Department of Veterans Affairs Cooperative Studies Program participated in the design and oversaw the conduct of the study. The Veterans Affairs Cooperative Studies Program, Forest Research Institute, and DSM Nutritional Products had no input into data collection, management, analysis, and interpretation of the data or preparation, review, or approval of the manuscript.
Glossary
| AChEI | acetylcholinesterase inhibitor |
| ADAS-cog | Alzheimer Disease Assessment Scale–Cognitive Subscale |
| ADCS-ADL | Alzheimer's Disease Cooperative Study/Activities of Daily Living |
| APOE | apolipoprotein E |
| CAS | Caregiver Activity Survey |
| MMSE | Mini-Mental State Examination |
| NPI | Neuropsychiatric Inventory |
Author Contributions
Dr Guarino had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dysken, Sano, Asthana, Vertrees, Schellenberg, Peduzzi, Guarino.
Acquisition of data: Asthana, Pallaki, Llorente, Love, Schellenberg, McCarten, Malphurs, Prieto, Chen, Loreck, Trapp, Bakshi, Mintzer, Heidebrink, Vidal-Cardona, Arroyo, Cruz, Zachariah, Kowall, Chopra, Craft, Thielke, Turvey, Woodman, Monnell, Gordon, Segal.
Analysis and interpretation of data: Dysken, Asthana, Sano, Vertrees, Pallaki, Llorente, Love, Schellenberg, McCarten, Malphurs, Chen, Loreck, Trapp, Bakshi, Mintzer, Heidebrink, Arroyo, Cruz, Kowall, Chopra, Craft, Turvey, Woodman, Monnell, Gordon, Tomaska, Segal, Peduzzi, Guarino.
Drafting of the manuscript: Dysken, Guarino.
Critical revision of the manuscript for important intellectual content: Dysken, Sano, Asthana, Vertrees, Pallaki, Llorente, Love, Schellenberg, McCarten, Malphurs, Prieto, Chen, Loreck, Trapp, Bakshi, Mintzer, Heidebrink, Vidal-Cardona, Arroyo, Cruz, Zachariah, Kowall, Chopra, Craft, Thielke, Turvey, Woodman, Monnell, Gordon, Segal, Peduzzi, Guarino.
Statistical analysis: Peduzzi, Guarino.
Obtained funding: Dysken, Vertrees, Peduzzi, Guarino.
Administrative, technical, or material support: Dysken, Sano, Asthana, Vertrees, Pallaki, Llorente, Love, Schellenberg, McCarten, Malphurs, Prieto, Chen, Loreck, Trapp, Bakshi, Mintzer, Vidal-Cardona, Arroyo, Cruz, Zachariah, Kowall, Chopra, Thielke, Woodman, Gordon, Tomaska, Segal, Guarino.
Study supervision: Dysken, Sano, Asthana, Vertrees, Pallaki, Llorente, Love, Schellenberg, McCarten, Malphurs, Chen, Loreck, Trapp, Bakshi, Mintzer, Heidebrink, Vidal-Cardona, Arroyo, Cruz, Kowall, Chopra, Craft, Turvey, Monnell, Gordon, Guarino.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Sano reported having been a board member for Medication and a data and safety monitoring board member for the ASPREE study; having received consulting fees from Takeda, Medpace, sanofi-aventis, Trans-tech Pharma, Elsai Pharmaceutical, Eli Lilly, Neurocog, Targacept, and Vital Images; having provided expert testimony for McDermott Will & Emory; having received grants from the Alzheimer's Association; and having received speakers' fees or travel expenses from the University of New England, Medical College of South Carolina, International Psychogeriatric Association, and Alzheimer's Association. Dr Asthana reported having received grant support from Pfizer, Elan, Merck, Eli Lilly, Lilly, Eisai, and Wyeth and having served as associate editor for a textbook from McGraw Hill Publishers. Dr Mintzer reported having been vice president of clinical affairs for NeuroQuest; being majority owner of BioPharma Connex; and having received grants from the National Institute on Aging, Takeda Global Research and Development Center, Pfizer, Genentech, Merck, Accera, Elan Pharma International, Avanir Pharmaceuticals, Hoffmann-LaRoche, Eli Lilly, Novartis, Baxter Healthcare, Wyeth, and Janssen AI Research and Development. Dr Chopra reported having received a Boston University Alzheimer's Disease Core Center Pilot Grant. No other disclosures were reported.
