Abstract
Free full text

Insulin-like peptide 5 is an orexigenic gastrointestinal hormone
Associated Data
Significance
Hormonal factors from specialized enteroendocrine cells in the gut epithelium link the availability of dietary nutrients to energy utilization and storage. Many gut hormones also affect behaviors such as appetite and foraging, conveying for example the satiating effects of food consumption. Here we identify insulin-like peptide 5 (Insl5) as a product of colonic endocrine L-cells, and show that levels were elevated in calorie-restricted mice and reduced after feeding. Consistent with this profile Insl5 administration stimulated food intake in mice, indicating it should join ghrelin as only the second identified gut hormone that enhances appetite. Modulating the Insl5 axis presents a new strategy for the treatment of metabolic disease and obesity.
Abstract
The gut endocrine system is emerging as a central player in the control of appetite and glucose homeostasis, and as a rich source of peptides with therapeutic potential in the field of diabetes and obesity. In this study we have explored the physiology of insulin-like peptide 5 (Insl5), which we identified as a product of colonic enteroendocrine L-cells, better known for their secretion of glucagon-like peptide-1 and peptideYY. i.p. Insl5 increased food intake in wild-type mice but not mice lacking the cognate receptor Rxfp4. Plasma Insl5 levels were elevated by fasting or prolonged calorie restriction, and declined with feeding. We conclude that Insl5 is an orexigenic hormone released from colonic L-cells, which promotes appetite during conditions of energy deprivation.
The success of bariatric surgery in the treatment of morbid obesity and its surprising ability to resolve a high percentage of cases of type 2 diabetes has sparked a renewal of interest in the intestinal endocrine system (1, 2). Gut hormones have a variety of physiological actions outside the intestine, and play a central role in linking food ingestion to peripheral nutrient disposal and appetite (3, 4). Indeed, glucagon-like peptide-1 (GLP-1) analogs and inhibitors of GLP-1 degradation are now widely prescribed for the treatment of type 2 diabetes, offering additional beneficial effects on body weight compared with conventional insulin secretagogues (5, 6). As increasing evidence implicates enteroendocrine L-cells, which secrete GLP-1 together with the anorectic peptides oxyntomodulin and peptideYY (PYY), as substantial players in postbariatric physiology (7), a question that has raised considerable interest is whether L-cells or their close relatives (8, 9) produce additional peptides of therapeutic significance.
Insulin-like peptide 5 (Insl5) is a member of the relaxin family of peptides, similar in tertiary structure to insulin and the insulin-like growth factors (10). It has been identified recently in colonic tissue and neuroendocrine tumors (11, 12), but its function remains unclear. Although some members of the relaxin family have important roles in reproductive physiology and remodeling of connective tissue, the functions of others, including Insl5, have remained elusive (13). One recent report suggested a role of Insl5 in insulin secretion, based on the initial observation of impaired glucose tolerance in aging Insl5−/− mice; however, the phenotype was mild and only observed on a 129/Sv, but not on a C57B6, genetic background (14).
Insl5 has been reported to act through the G protein coupled relaxin/insulin-like family peptide receptor-4 (Rxfp4). Human and murine Insl5 have been shown to reduce cAMP through a pertussis toxin sensitive Gαi signaling pathway in cells heterologously expressing Rxfp4. By contrast, Insl5 does not stimulate the related Rxfp3, unlike relaxin-3, which is an agonist at both receptors (15, 16). The pairing of receptor and ligand is further supported by the observation that both Insl5 and Rxfp4 are inactivated in tandem by pseudogenization in some species such as rat and dog (17).
We show here that Insl5 is produced by a subset of enteroendocrine L-cells in the colon in close proximity to Rxfp4 positive enteric neurons. However, unlike the other known hormonal products of L-cells, Insl5 acts as an orexigenic signal in mice and is up-regulated under conditions of calorie restriction (CR).
Results
Identification of Insl5 as an Enteroendocrine Hormone in Mouse and Human.
Insl5 was identified as an L-cell transcript in a microarray analysis of mouse L-cell populations purified by their transgenic expression of a fluorescent protein (Venus) driven by the proglucagon (Gcg) promoter (GLU-Venus mice) (18). Quantitative RT-PCR (qRT-PCR) analysis of FACS-purified murine L-cells from different regions of the intestine confirmed that L-cells from jejunum, ileum and colon expressed the expected transcripts for Gcg and Pyy, but that Insl5 message was largely restricted to the colonic L-cell population (Fig. 1A). Insl5 message was ~500-fold lower in non–L-cells compared with colonic L-cells (Fig. 1A). By flow cytometric analysis, Insl5 immunoreactivity was detected in 56 ± 2% (n = 3) of L-cells isolated from mouse colon/rectum (Fig. 1B). In tissue homogenates from different regions of the mouse large intestine, we detected high Insl5 expression in the ascending, transverse and descending colon and proximal rectum, with lower levels in the cecum and distal rectum (Fig. 1C). Outside the gastrointestinal tract low levels of Insl5 mRNA were detected in the pancreas, thymus, and eye (Fig. S1A). No Insl5 message was detected in pancreatic islet cells (Fig. S1B). Immunostaining of murine primary colonic cultures (Fig. 1D), and of colonic sections from human (Fig. 1E) or mouse (Fig. S1C, controls in Fig. S1 D and E), revealed cells costained for Insl5 together with GLP-1 and PYY.
