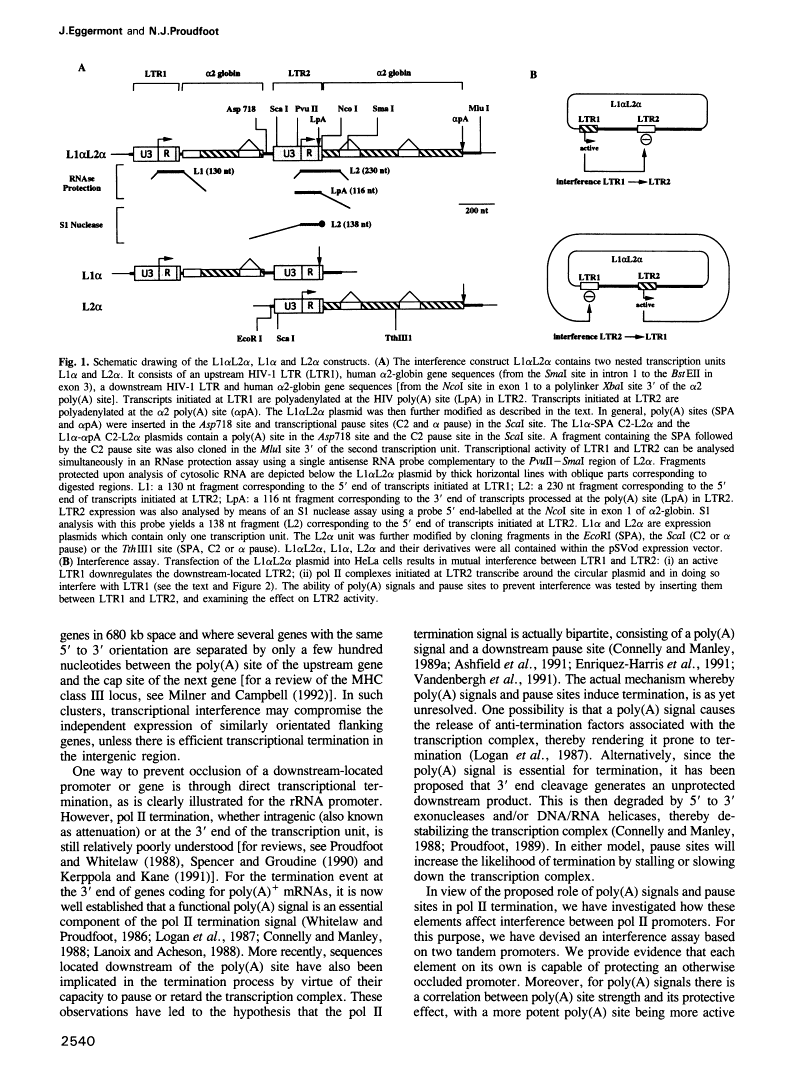
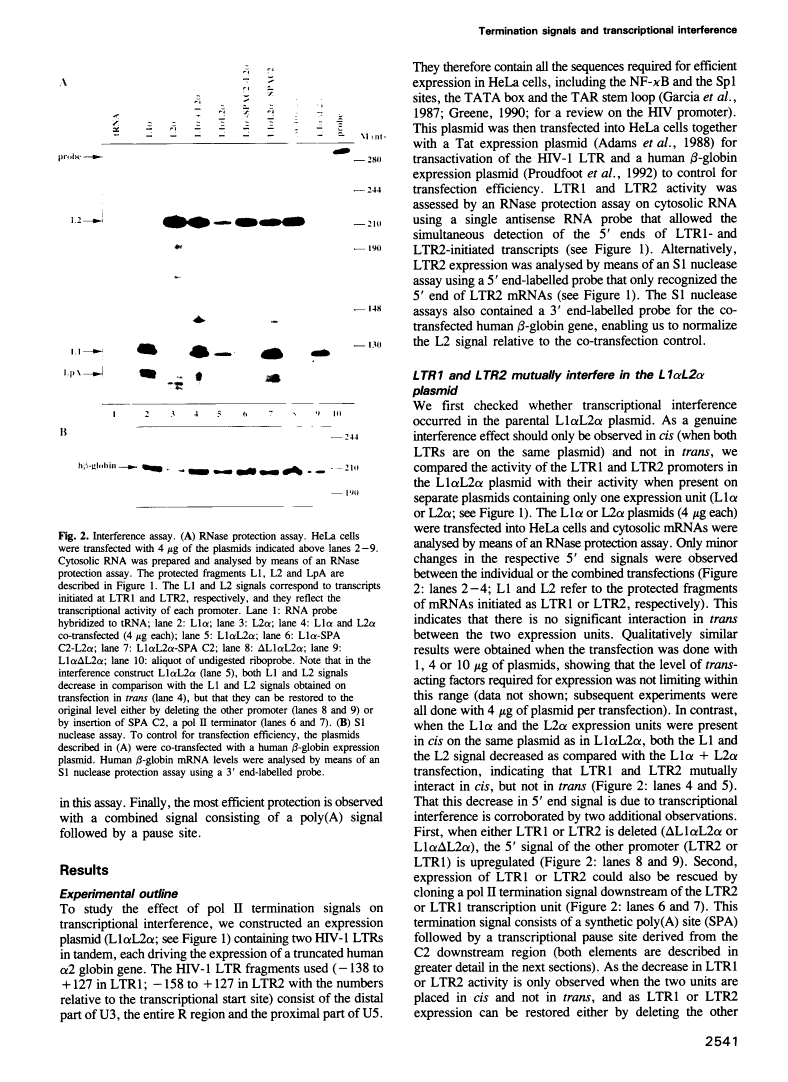
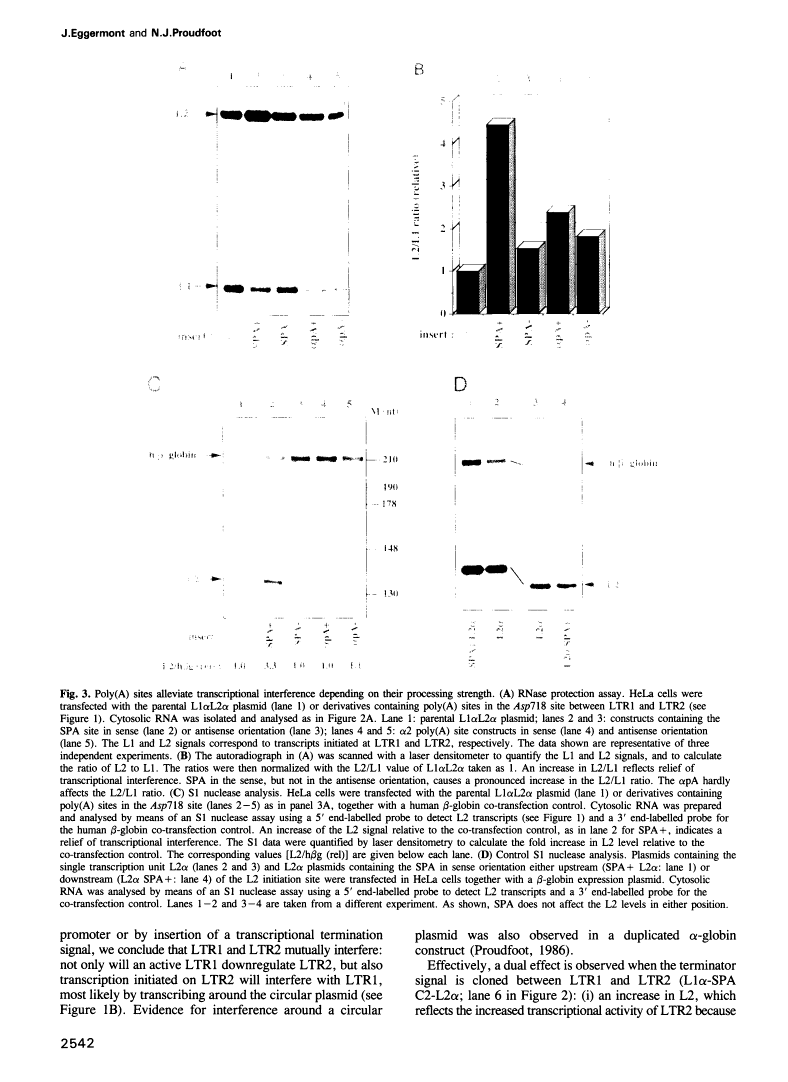
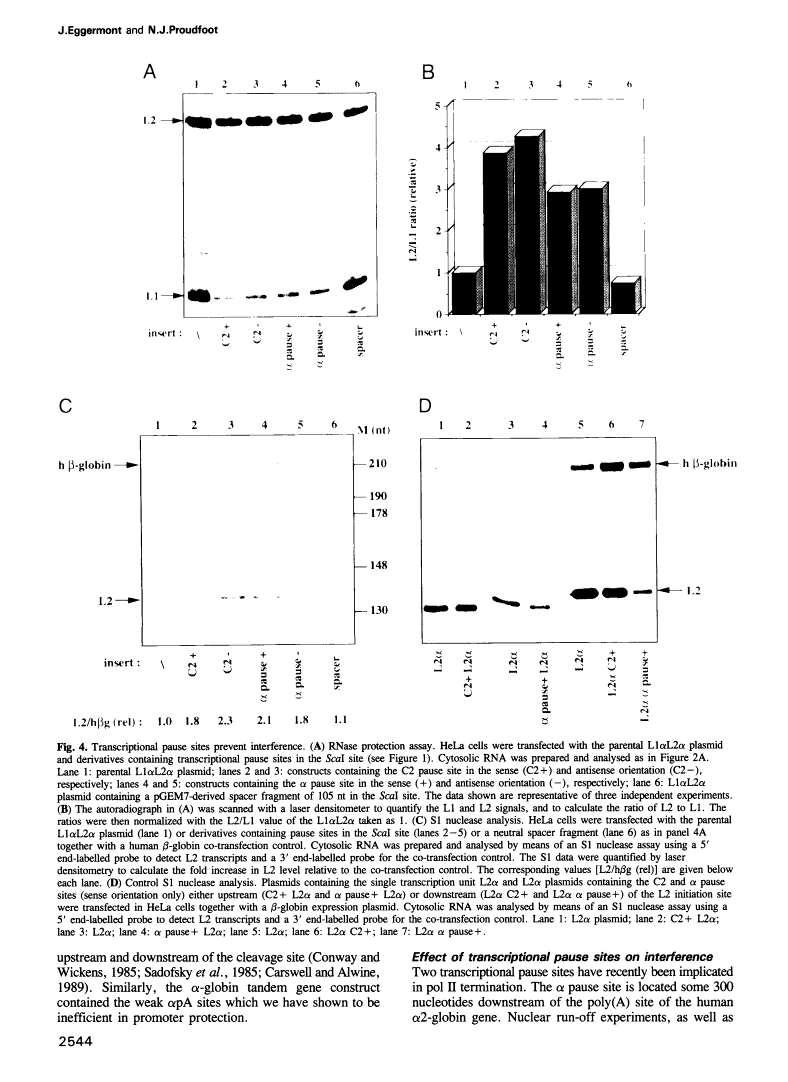
Abstract
Free full text

Poly(A) signals and transcriptional pause sites combine to prevent interference between RNA polymerase II promoters.
Abstract
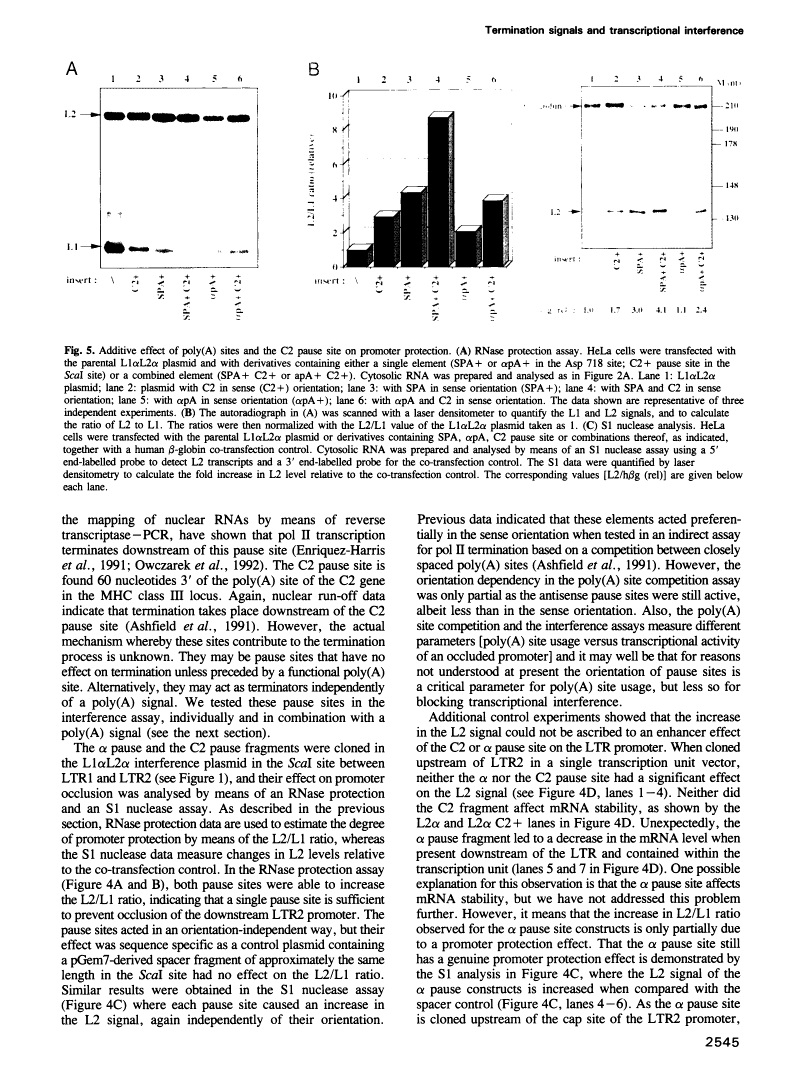
Transcriptional termination by RNA polymerase II at the 3' end of genes encoding poly(A)+ mRNAs is thought to require two distinct cis-active elements: a functional poly(A) signal and a downstream transcriptional pause site. An important requirement for efficient termination is to prevent transcriptional interference of downstream-located promoters. We have therefore investigated whether these two elements, individually or in combination, can prevent transcriptional interference of RNA polymerase II-activated promoters. For this purpose, we constructed an expression plasmid containing two tandem retroviral long terminal repeats (LTRs) derived from HIV-1. When transfected into HeLa cells, this construct resulted in transcriptional interference of the LTR promoters. Using this assay, we were able to show that a single poly(A) signal was able to protect an otherwise occluded promoter. This effect depended on the RNA-processing strength of the poly(A) signal. Furthermore, transcriptional pause sites provided adequate protection against promoter occlusion even when tested alone. Finally, a combined element consisting of a poly(A) signal followed by a pause site was more efficient in promoter protection than either element on its own. These results indicate that an interference-blocking element can take various forms: a poly(A) signal, a transcriptional pause site or a combination of both.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greene WC. Regulation of HIV-1 gene expression. Annu Rev Immunol. 1990;8:453–475. [Abstract] [Google Scholar]
- Hausler B, Somerville RL. Interaction in vivo between strong closely spaced constitutive promoters. J Mol Biol. 1979 Jan 25;127(3):353–356. [Abstract] [Google Scholar]