VA TEAM-AD Study
Planning Committee: M. Dysken (Study Chair), S. Asthana, P. Guarino, J. Hanlon, M. Kunik, P. Lavori, P. Peduzzi, E. Perry, M. Sano, G. Schellenberg, T. Sunderland, G. Vatassery (deceased), J. Vertrees, L. Volicer. Executive Committee: M. Dysken (Chair), S. Asthana, P. Guarino, M. Llorente, S. Love, M. Pallaki, M. Sano, G. Schellenberg, G. Vatassery (deceased), J. Vertrees. Data Monitoring Committee: K. Kieburtz (Chair), C. Kawas, E. Lonn (resigned), P. Rabins, J. Rochon, D. Sultzer, R. Thomas. VA Cooperative Studies Program Human Rights Committee, West Haven, Connecticut: R. Marottoli (Chair), H. Allore, D. Beckwith, W. Farrell, R. Feldman, R. Mehta, J. Neiderman, E. Perry, S. Kasl, M. Zeman. VA Site Investigators and Coordinators: Ann Arbor, Michigan: J. Heidebrink, R. S. Turner, N. Barbas, C. Bloehm, J. Lord, K. Belanger, N. Ricci, C. Nwankwo, C. Fletcher; Baltimore, Maryland: D. Loreck, L. Katzel, K. Anderson, G. Kavanagh, S. Carney, A. Loreck. (Bay Pines, Florida) A. Cruz, S. Reddy, N. Purohit, R. Tamayo, K. Monnell, S. Huda, S. Zachariah, W.C. McCarthy; Boston, Massachusetts: N. Kowall, M. Chopra, B. Seltzer (deceased), K. Kolbe; Charleston, South Carolina: J. Mintzer, O. Brawman-Mintzer, A. Senseney, D. Courtney, M. Stuckey, S. Russell, J. A. Sweeney;. Cleveland, Ohio: M. Pallaki, P. Chen, T. Hornick, T. Dolinar, L. Abood, A. Coulter, S. Truax, D. Davis; Dallas, Texas; G. Trapp, R. Bakshi, L. Moody, N. Flye, D. Turner-Knight; Iowa City, Iowa: C. Turvey, C. Woodman, A. Ray, K. Ekstam Smith, N. Suiter; Madison, Wisconsin: S. Asthana, C. Gleason, S. Barczi, C. Carlsson, N. Lane, M. Wroblewski, Z. Zugin, J. J. Fruehling; Miami, Florida: J. Malphurs, M. Llorente, F. Adan, S. Prieto, M. Horvath, D. Santiago, G. Athappilly, A. Cortes, A. Vazquez, R. Dreize, F. Ostovary, E. Palaois, M. Oliveira, J. Pino, L. Claude; Minneapolis, Minnesota: J. McCarten, H. Fink, C. Erickson, L. Becker-Grandle; Salisbury, North Carolina: K. Monnell, K. Gordon, K. Phillips, D. Eknoyan; San Juan, Puerto Rico: A. Vidal-Cardona, L. Arroyo, A. Melendez, L. Santiago, B. Padilla; Seattle, Washington: S. Craft, J. Breitner, S. Thielke, K. Enstrom, J. Tidwell, R. Bridgan, K. Bowton, D. Dahl. Study Chair's Office, VA Health Care System, Minneapolis: M. Dysken (Study Chair), S. Love, J. Tomaska. Central Laboratory, VA Health Care System, Minneapolis: G. Vatassery (deceased), Y. Segal, E. Smith, H. Quach. VA Cooperative Studies Program Coordinating Center, VA Connecticut Healthcare System, West Haven: P. Guarino (Director, Study Biostatistician), M. Antonelli, E. Jobes, C. Joncas, S. Joyner, K. Kirkwood, P. Peduzzi, M. Perry, E. Petrokaitis, J. Russo, J. Scholl, S. Yang, S. Zellner. VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, New Mexico: M. Sather (Director), J. Vertrees (Study Clinical Research Pharmacist), S. Campbell, D. Conner, E. Copeland, A. Davis S. Jenkins, B. Matura. VA