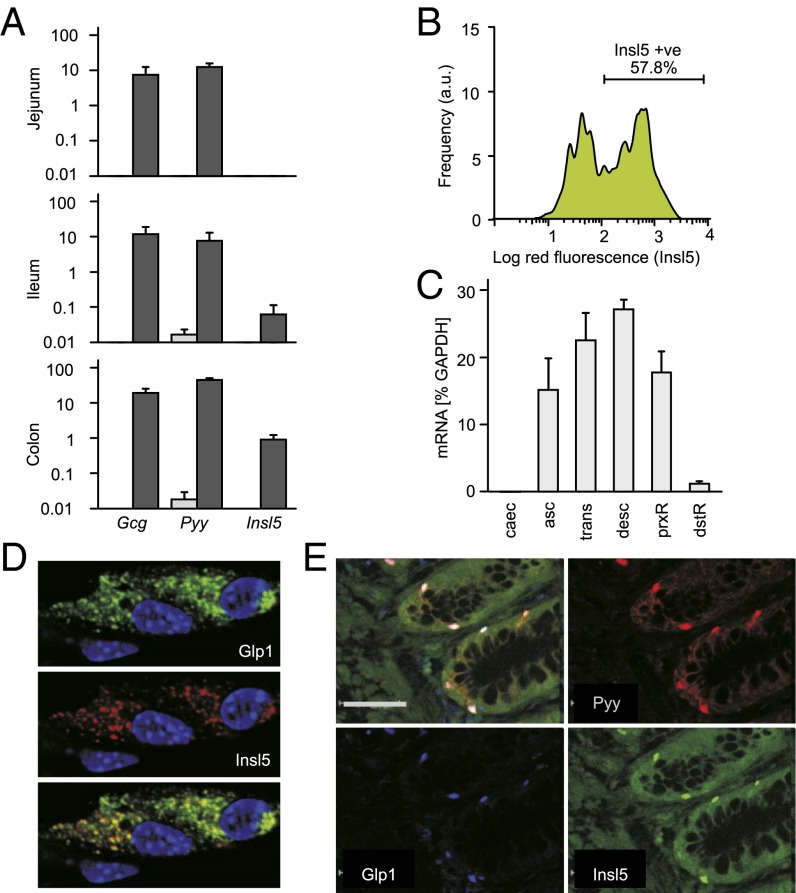
Insl5 is expressed in GLP-1–positive enteroendocrine cells. (A) L-cells (dark gray) and control cells (light gray) were isolated by flow cytometry from small and large intestine of transgenic GLU-Venus mice (18). Expression of Gcg, Pyy and Insl5 mRNAs were analyzed by qRT-PCR, and presented relative to β-actin (n = 3). (B) Primary colon/rectal tissue suspensions from GLU-Venus mice were immunostained for Insl5 with a red fluorescent secondary, and Insl5 staining in Venus positive L-cells was recorded by FACS analysis. (C) Expression of Insl5 in the mouse ascending (asc), transverse (trans) and descending (desc) colon and proximal (prxR) and distal rectum (dstR), but not cecum (Caec), by qRT-PCR. (D) Primary murine colonic cultures immunostained for GLP-1 (Top) and Insl5 (Middle), revealing colocalization by confocal microscopy (Bottom). Nuclear stain: DAPI. (E) Immunohistochemistry of human colon demonstrated colocalization of Insl5 with PYY and GLP-1. (Scale bar: 500 μm.)
Sites of Expression of the Insl5 Receptor, Rxfp4.
By qRT-PCR, we detected Rxfp4 expression throughout the length of the mouse colon and cecum, as well as in the nodose ganglion. Expression was low in the distal rectum, and not detected in the hypothalamus (Fig. 2 A and B). A similar profile in the intestine and nodose ganglion was found for the GLP-1 receptor (Glp1r, Fig. 2B). Rxfp3, by contrast, was expressed at higher levels in the hypothalamus than colon (Fig. 2A) (16). Within the mouse colon, in situ hybridization revealed Rxfp4 signal in a pattern consistent with expression in submucosal and myenteric ganglia (Fig. 2C).
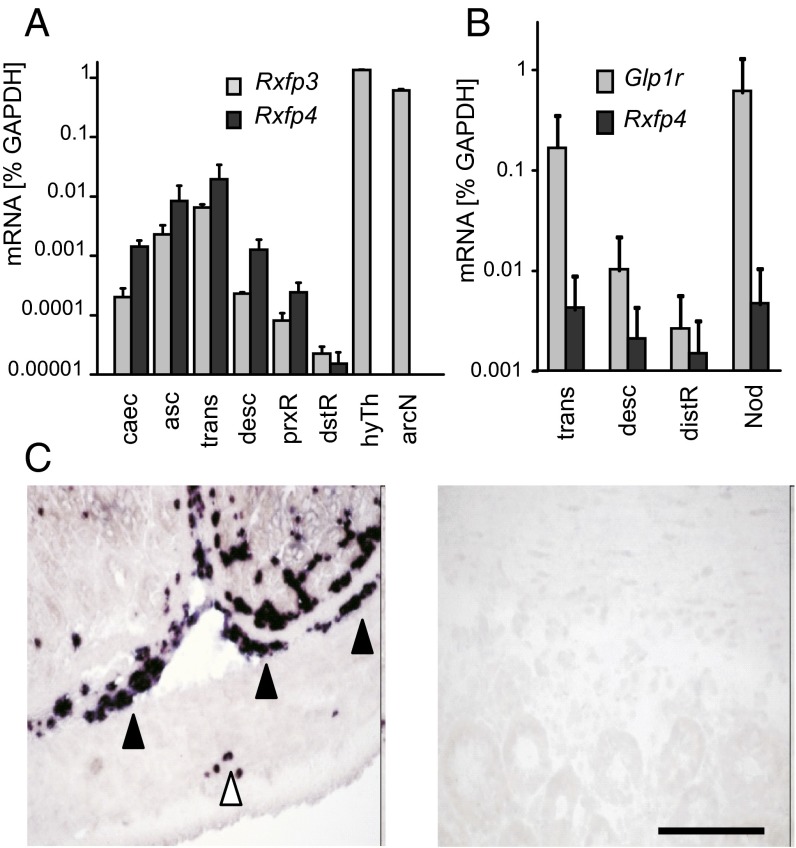
Patterns of Rxfp4 expression. (A) mRNAs for Rxfp4 and Rxfp3 were detected by qRT-PCR in the different regions of large intestine: asc, ascending colon; caec, cecum; desc, descending colon; distR, distal rectum; prxR, proximal rectum; trans, transverse colon. Rxfp3 but not Rxfp4 was detectable in the hypothalamus (hyTh) and the arcuate nucleus (arcN). Data are presented as mean ± SEM (n = 3). (B) Glp1r and Rxfp4 are expressed in a similar pattern in the gastrointestinal tract (abbreviations as in A) and in the nodose ganglion (Nod). Data are presented as mean ± SEM (n = 3). (C) In situ hybridization of mouse colon using a probe for Rxfp4 (Left) revealed a pattern consistent with expression in the myenteric (open arrow heads) and submucosal (filled arrow heads) plexus of the enteric nervous system. No labeling was observed using a sense Rxfp4 control probe (Right; scale bar: 100 μm).
