Abstract
Free full text

Oxytocin Receptor Gene (OXTR) Polymorphism, Perceived Social Support, and Psychological Symptoms in Maltreated Adolescents
Abstract
Despite the detrimental consequences of child maltreatment on developmental processes, some individuals show remarkable resilience, with few signs of psychopathology, while others succumb to dysfunction. Given that oxytocin has been shown to be involved in social affiliation, attachment, social support, trust, empathy, and other social or reproductive behaviors, we chose to examine the possible moderation of maltreatment effects on perceived social support and on psychological symptoms by a common SNP (rs53576) in the oxytocin receptor gene (OXTR). We studied adolescents (N = 425) aged approximately 13-15, including participants with objectively documented maltreatment histories (N = 263) and a non-maltreated comparison group from a comparable low-socioeconomic status background (N = 162). There was a significant genotype by maltreatment interaction such that maltreated adolescents with the G/G genotype perceived significantly lower social support compared to maltreated A-carriers, with no effect of genotype in the comparison group. Maltreated G/Gs also reported higher levels of Internalizing symptoms than A-carriers, even though they did not differ from them on objective measures of maltreatment (type, duration, or severity). G/G homozygotes may be more attuned to negative social experiences such as family maltreatment, while maltreated A-carriers were indistinguishable from non-maltreated adolescents in levels of mental health symptoms.
Even though maltreatment is arguably one of the most stressful experiences that children can encounter, some maltreated children exhibit remarkable resilience –e.g., they do not display maladaptive behaviors or psychopathology in the face of adversity (Cicchetti & Lynch, 1995; Cicchetti & Valentino, 2006). The field of developmental psychopathology has expended much effort to try to understand these individual differences and contextual factors that could explain both developmental trajectories leading to mental health problems in maltreated individuals and the absence of signs of psychopathology in some of them (Cicchetti, 2013; Cicchetti & Rogosch, 1997). Resilience is not “magic” (Masten, 2001) and research has suggested that individuals who are less scathed by adversity can be understood by examining the interactions between protective and risk factors at multiple levels of analysis including environmental inputs and biological parameters (for a more comprehensive and recent review on the topic, see Cicchetti, 2013). The same developmental cascades which can amplify maladaptive outcomes over time can perpetuate or amplify positive outcomes when the individual benefits from some combination of experiences and/or biological propensities that tip the initial balance towards adaptive outcomes.
For instance, it is increasingly recognized that genetic variations and their downstream effects on physiology and behavior may interact with environmental risk factors such as maltreatment to create vulnerability to mental health symptoms (Caspi & Moffitt, 2006; Karg, Burmeister, Shedden, & Sen, 2011) and may help to explain some of these individual differences, as well as the positive outcomes when they do occur.
It is important that research on gene-environment interactions as predictors of psychopathology pursue the study of biological systems that are plausibly related to the environmental exposure of interest to avoid spurious findings (Rutter, 2010). We chose the oxytocin system as a target for investigation in this sample of maltreated adolescents due to the well-recognized role of oxytocin in social-affiliative behaviors such as attachment, social support, trust, and empathy (Ebstein, Knafo, Mankuta, Chew, & Lai, 2012; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). Genetic variation in the oxytocinergic system is thus plausibly involved in shaping individual responses to an interpersonal trauma such as maltreatment. Indeed, there is increasing evidence implicating the sensitivity of the oxytocin system to maltreatment. For instance, adult women with a history of childhood maltreatment exhibit lower levels of oxytocin in cerebrospinal fluid (Heim et al., 2009). The second theoretical justification for this investigation lies in the role of oxytocin in the stress-reducing properties of social support (e.g., Chen et al., 2011). Given the important role of stress in the development, maintenance or aggravation of many types of child and adolescent psychopathology (Grant et al., 2003), we hypothesized that variation in the oxytocinergic system could be linked to higher levels of distress or psychopathology when social support levels are low (i.e., in maltreating families). Below we review the theoretical and basic science justification for the present study, beginning with a review of the roles of oxytocin and of variation in the oxytocin receptor gene in social behavior and continuing with their more specific roles in stress reduction and protection from psychopathology.
The Roles of Oxytocin and of Variation in the OXTR Gene in Social Behavior
Oxytocin (OT) is an evolutionarily conserved neuropeptide that regulates various aspects of social and reproductive behavior in humans and many species (Donaldson & Young, 2008; Heinrichs & Domes, 2008). Aside from its role in parturition and milk production, OT also has been implicated in a broader range of social and affiliative behaviors. For instance, increases in human participants’ plasma or urinary OT have been noted in tasks involving empathy with a stranger’s distress (Barraza & Zak, 2009), trust and generosity in monetary games (Zak, Kurzban, & Matzner, 2005), receiving a massage (Morhenn, Park, Piper, & Zak, 2008), natural variations in maternal and paternal behaviors with infants (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010; Gordon, Zagoory-Sharon, Leckman, & Feldman, 2010), and mother-daughter communication subsequent to a stressor (Seltzer, Ziegler, & Pollak, 2011). OT plasma levels have also been used to predict behavior –e.g., maternal-infant interactions (for a review, see Galbally, Lewis, IJzendoorn, & Permezel, 2011). Experimental manipulations involving intranasal administration of OT also reveal effects consistent with the presumed role of OT in prosocial behavior: increases in trust (Kosfeld , Heinrichs, Zak, Fischbacher, & Fehr, 2005), generosity (Zak, Stanton, & Ahmadi, 2007), self-reported attachment security in adulthood (Buchheim et al., 2010), ability to “read the mind” of another person (Domes et al., 2007), paternal responsiveness to infants during play (Naber, van Ijzendoorn, Deschamps, van Engeland, & Bakermans-Kranenburg, 2010), and positive communication during couple conflict (Ditzen et al., 2009).
Variation in OT receptor function is also related to social behavior. Rodent models show that the distribution of OT receptors across the brain is related to the social organization of a species (Insel & Shapiro, 1992) and to natural variations in maternal care behavior, variations which are eliminated by oxytocin receptor antagonists (Champagne, Diorio, Sharma, & Meaney, 2001). Furthermore, knockout mice deficient in the oxytocin receptor gene (OXTR) display pervasive social deficits (Takayanagi et al., 2005). The human genotype has a single oxytocin receptor gene, which is located on chromosome 3p25, spanning 17 kb, and containing three introns and four exons (Gimpl & Fahrenholz, 2001; Inoue et al., 1994). The gene encodes a 391-amino acid protein that belongs to the class I G-protein-coupled receptor family (Inoue et al., 1994). A single nucleotide polymorphism (SNP) with unknown function in the third intron of OXTR has been garnering increased attention recently, rs53576 (G/A). This SNP was associated with autism in a family transmission study of a Chinese Han population, with preferential transmission of the A allele among autistic individuals (Wu et al., 2005). This association was not found in Caucasians (Jacob et al., 2007). Compared to A-carriers, individuals with the G/G genotype perform better on laboratory measures of empathy (Rodrigues, Saslow, Garcia, John, & Keltner, 2009, but not on self-rated scales of empathic concern in patients with schizophrenia, Montag et al., 2012), manifest more trust during laboratory economic games (Krueger et al., 2012), are rated as more prosocial according to self-reports (Tost et al., 2010) and to observers (Kogan et al., 2011 –please see correction of the statistics and effect size from this study in Jostins, Pickrell, MacArthur, & Barretta, 2012). Furthermore, higher levels of perceived threat from others predict lower engagement in charitable activities for A-allele carriers, but not for G/Gs (Poulin, Holman, & Buffone, 2012). In the parenting domain, G/Gs exhibit higher levels of maternal sensitivity towards their toddlers compared to parents with the AA or AG genotype (Bakermans-Kranenburg & van IJzendoorn, 2008) and more pronounced physiological reactivity to recordings of infant crying in childless women of reproductive age (Riem, Pieper, Out, Bakermans-Kranenburg, & van IJzendoorn, 2011). There have also been some null results among studies examining associations of OXTR rs 53576 with social behavior, for instance a failure to replicate its links with behavior in an economic game (Apicella et al., 2010) and a lack of significant effects in predicting breastfeeding duration for new mothers (Tharner et al., 2012).