- Henderson SL, Ryan K, Sollner-Webb B. The promoter-proximal rDNA terminator augments initiation by preventing disruption of the stable transcription complex caused by polymerase read-in. Genes Dev. 1989 Feb;3(2):212–223. [Abstract] [Google Scholar]
- Irniger S, Egli CM, Kuenzler M, Braus GH. The yeast actin intron contains a cryptic promoter that can be switched on by preventing transcriptional interference. Nucleic Acids Res. 1992 Sep 25;20(18):4733–4739. [Europe PMC free article] [Abstract] [Google Scholar]
- Kerppola TK, Kane CM. RNA polymerase: regulation of transcript elongation and termination. FASEB J. 1991 Oct;5(13):2833–2842. [Abstract] [Google Scholar]
- Lanoix J, Acheson NH. A rabbit beta-globin polyadenylation signal directs efficient termination of transcription of polyomavirus DNA. EMBO J. 1988 Aug;7(8):2515–2522. [Europe PMC free article] [Abstract] [Google Scholar]
- Levitt N, Briggs D, Gil A, Proudfoot NJ. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989 Jul;3(7):1019–1025. [Abstract] [Google Scholar]
- Logan J, Falck-Pedersen E, Darnell JE, Jr, Shenk T. A poly(A) addition site and a downstream termination region are required for efficient cessation of transcription by RNA polymerase II in the mouse beta maj-globin gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8306–8310. [Europe PMC free article] [Abstract] [Google Scholar]
- McStay B, Reeder RH. An RNA polymerase I termination site can stimulate the adjacent ribosomal gene promoter by two distinct mechanisms in Xenopus laevis. Genes Dev. 1990 Jul;4(7):1240–1251. [Abstract] [Google Scholar]
- Maire P, Gautron S, Hakim V, Gregori C, Mennecier F, Kahn A. Characterization of three optional promoters in the 5' region of the human aldolase A gene. J Mol Biol. 1987 Oct 5;197(3):425–438. [Abstract] [Google Scholar]
- Marcu KB, Bossone SA, Patel AJ. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. [Abstract] [Google Scholar]