Influence of Meal Ingestion and Chronic Energy Balance on Insl5 Production in the Mouse.
As Insl5 is a peptide secreted by L-cells, we asked whether its plasma level is dependent on food intake. Mice on an ad libitum chow diet were fasted overnight, and blood samples taken before and at different times after refeeding. Insl5 concentrations fluctuated initially, but were consistently suppressed at later times of the refeeding phase (Fig. 3A). To validate these results, we repeated the fasting and late refeeding time points on a separate cohort of animals, using an Insl5 ELISA instead of the RIA. Insl5 concentrations obtained with the ELISA were generally lower, but a similar pattern was observed with high levels in the fasting state that were suppressed by refeeding (Fig. 3B).
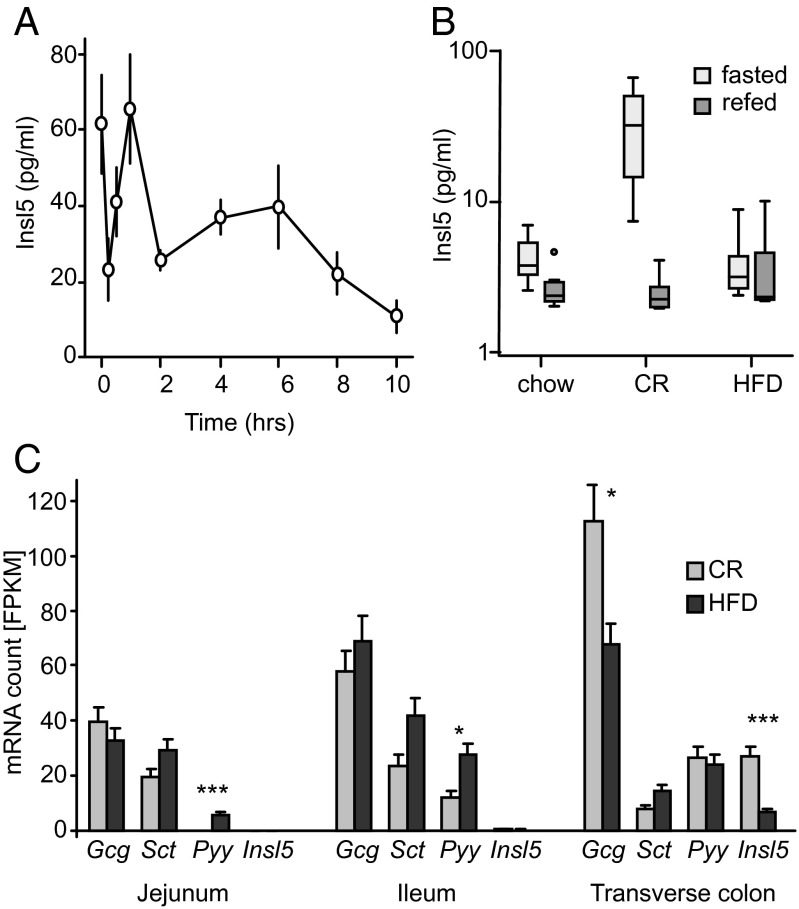
Insl5 responses to acute and chronic fasting. (A) Plasma levels of Insl5 in mice during refeeding with chow following an overnight fast, determined using an Insl5 RIA (Phoenix). Data are represented as the mean ± 1 SEM. Concentrations fell significantly after reintroduction of food, as assessed by Kruskal-Wallis test for independent samples (P < 0.001; n ≥ 10). (B) Male C57BL/6 mice were either on chow ad libitum, 60% caloric restriction or HFD for 2 wk. After an overnight fast or after 10hrs refeeding plasma Insl5 were measured by ELISA (Kamiya). Fasting Insl5 levels were higher in the CR cohort than in the HFD or chow cohorts (Kruskal Wallis test, P < 0.001). Comparing fasted and refeeding Insl5 levels within each diet group, significant differences were detected for chow and CR, but not the HFD cohort (P = 0.002 and P < 0.001, respectively. Mann–Whitney u test; n = 10, boxplot with median). (C) Expression of Gcg, secretin (Sct), Pyy and Insl5 mRNAs after CR or HFD in the distal intestine. C57BL/6 mice were either fed a HFD or were restricted to 60% caloric intake of a control group fed chow ad libitum (CR) for 4 wk. mRNA was harvested just before provision of the daily food ration in the CR cohort and at the same time in the ad libitum fed HFD group. Pooled cDNA (n = 6) was quantified by sequencing and data are represented as fragments per kilobase exon per million fragments (FPKM + SD). Data were analyzed using Cuffdiff, which provides the following P values adjusted for multiple testing: Colon: Insl5: 1.56 × 10−7; Gcg: 0.0449; Jejunum: Pyy: 8.58 × 10−6; Ileum: Pyy: 0.0119. *P < 0.05, ***P < 0.01.
We explored the response to chronic changes in energy balance in groups of male C57BL/6J mice that were either fed on high-fat diet (HFD, 45% fat) or subjected to CR (60% of the ad libitum chow consumption) for 2 wk. Fasting plasma levels were significantly higher in the CR group compared with ad libitum and HFD-fed mice (Fig. 3B). In the CR group, as in the ad libitum fed mice, Insl5 concentrations fell after refeeding. This pattern was lost in the HFD cohort, and no significant difference was detected between the 10-h-refed concentrations across all three groups. Interestingly, when in an independent experiment mice were subjected to prolonged calorie restriction for 10 wk instead of 2, Insl5 concentrations remained high at 10-h refeeding (Fig. S2B).