The OXTR Gene, Distress, and Psychopathology
The effect of the rs53576 OXTR alleles on brain function is currently unknown, but one study found smaller hypothalamic volumes and increased coupling of the hypothalamus with the amygdala and the dorsal anterior cingulate during a face emotion processing task in A-carriers compared to G/Gs, suggesting alterations in limbic-hypothalamic structure and function that may have clinical significance given the involvement of these regions in stress and emotion (Tost et al., 2010). Studies focusing on clinically-relevant outcomes suggest that the A-allele is associated with lower levels of self-reported positive affect (in German males, Lucht et al., 2009), as well as decreased levels of optimism, mastery and self-esteem, which in turn predict higher levels of depressive symptoms in a general nonclinical sample (Saphire-Bernstein, Way, Kim, Sherman, & Taylor, 2011). However, the G/G genotype was found to be overrepresented in a sample of clinically depressed patients, and these depressed homozygotes also reported higher levels of adult anxious attachment (Costa et al., 2009). However, the same study reported that there was no link between OXTR rs53576 and adult attachment status in their non-depressed control sample, which is consistent with null findings in another study (Gillath, Shaver, Baek, & Chun, 2008).
Interestingly, OXTR genotype seems to play differential roles under high and low distress conditions. For instance, Americans who are G-allele carriers report more emotional support seeking under distress compared to A-carriers, whereas in Koreans there is no difference by genotype, with neither group exhibiting differences by genotype under low distress (Kim et al., 2010). In another investigation showing differential susceptibility across contexts, G/G mothers exhibited greater sensitivity to their 2-year old toddlers under low levels of interparental conflict compared to high levels of conflict, whereas A-carriers did not differ across levels of conflict (Sturge-Apple, Cicchetti, Davies, & Suor, 2012). Another study showed that the OXTR genotype (G/G vs. A-carriers) related differentially to variation in social competence among non-maltreated children aged 6-12, whereas maltreated children did not differ by genotype (Cicchetti & Rogosch, 2012). This is likely because maltreated children showed significantly lower levels of social competence, suggesting a possible overpowering of genetic effects by maltreatment. Since we would predict G/Gs to have higher social competence than A-carriers, the finding that maltreated individuals did not show any difference by genotype suggests a possible vulnerability factor for G/Gs, who perhaps experience higher need for social interaction/prosocial behavior than A-carriers but do not have the behavioral skills and social competence (based on peer nominations, counselor and teacher reports in this study) to be effective in close interactions, due to their disruptive caregiving.
It is not surprising that OXTR seems to have different effects in low and high distress contexts given the roles that OT plays in stress and anxiety reduction at the neural, hormonal, and behavioral level (Neumann, 2008). Studies have shown that social partners can minimize stress responses across development and in many species (Hennessy, Kaiser, & Sachser, 2009), while in humans social support has been associated with beneficial health outcomes across the lifespan (Cohen, 2004). However, there are individual differences in the propensity to benefit from the stress-buffering effects of OT. One investigation found that adults who had experienced early parental separation did not show decreases in cortisol with intranasally-administered OT, whereas the comparison group did (Meinlschmidt & Heim, 2007). Based on the vast literature linking social support to stress reduction, individuals with the G/G genotype would be expected to more readily harness the benefits of social support, given that they seem to be more receptive to social cues and more prosocial. Indeed, a recent study showed that individuals carrying the G allele exhibited reduced cortisol responses to a laboratory stressor after social support, whereas A/A homozygotes did not (Chen et al., 2011).
However, few studies have examined whether the OXTR G/G polymorphism (rs53576) confers vulnerability under destructive relationship contexts. Relevant to this question, Poulin et al. (2012) reported that higher levels of perceived threat from others predicted lower engagement in charitable activities for A-allele carriers, but not for G/Gs. More directly pertinent to maltreated samples, a recent report examining African American adults showed that G/G carriers who reported being exposed to the most severe levels of childhood abuse described higher levels of symptoms on an Emotional Dysregulation Scale and increased levels of self-reported attachment disorganization (Bradley et al., 2011) compared to A-allele carriers. Individuals experiencing childhood maltreatment are in general at higher risk for experiencing physical and emotional symptoms (Rogosch, Dackis, & Cicchetti, 2011). Furthermore, they experience social relationships that are detrimental to their development, beginning with insecure attachment patterns in infancy and preschool (Cicchetti & Barnett, 1991; Cicchetti, Rogosch, & Toth, 2006) and extending to problems with peers which suggest a transfer of social dysfunction from the family context to new relationships that place them at risk for future maladaptation (Bolger, Patterson, & Kupersmidt, 1998; Rogosch, Cicchetti, & Aber, 1995). Adolescents who were maltreated as children similarly exhibit relational problems with peers, as well as higher rates of drug use, depression, and self-harm (Rogosch, Oshri, & Cichetti, 2010; Scott, Wolfe, & Wekerle, 2003). For these reasons, we predicted that maltreated adolescents would not only report less total social support, but less support from both family and non-family (i.e., peers).
Additionally, we expected psychological symptoms and perceived social support to vary according to maltreatment parameters such as duration, developmental timing/onset, and type, based on previous research suggesting that variation along these categories is important to consider when predicting the adjustment of maltreated children (Kaplow & Widom, 2007; Manly, Kim, Rogosch, & Cicchetti, 2001). One of the strengths of the present study is the focus on an adolescent sample, given that this developmental period is marked by the emergence of many types of psychopathology (Merikangas et al., 2010) and by multiple puberty-related alterations in brain affective systems (Crone & Dahl, 2012). Furthermore, animal models suggest that the oxytocin system matures during adolescence due to interactions with gonadal steroids that rise with puberty (Chibbar, Toma, Mitchell, & Miller, 1990). Thus, this period may be ideal for beginning to observe the interaction of maltreatment and oxytocin receptor genotype in predicting psychopathology.
Hypotheses
Based on the prior literature, the present study aimed to examine the following hypotheses. 1) First, we predicted that social support and conflict would be associated with emotional problems in adolescents with or without maltreatment histories. 2) Maltreatment was expected to be correlated with higher levels of total psychological symptoms, including Internalizing and Externalizing. Furthermore, we predicted based on prior literature (Kaplow & Widom, 2007; Manly et al., 2001; Manly, Cicchetti & Barnett, 1994) that symptoms would be related to maltreatment type, number of developmental periods with maltreatment, and onset, such that adolescents would report more symptoms if they experienced more maltreatment types, greater number of developmental periods when maltreated, or if their maltreatment started early in life. 3) OXTR rs53576 was hypothesized to be related to perceived social support from one’s social network (total support, as well as support from family and from non-family) in each group. Based on one finding in maltreated adults (Bradley et al., 2011), we predicted that G/Gs in the maltreated group would perceive less support whereas G/Gs in the non-maltreated group would report more social support. 4) Lastly, OXTR genotype was expected to moderate the relation between maltreatment and total psychological symptoms. Given the prediction for G/Gs to report less support if maltreated, this group is also expected to report more symptoms than maltreated A-carriers, whereas non-maltreated G/Gs are expected to have lower symptoms given the expectation that they would perceive higher support and the known associations between social support and well-being already reviewed above. We also examined if the moderation effect would be uniquely related to Internalizing or Externalizing symptoms, to provide further useful clinical information regarding specificity. Given gender differences in psychopathology (Nolen-Hoeksema, 2012), as well as sexually dimorphic roles of oxytocin in many species (Carter, 2007), we included gender as a covariate in all analyses.
Methods
Participants
Recruitment
Maltreating families were randomly contacted by a Department of Human Services recruitment liaison who explained the study. Interested parents signed consent forms releasing their contact information to research staff, who subsequently recruited families for participation. Indication of interest and/or participation was voluntary and could be declined at any time without consequence. Parents provided written consent for youth participation and for complete access to DHS family records. Adolescents aged approximately 13-15 were recruited and provided signed assent for their own participation. Records of DHS investigations of child abuse and/or neglect were used to identify all maltreated participants. The resulting sample of maltreating families was representative of the local DHS population.