- Meulia T, Krumm A, Spencer C, Groudine M. Sequences in the human c-myc P2 promoter affect the elongation and premature termination of transcripts initiated from the upstream P1 promoter. Mol Cell Biol. 1992 Oct;12(10):4590–4600. [Europe PMC free article] [Abstract] [Google Scholar]
- Milner CM, Campbell RD. Genes, genes and more genes in the human major histocompatibility complex. Bioessays. 1992 Aug;14(8):565–571. [Abstract] [Google Scholar]
- Owczarek CM, Enriquez-Harris P, Proudfoot NJ. The primary transcription unit of the human alpha 2 globin gene defined by quantitative RT/PCR. Nucleic Acids Res. 1992 Feb 25;20(4):851–858. [Europe PMC free article] [Abstract] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. [Abstract] [Google Scholar]
- Proudfoot NJ. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. [Abstract] [Google Scholar]
- Proudfoot NJ. How RNA polymerase II terminates transcription in higher eukaryotes. Trends Biochem Sci. 1989 Mar;14(3):105–110. [Abstract] [Google Scholar]
- Proudfoot NJ, Lee BA, Monks J. Multiple SP1 binding sites confer enhancer-independent, replication-activated transcription of HIV-1 and globin gene promoters. New Biol. 1992 Apr;4(4):369–381. [Abstract] [Google Scholar]
- Roberts S, Purton T, Bentley DL. A protein-binding site in the c-myc promoter functions as a terminator of RNA polymerase II transcription. Genes Dev. 1992 Aug;6(8):1562–1574. [Abstract] [Google Scholar]
- Sadofsky M, Connelly S, Manley JL, Alwine JC. Identification of a sequence element on the 3' side of AAUAAA which is necessary for simian virus 40 late mRNA 3'-end processing. Mol Cell Biol. 1985 Oct;5(10):2713–2719. [Europe PMC free article] [Abstract] [Google Scholar]
- Schibler U, Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. [Abstract] [Google Scholar]
- Sollner-Webb B, Mougey EB. News from the nucleolus: rRNA gene expression. Trends Biochem Sci. 1991 Feb;16(2):58–62. [Abstract] [Google Scholar]
- Spencer CA, Groudine M. Transcription elongation and eukaryotic gene regulation. Oncogene. 1990 Jun;5(6):777–785. [Abstract] [Google Scholar]