To explore the transcriptional regulation of enteroendocrine hormones in different parts of the gut, another cohort of C57BL/6J males was placed on HFD or 60% CR (chow) for 4 wk. At the end of the study period, intestinal tissue from different regions was harvested and the transcriptome sequenced and quantitatively analyzed. Transcripts of the anorexigenic peptide Pyy were elevated in the HFD group in both jejunum and ileum, but were not different in the colon (Fig. 3C). By contrast, in the colon of the CR group we detected a marked increase in Insl5 as well as an increase in Gcg transcripts. The combined findings that CR increased Insl5 transcripts and plasma Insl5 concentrations, and that circulating levels were lowered by refeeding, suggest that this hormone might help to shape the behavioral response to short and longer term energy balance.
Physiological Roles of Exogenous and Endogenous Insl5.
We examined the physiological role of Insl5 by injecting a commercial preparation of the recombinant peptide. i.p. Insl5 dose-dependently increased intake of chow in nonfasted wild-type mice (Fig. 4A). Mice adapted to receive a highly palatable meal daily at a fixed time also showed increased food consumption (Fig. S3A). Similar results were observed with an Insl5 preparation produced in-house (Fig. S3 B and C). Consistent with Rxfp4 being the cognate receptor for Insl5, the orexigenic action of Insl5 was not observed in mice lacking Rxfp4 (Fig. 4B). To examine whether the action of Insl5 was mediated by a peripheral or central effect, mice were injected intracerebroventricularly (ICV) with Insl5 or relaxin-3, or with ghrelin or neuropeptide Y (NPY) as positive controls. Whereas ICV ghrelin or NPY triggered robust feeding responses, no significant effect was observed with Insl5 (Fig. 4C), presumably reflecting the lack of central expression of Rxfp4 (Fig. 2A).

Dose-dependent stimulation of food intake by Insl5. (A) Effects of a commercial preparation of Insl5 (Phoenix) injected intraperitoneally at concentrations of 0 (white), 8, 40, 200 (light to dark gray), or 1,000 (black) ng per 25 g of body weight, on intake of chow, at 20 and 60 min after administration in free-feeding mice [General linear model (GLM) for repeated measures; n = 8; P < 0.001]. Significantly increased food intake was observed for the doses of 200 and 1000 ng/25g BW (P < 0.001, Tamhane’s T2 post hoc test). Data are presented as mean + 1 SEM. (B) Insl5 (Phoenix, 1000 ng/25g, black bars), injected as in A, induced food intake in wild-type but not Rxfp4−/− mice, compared with saline control (gray bars). (GLM for repeated measures in wild type P = 0.003; n ≥ 6, and in Rxfp4−/− P = 0.7; n ≥ 8). Data are presented as mean + 95% CI. (C) Twelve-wk-old C57/Bl6 mice were administered 4 μg of peptide or PBS as a sham control, by single injection via an implanted ICV cannula. Bar colors: sham, white; Insl5, midgray; relaxin-3, black; ghrelin, light gray; NPY, dark gray. Food intake was measured at the time points indicated. Data are shown as mean + SEM. Significance was tested by two-way ANOVA with post hoc Bonferroni test. *P < 0.05, ***P < 0.001 compared with sham control at the corresponding time point. (D) Wild-type mice were fasted overnight and injected via the tail vein with rabbit normal serum (open bars), or a polyclonal Insl5 antibody at a dose of 10 (light gray), 30 (dark gray), or 100 (black bars) μg per mouse. Food was reintroduced and cumulative food intake monitored over the following 24 h. Food intake was reduced in a dose dependent manner (P < 0.001, GLM for repeated measures, n = 5) at all 3 doses (P < 0.05 in Dunnett’s T post hoc test vs. control). Data are presented as mean + 95% CI.
To examine the role of endogenous Insl5, we performed studies involving immunoneutralization of circulating Insl5 or genetic disruption of Rxfp4. i.v. injection of a polyclonal antibody against Insl5 in overnight-fasted wild-type mice reduced food intake over the following 24-h period (Fig. 4C). The effect of the antibody injection on daily food intake was still evident at 3 d but faded thereafter, in line with common antibody half lives (Fig. 4D). Mice with a homozygous deletion of Rxfp4 (Rxfp4−/−; Fig. S4) were viable and fertile without any obvious differences in a number of morphological, neurological, behavioral assays, clinical chemistry or hematology. Glucose metabolism was assessed in fasted mice (male and female, 3 and 9 mo old, chow diet), in oral glucose tolerance tests, and during a postprandial fast (hourly readings for 9 h) without detecting reproducible differences in either glucose or insulin levels (data not shown). As well as showing no response to i.p. Insl5 (Fig. 4B), Rxfp4−/− mice exhibited no alteration in food intake after injection of anti-Insl5 antibody (Fig. S3D).
To further investigate the effects of Rxfp4 knockout on food intake, Rxfp−/− and wild-type animals were fasted overnight and feeding patterns monitored with two different diet options, a HFD and a high-carbohydrate diet (HCD), offered in parallel. Rxfp−/− animals exhibited shorter meal durations, particularly pronounced for the HFD, and appeared to have lost the preference for HFD seen in wild-type animals (Fig. 5A). In a further experiment, mice were fasted overnight before being offered only a HFD for the first 24 h, and then an additional choice of HCD for a further 2 d. Rxfp4−/− mice consumed less HFD than wild-type animals throughout the 3 d observation period (Fig. 5B). When additionally offered HCD, the cumulative combined intake of both diets remained lower in Rxfp4−/− animals, although they eventually consumed more HCD than the wild-type mice (Fig. 5C). These data suggest that Insl5 levels may not only affect hunger after fasting but might also contribute to the normal preference for HFD over HCD, particularly in states of calorie restriction.