Because the preponderance of maltreated youth represent low income families (Sedlak et al., 2010), demographically comparable families without maltreatment experiences were recruited through the Aid to Families with Dependent Children program. Non-maltreating families also were randomly contacted by a DHS liaison and recruited in the same manner as maltreating families. The absence of maltreatment experiences was verified through searches of the child abuse registry prior to recruitment. In addition, study interviews were conducted with caregivers to confirm a lack of DHS involvement (i.e., documented maltreatment or receipt of preventive services). Mothers of youth identified as non-maltreated completed the Maternal Maltreatment Classification Interview developed by Cicchetti, Toth, and Manly (2003). The procedures in this investigation were approved by the Research Subjects Review Board at the University of Rochester.
Demographics
We recruited 432 adolescents including both maltreated (N = 270) and non-maltreated (N = 167) youth with a mean age of 13.78 years (SD = 1.05). This is an independent and non-overlapping sample to that described in Cicchetti & Rogosch (2012), which examined OXTR and maltreatment in the context of other psychosocial outcomes in younger children. We used the Add Health system to code race and ethnicity in a single variable (http://www.cpc.unc.edu/projects/addhealth/data/code/race; Accessed 7/28/2012), with the exception that ‘‘American Indian’’ was coded as ‘‘other’’ because only two participants were identified as such. The sample was diverse (60.4% Black, 26.4% White, 11.6% Hispanic, and 1.6% Other). We excluded 7 participants under the “Other” race/ethnicity category from all analyses in order to be able to include it as a categorical covariate since the cell size for this group was too small to identify a main effect. Thus the remaining sample size was N = 425, with N = 263 maltreated and N = 162 non-maltreated. Maltreated and non-maltreated youth did not significantly differ in sex distribution, χ2(1) = .61, p = .44 or on whether they had a history of receipt of public assistance, χ2(1) = .07, p = .80. The difference in race/ethnicity distribution was not statistically significant (χ2(2) = 5.61, p = .06). Nevertheless, race/ethnicity was included as a categorical covariate in all analyses (see data analysis plan). Controlling for race/ethnicity also was used to address potential population stratification differences. Marital status (i.e., 1) never married; 2) married or living with partner; or 3) no longer married [divorced, separated, widowed]) of the primary caregiver also differed across groups, χ2(2) = 25.06, p < .01, such that caregivers of non-maltreated youth were more likely to be married or living with partners (50.3%) compared to caregivers of maltreated youth (26.2%). However, the main results of the paper remained significant when controlling for caregiver marital status; the interaction of OXTR by maltreatment in predicting Social Support remained significant, F(1,400) = 11.97, p = .001, as it was in predicting Internalizing, F(1,402) = 6.38, p = .01). Thus, this factor did not bias our results and we exclude it from future analyses for simplicity.
Measures
Childhood maltreatment
Thorough searches for DHS maltreatment records were conducted, and the obtained information was coded in accordance with the criteria detailed in the Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993). The reliability and validity of the MCS have been established in previous research (e.g., Bolger & Patterson, 2001; Manly, 2005). The MCS utilizes comprehensive DHS information to categorize maltreatment experiences independently from legal classifications and case dispositions. Specifically, each maltreatment subtype (i.e., sexual abuse, physical abuse, physical neglect, emotional maltreatment) was identified and coded (inter-rater reliability was high, kappa = .93), with the severity of each maltreatment experience also being coded (reliability was high, intra-class correlation of .96). Information about onset and duration of maltreatment was also obtained. Table 1 shows these sample characteristics. For 98.6% of maltreated individuals, the mother was one of the perpetrators for at least one of the maltreatment subtypes. Participants experienced on average 2.17 maltreatment subtypes (SD = .97, range = 1- 4).
Table 1
Maltreatment sample characteristics.
| Maltreated Sample (N = 229 with available information) | ||
|---|---|---|
| Type* (several possible) | Sexual Abuse | 19.7% (N = 45) |
| Physical Abuse | 50.2% (N = 115) | |
| Physical Neglect | 81.2% (N =186) | |
| Emotional Maltreatment | 66.4% (N =152) | |
| Duration** | 1 | 29.3% (N = 67) |
| 2 | 21.8% (N = 50) | |
| 3 | 28.8% (N = 66) | |
| 4+ | 20.1% (N = 46) | |
| Onset*** | Early | 86.5% (N= 198) |
| Late | 13.5% (N = 31) | |
In this classification system, sexual abuse was defined as sexual relationships between children and adults, whereas physical abuse was recorded in situations when a caregiver inflicts a physical injury to the child by other than accidental means. Physical neglect was defined as failure to provide for the child’s nutritional, medical, and cleanliness needs and/or a lack of supervision –e.g., leaving a young child unattended or with a caregiver known to be inappropriate, for instance someone predisposed to violence. Emotional maltreatment was conceptualized as those acts deemed by Child Protective Services to be instances of maltreatment that involved thwarting of children’s basic emotional needs. These needs included needs to psychological safety and security in the environment, for acceptance and positive regard, and for age-appropriate autonomy.
Network of Relationships Inventory (NRI)
The revised NRI (Furman & Buhrmester, 1992) was designed to be used with children and adolescents and it assesses ten relationship qualities including seven aspects of support (reliable alliance, enhancement of worth, affection, companionship, instrumental help, intimacy, and nurturance) and three other relationship features (conflict, punishment, relative power). Subjects rated these qualities using three Likert-type questions for each of eight specific relationships: mother, father, sibling, grandparent, closest same-sex friend, closest opposite-sex friend, romantic partner, and any one extra individual. For instance, participants had to use 1-5 scales to rate “how much does this person like or love you” or “how often do you and this person get mad at or get in fights with each other.” Derived scales include support, conflict, punishment, and relative power, and Furman and Buhrmester (1992) reported a mean alpha for these scales of .81. Principal Components Analysis indicated that the seven aspects of support all loaded highly on a single factor (loadings between .75 and .89), whereas conflict and punishment loaded highly on a second factor (loadings of .82 and .65). Thus we created a total Social Support composite by averaging the means for the seven support scales (alpha for the scale was .94) and a total Conflict composite by averaging the means for the conflict and punishment scales (alpha for the scale was .84). Relative power was dropped from analyses as it did not load highly on any of the two factors and it was only measured by 3 questions. We also calculated separate composites for family and non-family members based on the information available (i.e., if an adolescent did not have any opposite-sex friends, this was treated as missing data and not included in the mean for non-family). All maltreated participants were still living with their original families (no foster care experiences) and described these relationships with the NRI.
Youth Self Report (YSR, Achenbach, 1991)
Internalizing and Externalizing symptoms were assessed using the YSR. The YSR is a self-report questionnaire designed for 11- to 18-year olds that measures a comprehensive set of behavioral disturbances subsumed under two broadband dimensions of Internalizing (i.e., anxious depressed, withdrawn, somatic complaints) and externalizing (i.e., aggressive and rule-breaking behavior) symptoms. Responses to the 118 items were provided on a 3-point scale (0 = Not True, 1 = Somewhat or Sometimes True, 2 = Very True or Often True). Raw summed scores for Internalizing and Externalizing symptoms were transformed to T-scores based on extensive normative data such that higher scores reflect higher levels of symptoms. We used the T-score for Total Problems (average of Internalizing and Externalizing) as an index of current youth symptoms. The YSR is a widely used, well-validated and reliable measure (Achenbach, 1991; Achenbach & Rescorla, 2001).
DNA collection, extraction, and genotyping
Using an established protocol, trained research assistants obtained DNA samples from participants by collecting buccal cells with the Epicentre Catch-All Collection Swabs. Subsequently, using the conventional method, DNA was extracted with the Epicentre BuccalAmp DNA Extraction Kit, in order to prepare DNA for PCR amplification. DNA was whole-genome amplified using the Repli-g kit (Qiagen, Catalogue No. 150043) per the kit instructions to provide sufficient material for this and other genotyping experiments without exhausting the original sample. Amplified samples were then diluted to a working concentration.