Cooperative Studies Program Site Monitoring, Auditing and Review Team, Albuquerque: C. Haakenson, D. Krueger. VA Cooperative Studies Program Central Biorepository (MAVERIC), VA Healthcare System, Boston: M. Brophy (Director), D. Humphries, D. Govan. VA Cooperative Studies Program DNA Bank Coordinating Center, VAMC Palo Alto, CA: J. Cockroft, S. Bobra, A. Baylosis, R. Dodson, I. Belitskaya-Levy. VA Office of Research and Development, Clinical Science Research and Development, Washington, DC: T. O'Leary (Director, Deputy Chief Research and Development Officer), G. Huang (Deputy Director, Cooperative Studies Program).
Footnotes
This supplementary material has been provided by the authors to give readers additional information about their work.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jama.2013.282834
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4109898?pdf=render
Citations & impact
Impact metrics
Article citations
Exploring the Role of Reactive Oxygen Species in the Pathogenesis and Pathophysiology of Alzheimer's and Parkinson's Disease and the Efficacy of Antioxidant Treatment.
Antioxidants (Basel), 13(9):1138, 20 Sep 2024
Cited by: 0 articles | PMID: 39334797 | PMCID: PMC11429442
Review Free full text in Europe PMC
Artemisinin inhibits neuronal ferroptosis in Alzheimer's disease models by targeting KEAP1.
Acta Pharmacol Sin, 09 Sep 2024
Cited by: 0 articles | PMID: 39251858
Associations between Brain Alpha-Tocopherol Stereoisomer Profile and Hallmarks of Brain Aging in Centenarians.
Antioxidants (Basel), 13(8):997, 17 Aug 2024
Cited by: 0 articles | PMID: 39199242 | PMCID: PMC11351880
Molecular mechanisms of mitochondrial homeostasis regulation in neurons and possible therapeutic approaches for Alzheimer's disease.
Heliyon, 10(17):e36470, 17 Aug 2024
Cited by: 0 articles | PMID: 39281517 | PMCID: PMC11401100
Review Free full text in Europe PMC
Targeting the NRF2 pathway for disease modification in neurodegenerative diseases: mechanisms and therapeutic implications.
Front Pharmacol, 15:1437939, 25 Jul 2024
Cited by: 0 articles | PMID: 39119604 | PMCID: PMC11306042
Review Free full text in Europe PMC
Go to all (259) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00235716
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Vitamin E and memantine in Alzheimer's disease: clinical trial methods and baseline data.
Alzheimers Dement, 10(1):36-44, 11 Apr 2013
Cited by: 32 articles | PMID: 23583234 | PMCID: PMC4128187
Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial.
JAMA, 291(3):317-324, 01 Jan 2004
Cited by: 699 articles | PMID: 14734594
Vitamin E for Alzheimer's dementia and mild cognitive impairment.
Cochrane Database Syst Rev, 4:CD002854, 18 Apr 2017
Cited by: 64 articles | PMID: 28418065 | PMCID: PMC6478142
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIA NIH HHS (3)
Grant ID: P50 AG005136
Grant ID: P30 AG013846
Grant ID: P50 AG005138