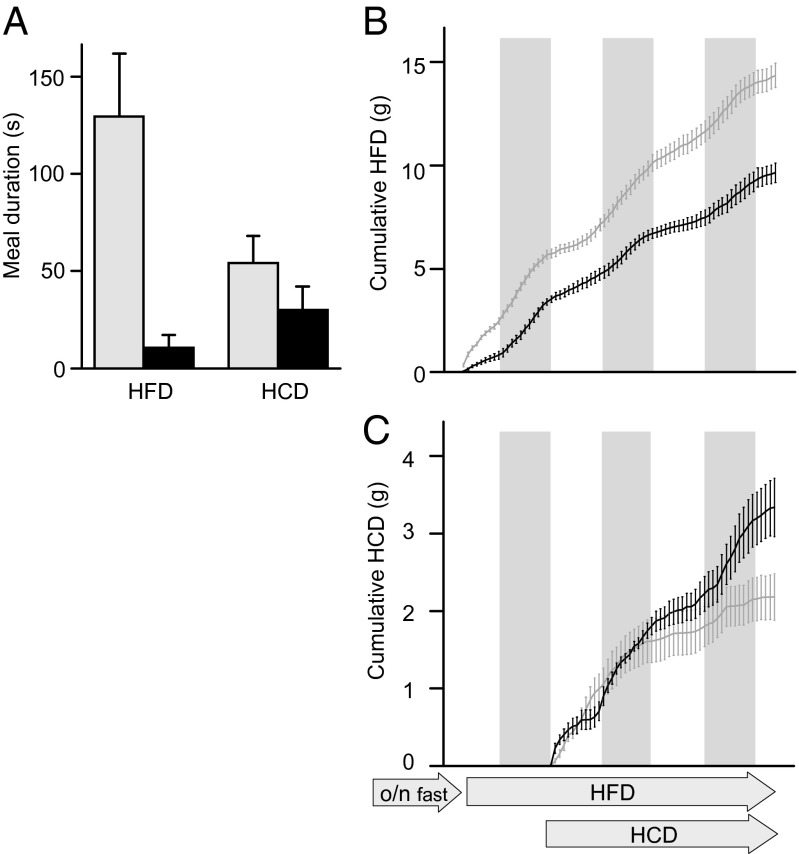
Deletion of Rxfp4 changes feeding patterns and macronutrient preference. (A) Single housed wild-type (gray bars) and Rxfp4−/− (black bars) mice were fasted overnight and presented with a choice of a HFD and HCD, and consumption of both diets was continuously monitored. Meal durations (exceeding 10s undisturbed feeding at the chosen food hopper) differed between genotype by median test (P < 0.001 for HFD, P = 0.005 for HCD). Data represent the mean of n = 4 and the 95% CI. (B and C) To explore the initial consumption of HFD without a choice after the overnight fast we performed an experiment where the HCD was introduced after a 24 h delay. Continuous monitoring of the consumption of HFD (B) and HCD (C) revealed that Rxfp4−/− mice (black lines) consumed less HFD throughout the 3-d observation period than wild-type mice (gray lines). Data are presented as mean ± 95% CI, n = 4; dark phases are indicated by gray boxes.
Consistent with a physiological role for the Insl5/Rxfp4 axis in the control of food intake, Rxfp4−/− mice tended to have a lower body weight than wild-type controls at an age of 3 mo (Fig. 6A). When fed on diets with high caloric density (HFD or HCD) for 22 wk, however, Rxfp4−/− mice eventually reached similar body weights to the wild-type controls (Fig. 6A). To examine further the influence of Rxfp4 on body composition, we analyzed body weight and composition of mice on chow diet at 8 and 11 wk of age (Fig. 6 B–E). Although no significant differences in body weight or lean mass were observed in this cohort, Rxfp4−/− animals had a trend toward a lower fat mass at 8 wk by NMR spectroscopy (EchoMRI), which reached significance at 11 wk, and had significantly decreased inguinal and gonadal fat pad masses observed ex vivo.
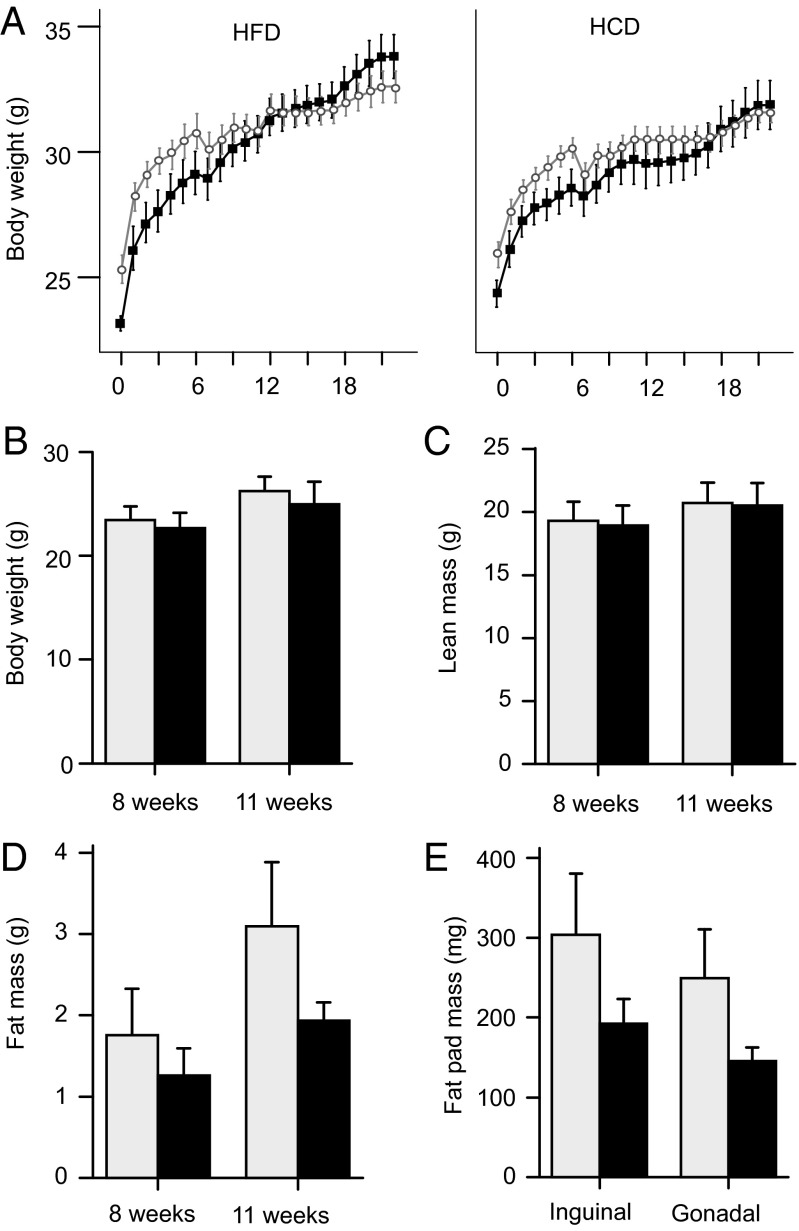
Effect of Rxfp4 knockout on body composition. (A) Three-month-old wild-type (open circles) and Rxfp4−/− (black squares) mice were put on either a HFD or HCD, and their body weights were recorded for the following 22 wk. No significant differences were detected over the full observation period, but a general linear model (GLM) for repeated measures for the first 6 wk revealed a significant effect of the genotype. (P < 0.01; n = 10; mean ± SEM). Note the difference in body weight at the beginning of the study (median test, P = 0.004, n = 20 as all mice had been on chow up to that time point). (B–E) In a separate cohort of wild-type (light gray bars) and Rxfp4−/− (black bars) mice, body composition was measured by NMR spectroscopy (EchoMRI-4in1, EchoMRI) in 8 and 11 wk old mice fed a chow diet. Body weight and lean mass were not different (B and C), but fat content (D) was lower in Rxfp4−/− mice at 11 wk (Mann–Whitney u test, P = 0.006, n = 8). At 11 wk of age, animals were killed and the complete inguinal and gonadal fat pads were dissected (E) and found to be smaller in Rxfp4−/− than wild-type mice (Mann–Whitney u test, P = 0.002 for each fat pad. Data presented as mean ± 95% CI).