The OXTR rs53576 SNP was genotyped for individual alleles using a TaqMan SNP assay from Applied Biosystems, Inc (C__3290335_10) with TaqMan Genotyping Master Mix (Applied Biosystems, Catalog No. 4371357) performing amplification on an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200 with JMP 8.0 (SAS, Inc.). Human DNA from immortalized cell lines was used as positive controls and “no template” served as the negative controls. The positive control samples genotypes were confirmed using DTCS chemistry on an ABI 3130xl. All genotyping controls represented 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated up to 4 times under the same conditions before a null result was reported.
The genotype distribution for OXTR was in Hardy-Weinberg equilibrium for both the comparison group [n = 11 A/A, n = 54 A/G, n = 97 G/G, χ2(2) = 1.38, p = .50] and the maltreated group [n = 15 A/A, n = 109 A/G, n = 139 G/G, χ2(2) = 1.1, p = .58]. As expected, the A/A genotype was relatively infrequent, thus we combined A/A and A/G carriers to form one group (A-allele carriers) in all analyses contrasting them to G/Gs. This approach also allows comparability with prior studies adopting the same grouping strategy (Kogan et al., 2011; Krueger et al., 2012; Rodrigues et al., 2009; Tost et al., 2010). Genotype group distribution (A-carriers versus G/Gs) did not differ significantly by maltreatment status (χ2(1) = 2.01, p = .16). We present sample size by race, genotype and maltreatment status in Table 2, showing also that genotype distributions did not differ by maltreatment status within any of the three race groups. This suggests that, at least in this sample, there is no evidence that OXTR genotype correlates with maltreatment status. Within the maltreated group, individuals with the G/G genotype did not differ significantly from A-carriers on any objective measures of maltreatment type and severity, such as mean number of maltreatment subtypes experienced (t(222) = .40, p = .69), sum of the severities for each maltreatment type encountered (t(222) = .05, p = .96), proportion of adolescents in each maltreatment subtype category (χ2(3) = 5.06, p = .17), number of developmental periods with documented maltreatment (t(222) = 1.41, p = .16), or developmental period for maltreatment onset (χ2(5) = 5.43, p = .37).
Table 2
Sample size by race, genotype and maltreatment status.
| Race | Genotype | Maltreatment Status | Total | Chi-square test | |
|---|---|---|---|---|---|
| Comparison | Maltreated | ||||
| Black | G/G | 70 | 91 | 161 | χ2(1) = .51 |
| A/A or A/G | 39 | 61 | 100 | p = .48 | |
| Total | 109 | 152 | 261 | ||
| White | G/G | 13 | 33 | 46 | χ2(1) = .02 |
| A/A or A/G | 20 | 48 | 68 | p = .89 | |
| Total | 33 | 81 | 114 | ||
| Hispanic | G/G | 14 | 15 | 29 | χ2(1) = 1.97 |
| A/A or A/G | 6 | 15 | 21 | p = .16 | |
| Total | 20 | 30 | 50 | ||
| Total | G/G | 97 | 139 | 236 | χ2(1) = 2.01 |
| A/A or A/G | 65 | 124 | 189 | p = .16 | |
| Total | 162 | 263 | 425 | ||
The genotype distributions differed by race (χ2(2) = 14.7, p < .001), with Whites having more A-carriers than G/Gs and the opposite pattern being true among Blacks and Hispanics (see Table 2 for frequencies), which was another rationale to control for the main effect of race in all analyses.
Data Analysis Plan
Data preparation
Variables were examined for normality of distributions. None of the continuous variables exhibited excessive skewness or kurtosis, thus untransformed scores were used in statistical analyses. There were no univariate outliers for NRI Social Support and Conflict or YSR measures (defined as scores more than 3 standard deviations from the mean). Mean-centered variables were used in regressions. Multivariate outliers exerting disproportionate effects on the outcomes of ANCOVAs (Cook’s distance > 4/n-k-1) were excluded from analyses. Regression diagnostics (Q-Q plots for assessing normality of residuals, Levene’s test for the equality of error variances) indicated that the assumptions needed for conducting ANCOVAs/MANCOVAs were met for each analysis conducted.
Analyses
We used multiple regression analysis, MANCOVAs and ANCOVAs to examine links between genotype, maltreatment, Social Support and Total Problems, while using sex, age and race/ethnicity as covariates. Race/ethnicity was included as a categorical covariate in all analyses. Our approach was to first conduct a separate ANCOVA testing for the effects of maltreatment status, genotype, and their interactions on Total Social Support, Total Conflict, and Total Problems, and then follow-up with MANCOVAs including subcomponents of these composites as dependent variables (i.e., Support from Family and from Non-family, Internalizing and Externalizing Problems).
Results
Associations among Social Support, Conflict, Total Problems, and Maltreatment
Means, standard deviations, and correlations for the main study variables are presented for the entire sample in Tables 3 and and44.
Table 3
Correlations and descriptives for main continuous study variables in the entire sample.
| Correlations | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. NRI Social Support (mean) | 1 | ||||
| 2. NRI Conflict (mean) | .15** | 1 | |||
| 3. YSR Total Problems (T-score) | −.22** | .33** | 1 | ||
| 4. YSR Internalizing (T-score) | −.16** | .28** | .87** | 1 | |
| 5. YSR Externalizing (T-score) | −.26** | .28** | .86** | .59** | 1 |
| Mean | 3.6 | 2.12 | 49.4 | 48.4 | 50.9 |
| SD | .65 | .52 | 10.8 | 10.4 | 11.9 |
| Range | 1.8-4.9 | 1-3.9 | 24-81 | 26-88 | 25-86 |
Table 4
Raw means and standard deviations for main study variables by group and genotype.
| Maltreatment Group | Genotype | Social Support Mean (SD) | Conflict Mean (SD) | Total Problems Mean (SD) |
|---|---|---|---|---|
| Maltreated | G/G | 3.49 (.66) | 2.14 (.51) | 50.85 (10.99) |
| Maltreated | A/A or A/G | 3.65 (.66) | 2.11 (.55) | 48.03 (11.55) |
| Comparison | G/G | 3.63 (.67) | 2.08 (.52) | 49.1 (10) |
| Comparison | A/A or A/G | 3.66 (.54) | 2.14 (.52) | 49.5 (10.3) |
In a multiple regression analysis with Total Problems as the dependent variable and sex, age and race as control variables (only sex was significant, β = .1, t = 2.12, p = .04, with adolescent females reporting more problems), we examined the following independent variables: Social Support, Conflict, maltreatment status, and the interaction terms of Support by maltreatment and Conflict by maltreatment. Social Support and Conflict were each independently and significantly correlated with Total Problems, with Support negatively associated (β = -.31, t = -4.06, p < .001) and Conflict positively associated (β = .33, t = 4.47, p < .001) with Total Problems. Maltreatment status was not a significant predictor of Problems (β = - .01, t = -.29, p = .77) and neither was the interaction of maltreatment by Support (β = .05, t = .64, p = .52) or maltreatment by Conflict (β = .08, t = 1.02, p = .31). Regression analyses within each of the maltreated groups revealed that Support and Conflict remained independently and significantly associated with Total Problems within each group. When analyzing Internalizing or Externalizing separately instead of Total Problems, the same pattern of results was obtained, with only Support, Conflict and sex being significant correlates in the same direction as described above. ANCOVAs conducted within the maltreated group revealed that Total Problems did not differ by maltreatment type, F(3, 230) = .62, p = .60, number of developmental periods with maltreatment, F(3, 230) = .48, p = .70, or by onset, F(1, 230) = 1.26, p = .26 (see Table 1 for categories included under each variable), while controlling for sex, age and race as in all other analyses. The same non-significant findings were obtained when analyzing Internalizing or Externalizing Problems separately instead of Total Problems.