- Vales LD, Darnell JE., Jr Promoter occlusion prevents transcription of adenovirus polypeptide IX mRNA until after DNA replication. Genes Dev. 1989 Jan;3(1):49–59. [Abstract] [Google Scholar]
- Vandenbergh DJ, James-Pederson M, Hardison RC. An apparent pause site in the transcription unit of the rabbit alpha-globin gene. J Mol Biol. 1991 Jul 20;220(2):255–270. [Abstract] [Google Scholar]
- Whitelaw E, Proudfoot N. Alpha-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3' end processing in the human alpha 2 globin gene. EMBO J. 1986 Nov;5(11):2915–2922. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu LC, Morley BJ, Campbell RD. Cell-specific expression of the human complement protein factor B gene: evidence for the role of two distinct 5'-flanking elements. Cell. 1987 Jan 30;48(2):331–342. [Abstract] [Google Scholar]
- Adams SE, Johnson ID, Braddock M, Kingsman AJ, Kingsman SM, Edwards RM. Synthesis of a gene for the HIV transactivator protein TAT by a novel single stranded approach involving in vivo gap repair. Nucleic Acids Res. 1988 May 25;16(10):4287–4298. [Europe PMC free article] [Abstract] [Google Scholar]
- Adhya S, Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. [Abstract] [Google Scholar]
- Ashfield R, Enriquez-Harris P, Proudfoot NJ. Transcriptional termination between the closely linked human complement genes C2 and factor B: common termination factor for C2 and c-myc? EMBO J. 1991 Dec;10(13):4197–4207. [Europe PMC free article] [Abstract] [Google Scholar]
- Bateman E, Paule MR. Promoter occlusion during ribosomal RNA transcription. Cell. 1988 Sep 23;54(7):985–992. [Abstract] [Google Scholar]
- Bossone SA, Asselin C, Patel AJ, Marcu KB. MAZ, a zinc finger protein, binds to c-MYC and C2 gene sequences regulating transcriptional initiation and termination. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7452–7456. [Europe PMC free article] [Abstract] [Google Scholar]
- Carswell S, Alwine JC. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol. 1989 Oct;9(10):4248–4258. [Europe PMC free article] [Abstract] [Google Scholar]