Discussion
The data we present here show that Insl5 is a hormonal product of colonic L-cells that enhances food intake in mice. In line with the expected properties of an orexigenic hormone, Insl5 plasma levels and colonic mRNA transcripts were elevated by caloric restriction, and circulating Insl5 concentrations were suppressed by refeeding. Knockout of the receptor Rxfp4 or treatment with antibodies against endogenous Insl5 correspondingly reduced refeeding responses after fasting. Taken together our data support the notion that Insl5 is an orexigenic hormone that plays a physiological role in driving food intake under conditions of energy restriction.
Insl5 was found to be produced by ~50% of L-cells in the colon, but mRNA transcripts in the ileum were much lower, and in the jejunum and duodenum were largely absent. This pattern differs from the other L-cell hormones, GLP-1 and PYY, which show increased production along the proximal-distal gut axis, but are still found in substantial amounts in the small intestine. Indeed the direct stimulation by ingested nutrients of L-cells in the upper small intestine would be sufficient to underlie the rapid rise in plasma concentrations of GLP-1 and smaller elevation in PYY after food intake. It remains debatable, however, whether colonic L-cells contribute substantially to postprandial gut hormone secretion, as they would not normally experience major fluctuations in local nutrient exposure. Although some evidence supports the concept that colonic L-cells can be stimulated by a neural or hormonal loop after food intake, the contribution of this pathway to plasma levels remains unclear. Indeed, our finding that circulating Insl5 concentrations were high in the fasting state and suppressed after food intake suggests instead that L-cells in the colon are not dramatically stimulated by food ingestion, but may rather signal longer term measures of energy balance.
The observed expression of Rxfp4 in enteric and nodose ganglia but not the hypothalamus, together with the lack of effect on food intake of ICV Insl5, suggests that the orexigenic effects of Insl5 are initiated peripherally and transmitted to the central nervous system by afferent sensory nerves. The nodose ganglion also expresses receptors for GLP-1, PYY and CCK, supporting the established view that the afferent vagus nerve contributes to the anorexigenic actions of a number of endogenous hormones released locally within the intestinal epithelium (19, 20). Acting on the intrinsic enteric plexus, Insl5, like several other metabolically relevant gut hormones, may contribute to the coordination of colon motility and secretion, ensuring the efficient mixing, fermentation and propulsion of ingesta. Whether and how the enteric nervous system influences afferent signals related to food intake remains to be resolved.
Altered gut hormone secretory profiles are thought to contribute substantially to the efficacy of bypass surgery for the treatment of obesity and type 2 diabetes (1, 2), and pharmacological modulation of gut peptide secretion is under intensive investigation as a potential pharmacological replacement for bariatric procedures. One of the major features of bypass surgery is that postprandial GLP-1 levels rise up to 10-fold, and correlate with improvements in glucose tolerance and insulin secretion (21). The high GLP-1 concentrations probably arise because ingested food is delivered directly into the jejunum after surgery, and makes contact with the large numbers of L-cells in the lower small intestine. How much nutrient is delivered to the colon after surgery depends on the anatomical rearrangement, but once Insl5 assays are developed that are adequately validated for measurements in human plasma, it will be very interesting to assess basal and postprandial plasma Insl5 concentrations in this patient population.
Low energy stores activate a variety of redundant and complex neuronal and entero-endocrine responses affecting many aspects of feeding behavior. The outcomes are directed at refilling energy stores, and act by modulation of hunger, taste, food preference, meal size, intermeal interval, satiation, and hedonic aspects of feeding (22). Although it was recently reported that Insl5-deficient mice were glucose intolerant and exhibited abnormal islet morphology (14), we did not observe an overt glucose intolerant phenotype in mice lacking Rxfp4. Our findings rather indicate that Insl5 is an orexigenic hormone, plasma levels of which are elevated by calorie restriction, and which may drive the ingestion of energy-dense high-fat food under conditions associated with low energy stores.
Methods
Ethics.
The use of human enteric tissues was approved by the ethics committee of the Canton of Fribourg. All animal studies were in accordance with the UK Home Office legislation and approved by the appropriate ethical committee. Mice were maintained under controlled temperature on a 12-h light–dark cycle with free access to food and water unless otherwise stated. All mice were 129SvEv unless indicated otherwise.
Insl5.