Variation in OXTR Genotype and Perceived Social Support
We used an ANCOVA to examine the effects of OXTR genotype, maltreatment, and their interaction on perceived total Support, while controlling for sex, age, and race. There was a significant interaction of maltreatment status and genotype in predicting total Social Support (F(1,401) = 4.04, p = .045, ηp2= .01), with a non-significant main effect of maltreatment (F(1,401) = 3.44, p =.06) and no main effect of genotype (F(1, 401) = .97, p = .33). We then conducted ANCOVAs within each maltreatment group to follow-up on the interaction effect. In the maltreated group, there was a significant main effect of genotype (F(1,251) = 5.47, p = .02, ηp2 = .02) such that carriers of the G/G genotype reported significantly less support (M = 3.48, SE = .06) compared to A-allele carriers (M = 3.66, SE = .06). In the comparison group, the effect of genotype was not significant (F(1,146) = .88 , p = .35), with G/Gs showing similar means (M = 3.74, SE = .07) compared to the A-allele carriers (M = 3.66, SE = .07). Estimated marginal means by group and genotype are presented in Fig. 1. Follow-up ANCOVAs also indicated that within the G/G genotype, the maltreated group reported significantly less support compared to the non-maltreated group (F(1,216) = 8.26, p = .004, ηp2= .04). However, among individuals with the AA or AG genotype, maltreated participants did not report different levels of support when compared to non-maltreated adolescents (F(1,181) = .003, p = 96)1.
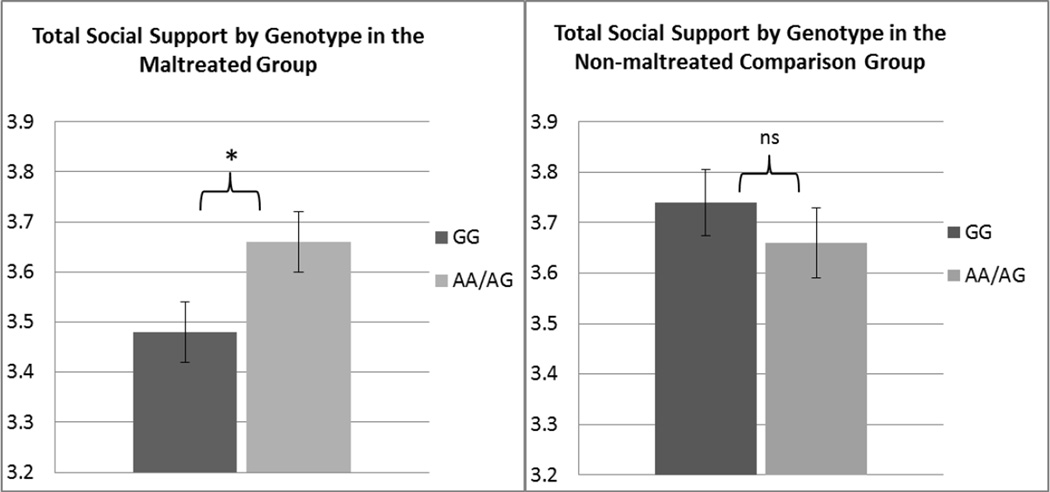
Estimated marginal means of perceived total Social Support after controlling for age, sex, and race. Error bars represent SEMs. Differences were considered significant (marked with *) if p < .05, whereas “ns” was used to label non-significant differences.
To investigate whether the effect on Total Support was carried by Support from Family or Non-family, A MANCOVA with Family Support and Non-family Support as the dependent variables was conducted. The analysis revealed that the interaction effect of genotype by maltreatment was non-significant for Social Support from Family (F(1,396) = 3.16, p =.076, ηp2 = .01) and significant for Non-family (F(1,396) = 5.01, p = .026, ηp2 = .01). The follow-up ANCOVA for the significant interaction predicting Support from Non-family revealed the same pattern of results as above, with main effects of genotype being significant in the maltreated group (F(1,246) = 5.01, p = .026, ηp2 = .02), whereas in the comparison group there was no effect of genotype (F(1,146) = .94, p = .33).
In a MANCOVA predicting total Conflict from genotype, maltreatment, and the interaction of maltreatment by genotype, with sex, age and race as covariates, there were no significant effects of genotype (F(1,399) = .32, p = .57), maltreatment, (F(1,399) = 2.56, p = .11), or the interaction of maltreatment by genotype (F(1,399) = 1.76, p = .19). Similar nonsignificant results were obtained for Family Conflict and Non-family Conflict separately.
OXTR Genotype and Internalizing, Externalizing Problems
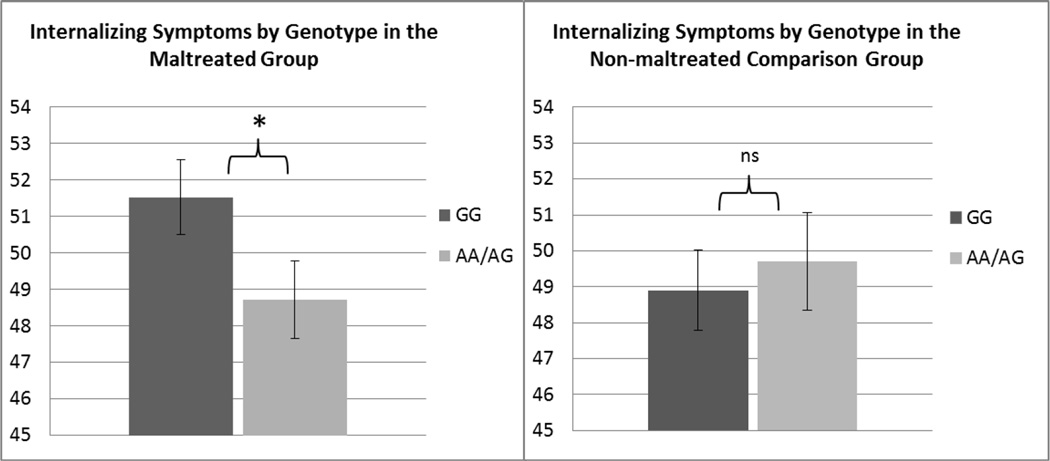
An ANCOVA was used to examine the effect of genotype, maltreatment, and their interaction on Total YSR Problems, while controlling for sex, age, and race. There was no main effect of genotype (F(1,402) = 2.44, p = .12) or maltreatment (F(1,402) = .002, p = .97), with a significant interaction effect of genotype by maltreatment (F(1,402) = 3.8, p = .05, ηp2 = .01). We then conducted a MANCOVA with Total Internalizing Problems and Total Externalizing Problems as the dependent variables to examine whether the interaction effect of gene × maltreatment was statistically significant for only one of these dimensions included in Total Problems. Indeed, the MANCOVA revealed that the interaction between genotype and maltreatment was significant for Internalizing (F(1,402) = 6.42, p = .01, ηp2 = .02) but not for Externalizing (F(1,402) = 2.58, p = .11). We then probed the interaction effect by running separate ANCOVAs to examine the effect of genotype on Internalizing within each maltreatment group, controlling for sex, age and race. In the maltreated group there was a main effect of genotype (F(1,249) = 7.03, p = .01, ηp2 = .03) such that G/Gs reported higher mean levels of Internalizing Symptoms (M = 50.3, SE = 1.02) compared to A-allele carriers (M = 46.9, SE = 1.07). In the non-maltreated comparison group there was no effect of genotype on Internalizing (F(1,153) = 1.31, p = .25). Follow-up ANCOVAs controlling for age, sex, and race also revealed that among individuals with the G/G genotype, maltreated participants reported significantly higher levels of Internalizing symptoms than the non-maltreated comparison group (F(1,221) = 5.06, p = .025, ηp2 = .02). Among the AA/AG carriers, mean levels of Internalizing symptoms did not differ by maltreatment status (F(1,169) = 1.86, p = .18)2. Estimated marginal means for each genotype and maltreatment group controlling for sex, age, and race, are presented in Fig.2.