- Chretien S, Dubart A, Beaupain D, Raich N, Grandchamp B, Rosa J, Goossens M, Romeo PH. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. [Europe PMC free article] [Abstract] [Google Scholar]
- Clark L, Matthews JR, Hay RT. Interaction of enhancer-binding protein EBP1 (NF-kappa B) with the human immunodeficiency virus type 1 enhancer. J Virol. 1990 Mar;64(3):1335–1344. [Europe PMC free article] [Abstract] [Google Scholar]
- Connelly S, Manley JL. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev. 1988 Apr;2(4):440–452. [Abstract] [Google Scholar]
- Connelly S, Manley JL. A CCAAT box sequence in the adenovirus major late promoter functions as part of an RNA polymerase II termination signal. Cell. 1989 May 19;57(4):561–571. [Abstract] [Google Scholar]
- Connelly S, Manley JL. RNA polymerase II transcription termination is mediated specifically by protein binding to a CCAAT box sequence. Mol Cell Biol. 1989 Nov;9(11):5254–5259. [Europe PMC free article] [Abstract] [Google Scholar]
- Conway L, Wickens M. A sequence downstream of A-A-U-A-A-A is required for formation of simian virus 40 late mRNA 3' termini in frog oocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3949–3953. [Europe PMC free article] [Abstract] [Google Scholar]
- Corbin V, Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. [Abstract] [Google Scholar]
- Cullen BR, Lomedico PT, Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. [Abstract] [Google Scholar]
- Enriquez-Harris P, Levitt N, Briggs D, Proudfoot NJ. A pause site for RNA polymerase II is associated with termination of transcription. EMBO J. 1991 Jul;10(7):1833–1842. [Europe PMC free article] [Abstract] [Google Scholar]
- Garcia JA, Wu FK, Mitsuyasu R, Gaynor RB. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. [Europe PMC free article] [Abstract] [Google Scholar]
- Gil A, Proudfoot NJ. Position-dependent sequence elements downstream of AAUAAA are required for efficient rabbit beta-globin mRNA 3' end formation. Cell. 1987 May 8;49(3):399–406. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1993.tb05909.x