Insl5 for i.p. administration was purchased from Phoenix Pharmaceuticals, dissolved according manufacturer’s recommendations. Insl5 effects on food intake were confirmed using Insl5 with an acidic C terminus (Mw 5268.6, 100% peptide, chemically synthesized at the Florey Institute of Neuroscience and Mental Health), prepared as a stock solution in 20% (vol/vol) dimethyl sulfoxide at 1 µg/µL by gentle agitation. To reduce adsorption of Insl5 we recommend use of siliconized glassware (syringes were flushed with 100 µL of Sigmacote (Sigma-Aldrich), and addition of 0.5% mouse serum albumin (A3139, Sigma-Aldrich) to dilutions of the stock (Fig. S3C).
Insl5 Antibodies.
The antibody used for mouse immunohistochemistry and FACS analysis was raised in guinea pig and affinity purified against a sequence of the C-peptide that is cleaved from prepro-Insl5 (LQLLDRHEPSKKTL). Neutralization of endogenous Insl5 was performed using polyclonal anti-Insl5 Ab (G-035–40, Phoenix Pharmaceuticals). The antibody for staining human tissue was raised in a rat immunized with mouse Insl5, producing the IgG1 hybridoma clone NSFL7-18H1, which was specific for both mouse and human Insl5 (Kd = 0.33 and 0.7 nM, respectively).
Primary Mouse L-Cells.
L-cells were isolated from the small and large intestine of transgenic C57BL6 mice expressing the Venus variant of yellow fluorescent protein under the control of the glucagon promoter (GLU-Venus). Intestinal tissue was prepared for primary culture, flow assisted cell sorting or FACS analysis as described (18), and purified cells were analyzed by Affymetrix 430 2.0 microarray (8), or qRT-PCR using primers for Actb, Gcg, Pyy, and Insl5 (Applied Biosystems). Three-day-old primary cultures were fixed with 4% (wt/vol) paraformaldehyde (PFA) and stained with anti-Insl5 (1:1,000) and anti-proglucagon (1:200; sc-13091, Santa Cruz Biotechnology) antibodies in 1% goat serum at 4 °C overnight, followed by 1:300 dilutions of secondary antibodies (Alexafluor488 anti-guinea pig and Alexafluor633 anti-rabbit; Invitrogen) for 1 h. PFA-fixed cell suspensions from the colon/rectum of GLU-Venus mice (18) were permeabilized and immunostained with anti-Insl5 (1:200 overnight) and Alexafluor633 anti-guinea pig (1:300, 1 h), and analyzed using a BD LSRFortessa analyzer (BD Biosciences) and FlowJo 7.6 software (Tree Star). Events with very low side and forward scatter or high pulse width were excluded. Immunopositivity in the Venus-positive subpopulation was quantified.
In Situ Hybridization.
Eight-month-old male mice were perfused with cold 0.9% NaCl (DEPC-treated water), the organs dissected and embedded in Tissue-tek. Sections (14 μm) were cut in a cryostat at −20 °C, mounted on “Superfrost gold” slides and stored at −80 °C. In situ hybridization was performed using DIG RNA Labeling kit (SP6/T7), anti-DIG-AP antibody, and NBT/BCIP according to provider’s instructions (Roche Applied Sciences).
Human Tissue Sample Preparation.
Tissue was fixed with 10% (wt/vol) buffered formalin, dehydrated, and embedded in paraplast. Sections were cut with a Leica microtome at nominal thicknesses of 3 µm and mounted on superfrost plus (Menzel) slides. Sections were deparaffinized and treated with H2O2/methanol (30 min), and antigens were retrieved by pressure cooking in citrate buffer, 0.001 M, pH 6. After cooling and washing with phosphate buffer (0.1 M, pH 7.4), immunohistochemical detection was performed using primary antibodies against Insl5 (as described in Insl5 Antibodies), PYY (Eud 5201, Acris), and GLP-1 (T4057, Bachem) followed by appropriate secondary antibodies (Alexa-488, Cy3, and Cy5). Triple immunofluorescence staining was recorded with a Leica confocal scanning microscope.
Mouse Diet Study.
High fat [45% Atwater Fuel Energy (AFE) fat; 824053], high carbohydrate (70% AFE carbohydrates; 824050), and chow diet (801722) were from Special Diets Services. Insl5 was prepared as above, and 5% (wt/vol) mouse serum albumin or water constituted sham injection. Peptide was delivered using an insulin syringe (BD Micro-Fine 0.3-mL syringe, Becton Dickinson Medical). All doses were given in a volume of 100 µL i.p. For all i.p. peptide administrations, animals were handled and given sham ip. injections on 3–5 d before the experiment. For refeeding studies shown in Fig. S3C, male C57BL/6 mice aged 10 wk (Charles River) were acclimatized for 1 wk to single housing, appearance of a highly palatable foodstuff [either wet mash (chow diet in water, 1:1.5 wt/wt) or Farley’s Breakfast “Peachy Porridge” (HJ Heinz), 1 part dry food to 1.3 parts water for 1 h/d], and handling procedures. In the final 3 d of the acclimatization, mice underwent a sham i.p. injection of 100 µL of PBS. Mice were randomly assigned to receive a different dose on three occasions over a week. At the onset of the 1-h test period, standard chow was removed, and 20 g of highly palatable diet was placed in a clear plastic dish on the floor of the cage. After 1 h, palatable food was removed, standard chow was returned to the hopper, and mice were returned to their holding room.
Intracerebroventricular (ICV) injections were performed on 12-wk-old C57/Bl6 mice (Charles River), mean body weight 27.3 g, using a protocol described previously (23). In brief, a lateral ventricle cannula was placed under isoflurane anesthesia, the animal was given 1 wk to recover, and studies were only performed further if body weight was at or above pre surgery weight. Mice were given ad libitum access to standard chow. Experiments were started at 9:00 AM, following ad libitum feeding the previous night. A single injection of peptide (4 μg) was given, and food intake was measured over the following 6 h. Sham mice were injected with PBS. Insl5 and Relaxin-3 were obtained from Phoenix Pharmaceuticals (catalog numbers 035–40 and 035–36, respectively), NPY and ghrelin were from Bachem (catalog numbers H6375 and H4862, respectively).
Mouse Plasma Analyses.