Discussion
To our knowledge, the present study is the first investigation of the role of OXTR in risk for psychopathology in a developmental sample –i.e., maltreated adolescents. The results provided evidence of a genotype by maltreatment interaction such that adolescents with maltreatment histories and the G/G variant of OXTR rs 53576 perceived significantly less social support compared to maltreated A-carriers. They also reported significantly higher levels of Internalizing symptoms compared to A-carriers. Importantly, the two genotype groups did not differ on any objective measures of maltreatment type, duration, or severity. Given that prior literature suggests that G/G homozygotes may be more receptive to social cues and more prosocial compared to A-allele carriers (Kogan et al., 2011; Tost et al., 2010), we interpret the present findings to indicate that G/Gs may be more attuned to and affected by negative social experiences such as being maltreated. Furthermore, being more likely to seek social interaction may be detrimental when living with an abusive caregiver, while being more empathic (Rodrigues et al., 2009) may expose children to higher levels of emotional arousal, stress, and anger displayed by their maltreating parents. It is known that maltreatment negatively impacts one’s ability to form relationships with peers (Bolger, Patterson, & Kupersmidt, 1998; Rogosch, Cicchetti, & Aber, 1995), thus it is consistent that G/Gs also reported less perceived support from non-family members. Another possible interpretation of these results is that adolescents with the G/G genotype might objectively elicit less support; however, this is unlikely given that the genotype has previously been associated with more prosocial, generous and empathic behavior in the general population.
The findings reported here replicate the pattern of results obtained in an adult sample from a prior study (Bradley et al., 2011) that similarly found higher levels of emotional dysregulation among G/Gs who experienced 3 or more types of childhood abuse compared to Aallele carriers in an urban African American sample. The present study extends these findings to adolescents and a more ethnically diverse group. There are a number of methodological advantages to the present report, including an objective documentation of child maltreatment based on DHS records. Measurement in adolescence is another advantage, since this is more proximal to the maltreatment experience, whereas assessment in adulthood may introduce the possibility of additional intervening factors that could compound the effects. In addition, we adopted highly reliable, widely utilized and nationally-normed scales for measuring Internalizing and Externalizing symptoms (the Achenbach’s Youth Self Report) and perceived support/conflict of adolescents (the NRI). It is important that our findings are consistent with those of Bradley et al. (2011) given difficulties in replicating other G × E findings in the literature. Difficulty in replicating other G × E findings may stem from inconsistency or lack of rigor in measuring the E (environment) component (Monroe & Reid, 2008). We believe that our use of objective measures of maltreatment and widely utilized measures of psychological symptoms and support/conflict should facilitate future replication in other laboratories.
It must be noted that we found no evidence of gene-environment correlation between OXTR genotype and maltreatment status in our sample. This is not surprising given that studies on human and non-human animals have not been able to identify evidence of genetic transmission of maternal maltreating behaviors. In contrast, experimental rodent models have provided evidence for environmental effects on maltreatment. For instance, rodent models have been shown to induce abusive maternal behavior towards the pups by limiting the availability of bedding materials for the nest, a chronic stressor for the mother (Avishai-Eliner, Gilles, Eghbal-Ahmadi, Bar-El, & Baram, 2001; Rainecki, Sullivan, & Moriceau, 2010; Roth & Sullivan, 2005). Furthermore, cross-fostering studies in rhesus monkeys have shown that females who were maltreated in early development are significantly more likely to become maltreating mothers, but if offspring of maltreating mothers are raised from birth by good adoptive mothers, then they will not maltreat their descendants, suggesting an environmental rather than genetic transmission of maltreatment (Maestripieri, 2005). This does not rule out the possibility that OXTR shapes normative variations in parenting sensitivity in humans, as has been suggested (Bakermans-Kranenburg & van IJzendoorn, 2008); however, the OXTR genotype cannot be the sole cause of maltreatment in our sample given the lack of differences in genotype distributions by maltreatment status.
It was surprising that we did not detect a main effect of maltreatment on psychological symptoms, Internalizing, or Externalizing; however, our gene-environment interaction results suggest that this is likely due to the fact that A-carriers (47% of the maltreated sample) exhibited comparable levels of symptoms to participants in the non-maltreated sample (Fig. 2). Furthermore, this is consistent with prior literature on resilience in maltreated populations which suggests that the experience of maltreatment does not deterministically lead to psychopathology (Cicchetti, in press; Cicchetti & Rogosch, 1997). Variation in outcomes is the very phenomenon that our study set out to understand. We must also remember that the comparison group was a sample of adolescents living in poverty, who could exhibit comparable levels of symptoms due to other types of adversity that are unmeasured here. The fact that we also did not find a main effect of maltreatment on Social Support, especially support from family, could have the same explanations (i.e., a high-poverty comparison group and the moderation effect by genotype). Another prediction that did not materialize was the fact that maltreatment type, duration, and onset did not have significant effects on our measures of symptoms (Total, Internalizing, or Externalizing) in the maltreated group.
Interestingly, we did not find an effect of genotype on perceived Social Support or Total Problems in the non-maltreated group. The effect size for the difference in mean total Social Support between G/Gs and A-carriers in this non-maltreated comparison group was so small (ηp2 = .006) that a power analysis indicated that we would need approximately 2155 participants to detect a significant effect with a power of .95 and a significance criterion of α = .05 in this group. Thus, the non-significant effect in the comparison group was not due to having a smaller sample compared to the maltreated group. The finding may be due to the fact that this low-income group of adolescents is quite heterogeneous, with some experiencing other social adversity associated with living in poverty (e.g., exposure to violence) that is unmeasured here and which is perhaps associated with higher levels of symptoms in G/Gs similar to the pattern observed with the maltreated group, whereas some of them may experience the more normative pattern of lower psychological symptoms for G/Gs, possibly averaging out to an absent main effect of OXTR on symptoms in the non-maltreated comparison group.
Strengths and Limitations of the Present Study
This study has a number of strengths, including the recruitment and assessment of a challenging population and reliance on objective DHS records to identify maltreatment and to conduct a thorough classification of maltreatment characteristics, as already described. The present investigation also has the advantage of including a racially and ethnically diverse population and a comparison group that is as closely matched as possible with respect to low family income, sex, and age. Lastly, this report addresses an important question regarding the sources of individual differences in mental health outcomes among maltreated youth and would be the first study conducted with a developmental sample to investigate the potential interaction of the OXTR gene with maltreatment in predicting psychopathology. However, this project also has a number of limitations described below.
One of the limitations that must be acknowledged was that both perceived support and Internalizing/Externalizing symptoms were derived from self-report measures. The specificity of our OXTR × maltreatment interaction in predicting perceived Social Support but not Conflict derived from the NRI scales gives us some confidence in the validity of this measure, given that it is biologically plausible to link oxytocin to perceived social support, whereas there would be less of a theoretical grounding for results involving Conflict. With respect to using the Youth Self-Report measure, emotional symptoms are often more accurately reported by the individuals themselves (especially Internalizing) than by external observers. This is perhaps why we did not find a G × E effect with respect to Externalizing symptoms, given that individuals may be less likely to accurately report their own aggressive and rule-breaking behaviors that were sampled by this measure. Another reason to expect this differentiation is that OXTR has been linked by a few prior studies with symptoms from the internalizing spectrum such as depression (Costa et al., 2009; Saphire-Bernstein et al., 2011), whereas the minimal information existing for links with externalizing (e.g., aggression, conduct problems) indicates no associations with OXTR function (Malik, Zai, Abu, Nowrouzi, & Beitchman, 2012; Sakai et al., 2012). It may be that additional environmental or genetic factors need to be accounted for in predicting externalizing.
A second limitation is the examination of a single gene given the recognition that resilience is a complex, dynamic process that likely encompasses multiple systems affecting emotional and physiological reactions to maltreatment –e.g., the serotonin, dopamine, or CRH systems (Cicchetti & Rogosch, 2012). However, given the lack of any developmental studies on the role of OXTR in predicting psychopathology in maltreatment, this is an important gap to fill in the literature.