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1993.tb05909.x
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1993.tb05909.x
Article citations
Mining the Utricularia gibba genome for insulator-like elements for genetic engineering.
Front Plant Sci, 14:1279231, 08 Nov 2023
Cited by: 2 articles | PMID: 38023853 | PMCID: PMC10663240
An optimized circular polymerase extension reaction-based method for functional analysis of SARS-CoV-2.
Virol J, 20(1):63, 07 Apr 2023
Cited by: 5 articles | PMID: 37029393 | PMCID: PMC10080526
Eomes function is conserved between zebrafish and mouse and controls left-right organiser progenitor gene expression via interlocking feedforward loops.
Front Cell Dev Biol, 10:982477, 25 Aug 2022
Cited by: 0 articles | PMID: 36133924 | PMCID: PMC9483813
A viral toolbox for conditional and transneuronal gene expression in zebrafish.
Elife, 11:e77153, 22 Jul 2022
Cited by: 6 articles | PMID: 35866706 | PMCID: PMC9307271
Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays.
Int J Mol Sci, 21(12):E4197, 12 Jun 2020
Cited by: 41 articles | PMID: 32545533 | PMCID: PMC7352801
Review Free full text in Europe PMC
Go to all (66) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pause sites promote transcriptional termination of mammalian RNA polymerase II.
Mol Cell Biol, 26(10):3986-3996, 01 May 2006
Cited by: 133 articles | PMID: 16648491 | PMCID: PMC1488997
A pause site for RNA polymerase II is associated with termination of transcription.
EMBO J, 10(7):1833-1842, 01 Jul 1991
Cited by: 82 articles | PMID: 2050120 | PMCID: PMC452858
The poly(A) signal, without the assistance of any downstream element, directs RNA polymerase II to pause in vivo and then to release stochastically from the template.
J Biol Chem, 277(45):42899-42911, 23 Aug 2002
Cited by: 39 articles | PMID: 12196547
Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter.
Nucleic Acids Res, 26(5):1294-1301, 01 Mar 1998
Cited by: 87 articles | PMID: 9469840 | PMCID: PMC147389
Funding
Funders who supported this work.