Mouse Insl5 was measured using a RIA kit from Phoenix Pharmaceuticals (Fig. 3A and Figs. S2B and S5) The specificity of the assay was confirmed by detection of synthetic Insl5 (Phoenix Pharmaceuticals) injected intravenously, which resulted in the expected plasma peak followed by a decaying signal (Fig. S5). New cohorts of mice were assessed using an Insl5 ELISA (Kamiya Biomedical KT-58379), which became available later in the study (Fig. 3B).
Quantitative mRNA Sequencing.
Double-stranded cDNA was synthesized from polyadenylated RNA and sheared. The 190- to 210-bp fraction was isolated and PCR amplified to generate the sequencing library, as per the Illumina Genome Analyzer paired end library protocol (Illumina). The resulting libraries were sequenced on an Illumina GAII and paired end reads were mapped to the mm9 mouse genome assembly using TopHat 1.3.1 (24). The resultant alignments were analyzed for differential expression using Cufflinks 1.0.3 (25). Gene definitions were supplied as a gtf file, which was downloaded from the UCSC Table Browser (26) and further processed with cuffcompare (25). Raw read counts were determined using htseq-count (www-huber.embl.de/users/anders/HTSeq/doc/count.html).
Statistics.
All tests and post hoc tests are two-sided with α = 0.05 unless indicated otherwise. Instead of extensive post hoc tests 95% confidence intervals are indicated where suitable. PASW Statistics 17 and IBM SPSS 19 (IBM) software packages were used.
Note Added in Proof.
A recent batch of the mouse Insl5 ELISA from Kamiya give high unspecific signals. We recommend validating assays with plasma spiked with Insl5 and plasma from Insl5−/− mice. Alternative ELISAs are available from BlueGene and Cusabio.
Acknowledgments
F.M.G., F.R., G.J.R., P.R., S.O., and A.M.H. are funded by the Wellcome Trust (088357, 084210, and 095515). A.P.C., R.L., D.R., and S.O. are funded by Medical Research Council (MRC) Programme Grant G9824984, MRC Centre for Obesity and Related Metabolic Disease (MRC CORD) (G0600717), and subsequently MRC Metabolic Diseases Unit (4050281695) and the National Institute for Health Research Cambridge Biomedical Research Centre (CG50826). J.D.W. is a National Health and Medical Research Council of Australia Principal Research Fellow. Studies at the Florey Institute of Neuroscience and Mental Health were supported by the Victorian Government's Operational Infrastructure Support Program and the Australian Research Council Linkage Grant LP120100654.
Footnotes
Conflict of interest statement: J.G., H.H., L.P., W.H.C., J.D., Y.T., and M.B.L.C. are employees of or have received consultancy fees (W.H.C.) from Takeda Pharmaceutical Co. Ltd. or its subsidiaries.
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1411413111/-/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.1411413111
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/111/30/11133.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
A homeostatic gut-to-brain insulin antagonist restrains neuronally stimulated fat loss.
Nat Commun, 15(1):6869, 11 Aug 2024
Cited by: 0 articles | PMID: 39127676 | PMCID: PMC11316803
Interaction between the gut microbiota and colonic enteroendocrine cells regulates host metabolism.
Nat Metab, 6(6):1076-1091, 22 May 2024
Cited by: 3 articles | PMID: 38777856
Effects of dietary fibre on metabolic health and obesity.
Nat Rev Gastroenterol Hepatol, 21(5):301-318, 07 Feb 2024
Cited by: 5 articles | PMID: 38326443
Review
Unveiling the Genetic Landscape of Feed Efficiency in Holstein Dairy Cows: Insights into Heritability, Genetic Markers, and Pathways via Meta-Analysis.
J Anim Sci, 102:skae040, 01 Jan 2024
Cited by: 1 article | PMID: 38354297 | PMCID: PMC10957122
Review Free full text in Europe PMC
The diverse influences of relaxin-like peptide family on tumor progression: Potential opportunities and emerging challenges.
Heliyon, 10(2):e24463, 10 Jan 2024
Cited by: 0 articles | PMID: 38298643 | PMCID: PMC10828710
Review Free full text in Europe PMC
Go to all (72) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells.
Mol Metab, 16:65-75, 30 Jul 2018
Cited by: 31 articles | PMID: 30104166 | PMCID: PMC6158034
Selective stimulation of colonic L cells improves metabolic outcomes in mice.
Diabetologia, 63(7):1396-1407, 27 Apr 2020
Cited by: 28 articles | PMID: 32342115 | PMCID: PMC7286941
INSL5 activates multiple signalling pathways and regulates GLP-1 secretion in NCI-H716 cells.
J Mol Endocrinol, 60(3):213-224, 01 Apr 2018
Cited by: 7 articles | PMID: 29535183
Free Fatty Acid Receptors in Enteroendocrine Cells.
Endocrinology, 159(7):2826-2835, 01 Jul 2018
Cited by: 27 articles | PMID: 29688303
Review
Funding
Funders who supported this work.
Medical Research Council (9)
Grant ID: MC_UU_12012/5/B
Grant ID: G0600717B
Grant ID: 4050281695
MRC Centre for Translational Research in Obesity and related Metabolic Diseases
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: G0600717
Molecular Mechanisms in Human Obesity
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: G9824984
Mechanisms in Disorders of Energy Balance
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: MC_UU_12012/1
The Enteroendocrine System in Health and Disease
Prof Fiona Gribble, University of Cambridge
Grant ID: MC_UU_12012/3
Molecular and pathophysiological mechanisms in human obesity
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: G0900554
Enabling technologies
Professor Giles Yeo, University of Cambridge
Grant ID: MC_UU_12012/5
National Institute for Health Research (NIHR) (1)
Grant ID: NF-SI-0507-10380
Wellcome Trust (4)
Insulin Resistance: Lessons from Extreme Phenotypes.
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: 095515
Mechanisms underlying Glucose-dependent Insulinotropic Polypeptide (GIP) secretion and function.
Professor Frank Reimann, University of Cambridge
Grant ID: 084210
Institute of Metabolic Science.
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: 100574
Enteroendocrine cell function in diabetes and obesity.
Prof Fiona Gribble, University of Cambridge
Grant ID: 088357