Given that our sample was 13-15 years old, most if not all participants provided data after the onset of puberty, thus unfortunately we were not able to examine the interaction of puberty onset with OT function and maltreatment. This is an important question for future studies, given the role of gonadal steroids, which rise with puberty, in the maturation of the oxytocin system (Chibbar et al., 1990).
Lastly, the fact that the majority of our sample (more than 60%) was African American limits the generalizability of these findings to Whites and Hispanics, especially given the fact that similar findings from the adult study by Bradley et al. (2011) also characterized an African American sample. We reported the main analyses within the African American sample in the results presented above to include in future meta-analyses and argue that we must be cautious in interpreting these results as applying to all ethnicities. Future studies should explore similar questions in ethnically homogeneous samples of White or Hispanic adolescents, given evidence that some G × E effects involving other genes differ by ethnicity (Van IJzendoorn, Belsky, & Bakermans-Kranenburg, 2012). Even though we are not able to rule out population stratification effects in this mixed-ethnicity sample, we statistically controlled for the main effect of race in all analyses, ascertained Hardy-Weinberg equilibrium, and noted that maltreatment did not correlate with genotype in any of the three ethnic groups. Future studies should incorporate ancestral proportion scores to replicate these findings while controlling for any possible stratification effects.
Conclusions
One of the most remarkable findings of this study was that maltreated adolescents who were A-carriers had similar levels of perceived Social Support and Internalizing Problems to non-maltreated adolescents, suggesting possible protective effects conferred by the allele in this adverse social rearing environment. These findings reinforce the perspective that developmental outcomes are shaped by complex interactions between environmental characteristics and genes across development (Cicchetti, 2010). The results of this study may be combined with other insights to discover underlying processes that may contribute to resilience. The effect of being an A-carrier in the context of maltreatment seems to be opposite to some of the findings in the general population, where G/Gs seem better adjusted according to some criteria such as levels of positive affect, optimism, and self-esteem (Lucht et al., 2009; Saphire-Bernstein et al., 2011), findings which are consistent with the overall beneficial effects of social support for human development and for coping with stress under typical rearing conditions. These results suggest that future mental health interventions will be more successful if they account for individual differences in genetics and in psychological patterns of responding to adverse environmental conditions (Cicchetti & Blender, 2006).
Acknowledgements
We thank the adolescents and families who participated in this research. This study was supported by NIH grant R01-DA12903 and the Spunk Fund, Inc.
Footnotes
1When examining results within the African American sample alone, OXTR interacted significantly with maltreatment status in predicting total Social Support (F(1, 246) =5.99, p = .015, ηp2= .024). Follow-up ANCOVAs revealed that within the maltreated group GGs reported non-significantly lower levels of social support than A carriers (F(1, 147) =2.33, p = .13, ηp2= .016), whereas in the comparison group GGs reported higher levels of total support than A-carriers (F(1, 99) = 4.21, p = .04, ηp2= .04).
2Within the African American sample, the OXTR × maltreatment interaction tested was still significant (F(1, 251) = 4.50, p = .035, ηp2 = .018), with follow-up ANCOVAs revealing that in the maltreated sample there was a statistical trend for GGs to report more Internalizing problems (F(1, 147) = 3.37 , p = .068, ηp2 = .022) and there were no significant differences by genotype within the comparison group (F(1, 102) = 1.43, p = .24, ηp2 = .014).
References
- Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Apicella CL, Cesarini D, Johannesson M, Dawes CT, Lichtenstein P, Wallace B, Westberg L. No association between oxytocin receptor (OXTR) gene polymorphisms and experimentally elicited social preferences. PLoS One. 2010;5(6):e11153. [Europe PMC free article] [Abstract] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. Journal of Neuroendocrinology. 2001;13:799–807. [Europe PMC free article] [Abstract] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3(2):128–134. [Europe PMC free article] [Abstract] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences. 2009;1167:182–189. [Abstract] [Google Scholar]
- Bolger KE, Patterson CJ. Pathways from child maltreatment to internalizing problems: Perceptions of control as mediators and moderators. Development and Psychopathology. 2001;13:913–940. [Abstract] [Google Scholar]
- Bolger KE, Patterson CJ, Kupersmidt JB. Peer relationships and self-esteem among children who have been maltreated. Child Development. 1998;69(4):1171–1197. [Abstract] [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, Heim C. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: Moderation by oxytocin receptor gene. Development and Psychopathology. 2011;23(2):439–452. [Europe PMC free article] [Abstract] [Google Scholar]
- Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, Gündel H. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 2010;34(9):1417–1422. [Europe PMC free article] [Abstract] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavior and Brain Research. 2007;176:170–186. [Abstract] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews Neuroscience. 2006;7(7):583–590. [Abstract] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the USA. 2001;98(22):12736–12741. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences of the USA. 2011;108(50):19937–19942. [Europe PMC free article] [Abstract] [Google Scholar]
- Chibbar R, Toma JG, Mitchell BF, Miller FD. Regulation of neural oxytocin gene expression by gonadal steroids in pubertal rats. Molecular Endocrinology. 1990;4:2030–2038. [Abstract] [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective. World Psychiatry. 2010;9:1–10. [Europe PMC free article] [Abstract] [Google Scholar]
- Cicchetti D. Resilient functioning in maltreated children: Past, present, and future perspectives. Journal of Child Psychology and Psychiatry. 2013;54(4):402–422. [Europe PMC free article] [Abstract] [Google Scholar]
- Cicchetti D, Barnett D. Attachment organization in maltreated preschoolers. Development and Psychopathology. 1991;3:397–411. [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. [Abstract] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. Vol. 2. Hoboken, NJ: Wiley; 1995. pp. 32–71. [Google Scholar]
- Cicchetti D, Rogosch FA. The role of self-organization in the promotion of resilience in maltreated children. Development and Psychopathology. 1997;9:799–817. [Abstract] [Google Scholar]
- Cicchetti D, Rogosch FA. Gene × Environment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development and Psychopathology. 2012;24(2):411–427. [Europe PMC free article] [Abstract] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18(3):623–649. [Abstract] [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal maltreatment interview. 2003 Unpublished manuscript. [Google Scholar]
- Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2nd ed. Vol. 3. Hoboken, NJ: Wiley; 2006. pp. 129–201. [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. [Abstract] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34(10):1506–1514. [Abstract] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. [Abstract] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–731. [Abstract] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–733. [Abstract] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. [Abstract] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Hormones and Behavior. 2012;61(3):359–379. [Abstract] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. [Abstract] [Google Scholar]
- Furman W, Buhrmester D. Age and sex differences in perceptions of networks of personal relationships. Child Development. 1992;63:103–115. [Abstract] [Google Scholar]
- Galbally M, Lewis AJ, IJzendoorn M, Permezel M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harvard Review of Psychiatry. 2011;19:1–14. [Abstract] [Google Scholar]
- Gillath O, Shaver PR, Baek J-M, Chun DS. Genetic correlates of adult attachment style. Personality & Social Psychology Bulletin. 2008;34(10):1396–1405. [Abstract] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81(2):629–683. [Abstract] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. [Europe PMC free article] [Abstract] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psychological Bulletin. 2003;129(3):447–466. [Abstract] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller, A H, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry. 2009;14(10):954–958. [Abstract] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: Effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170(8):337–350. [Abstract] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–482. [Abstract] [Google Scholar]
- Inoue T, Kimura T, Azuma C, Inazawa J, Takemura M, Kikuchi T, Saji F. Structural organization of the human oxytocin receptor gene. Journal of Biological Chemistry. 1994;269:32451–32456. [Abstract] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences of the USA. 1992;89(13):5981–5985. [Europe PMC free article] [Abstract] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters. 2007;417:6–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Jostins L, Pickrell JK, MacArthur DG, Barretta JC. Misuse of hierarchical linear models overstates the significance of a reported association between OXTR and prosociality. Proceedings of the National Academy of Sciences of the USA. 2012;109(18):E1048. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. Journal of Abnormal Psychology. 2007;116:176–187. [Abstract] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–454. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu, Taylor SE. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences of the USA. 2010;107(36):15717–15721. [Europe PMC free article] [Abstract] [Google Scholar]
- Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Rodrigues Saturn S. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proceedings of the National Academy of Sciences of the USA. 2011;108(48):19189–19192. [Europe PMC free article] [Abstract] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. [Abstract] [Google Scholar]
- Krueger F, Parasuraman R, Iyengar V, Thornburg M, Weel J, Lin M, Lipsky RH. Oxytocin receptor genetic variation promotes human trust behavior. Frontiers in Human Neuroscience. 2012;6:4. [Europe PMC free article] [Abstract] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in neuropsychopharmacology & biological psychiatry. 2009;33(5):860–866. [Abstract] [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences of the USA. 2005;102(27):9726–9729. [Europe PMC free article] [Abstract] [Google Scholar]
- Malik AI, Zai CC, Abu Z, Nowrouzi B, Beitchman JH. The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes, Brain, and Behavior. 2012;11(5):545–551. [Abstract] [Google Scholar]
- Manly TJ. Advances in research definitions in child development. Child Abuse and Neglect. 2005;29:425–439. [Abstract] [Google Scholar]
- Manly JT, Cicchetti D, Barnett D. The impact of subtype, frequency, chronicity, and severity of child maltreatment on social competence and behavior problems. Development and Psychopathology. 1994;6:121–143. [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [Abstract] [Google Scholar]
- Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56(3):227–238. [Abstract] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological psychiatry. 2007;61(9):1109–1111. [Abstract] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication –Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. [Europe PMC free article] [Abstract] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524–538. [Abstract] [Google Scholar]
- Monroe SM, Reid MW. Gene-environment interactions in depression: Genetic polymorphisms and life stress polyprocedures. Psychological Science. 2008;19(10):947–956. [Abstract] [Google Scholar]
- Montag C, Brockmann EM, Lehmann A, Müller DJ, Rujescu D, Gallinat J. Association between oxytocin receptor gene polymorphisms and self-rated 'empathic concern' in schizophrenia. PLoS One. 2012;7(12):e51882. [Europe PMC free article] [Abstract] [Google Scholar]
- Morhenn V, Park J, Piper E, Zak P. Monetary sacrifice among strangers is mediated by endogenous oxytocin release after physical contact. Evolution and Human Behavior. 2008;29:375–383. [Google Scholar]
- Naber F, van Ijzendoorn MH, Deschamps P, van Engeland H, Bakermans-Kranenburg MJ. Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: a double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35(10):1583–1586. [Abstract] [Google Scholar]
- Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. Journal of Neuroendocrinology. 2008;20(6):858–865. [Abstract] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: The role of gender. Annual Reviews in Clinical Psychology. 2012;8:161–187. [Abstract] [Google Scholar]
- Poulin M, Holman EA, Buffone A. The neurogenetics of nice: Receptor genes for oxytocin and vasopressin interact with threat to predict prosocial behavior. Psychological Science. 2012;23(5):446–452. [Abstract] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67:1137–1145. [Europe PMC free article] [Abstract] [Google Scholar]
- Riem MME, Pieper S, Out D, Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Social Cognitive and Affective Neuroscience. 2011;6(3):294–300. [Europe PMC free article] [Abstract] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the USA. 2009;106(50):21437–21441. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogosch FA, Cicchetti D, Aber JL. The role of child maltreatment in early deviations in cognitive and affective processing abilities and later peer relationship problems. Development and Psychopathology. 1995;7(04):591. [Google Scholar]
- Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23(4):1107–1124. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogosch FA, Oshri A, Cicchetti D. From child maltreatment to adolescent cannabis abuse and dependence: A developmental cascade model. Development and Psychopathology. 2010;22:883–897. [Europe PMC free article] [Abstract] [Google Scholar]
- Roth T, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. [Abstract] [Google Scholar]
- Rutter M. Gene-environment interplay. Depression and Anxiety. 2010;27:1–4. [Abstract] [Google Scholar]
- Sakai JT, Crowley TJ, Stallings MC, McQueen M, Hewitt JK, Hopfer C, Ehringer MA. Test of association between 10 single nucleotide polymorphisms in the oxytocin receptor gene and conduct disorder. Psychiatric Genetics. 2012;22(2):99–102. [Europe PMC free article] [Abstract] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proceedings of the National Academy of Sciences of the USA. 2011;108(37):15118–15122. [Europe PMC free article] [Abstract] [Google Scholar]
- Scott KL, Wolfe DA, Wekerle C. Maltreatment and trauma: tracking the connections in adolescence. Child and Adolescent Psychiatric Clinics of North America. 2003;12(2):211–230. [Abstract] [Google Scholar]
- Sedlak AJ, et al. The Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences. 2011;277:2661–2666. [Europe PMC free article] [Abstract] [Google Scholar]
- Sturge-Apple ML, Cicchetti D, Davies PT, Suor JH. Differential susceptibility in spillover between interparental conflict and maternal parenting practices: Evidence for OXTR and 5-HTT Genes. Journal of Family Psychology. 2012;26(3):431–442. [Europe PMC free article] [Abstract] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the USA. 2005;102(44):16096–16101. [Europe PMC free article] [Abstract] [Google Scholar]
- Tharner A, Luijk MP, Raat H, Ijzendoorn MH, Bakermans-Kranenburg MJ, Moll H, Tiemeier H. Breastfeeding and its relation to maternal sensitivity and infant attachment. Journal of Developmental and Behavioral Pediatrics. 2012;33(5):396–404. [Abstract] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski Ba, Mattay VS, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the USA. 2010;107(31):13936–13941. [Europe PMC free article] [Abstract] [Google Scholar]
- van Ijzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;2(8):e147. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58(1):74–77. [Abstract] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48(5):522–527. [Abstract] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PloS one. 2007;2(11):e1128. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1017/s0954579414000066
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4141414?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1017/s0954579414000066
Article citations
Proposed Physiological Mechanisms Underlying the Association between Adverse Childhood Experiences and Mental Health Conditions: A Narrative Review.
Children (Basel), 11(9):1112, 12 Sep 2024
Cited by: 0 articles | PMID: 39334644 | PMCID: PMC11430311
Review Free full text in Europe PMC
The Multifaceted Role of Oxytocinergic System and OXTR Gene.
Glob Med Genet, 11(1):29-33, 18 Jan 2024
Cited by: 0 articles | PMID: 38239807 | PMCID: PMC10796195
Review Free full text in Europe PMC
Resilience by design: How nature, nurture, environment, and microbiome mitigate stress and allostatic load.
World J Psychiatry, 13(5):144-159, 19 May 2023
Cited by: 2 articles | PMID: 37303926 | PMCID: PMC10251360
Review Free full text in Europe PMC
Hunting for Genes Underlying Emotionality in the Laboratory Rat: Maps, Tools and Traps.
Curr Neuropharmacol, 21(9):1840-1863, 01 Jan 2023
Cited by: 0 articles | PMID: 36056863 | PMCID: PMC10514530
Review Free full text in Europe PMC
Genetic Variants Associated With Resilience in Human and Animal Studies.
Front Psychiatry, 13:840120, 20 May 2022
Cited by: 9 articles | PMID: 35669264 | PMCID: PMC9163442
Review Free full text in Europe PMC
Go to all (39) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs
- (3 citations) dbSNP - rs53576
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Gene × Environment interaction and resilience: effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes.
Dev Psychopathol, 24(2):411-427, 01 May 2012
Cited by: 81 articles | PMID: 22559122 | PMCID: PMC3684053
Disadvantage of Social Sensitivity: Interaction of Oxytocin Receptor Genotype and Child Maltreatment on Brain Structure.
Biol Psychiatry, 80(5):398-405, 18 Dec 2015
Cited by: 29 articles | PMID: 26858213
Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene.
Dev Psychopathol, 23(2):439-452, 01 May 2011
Cited by: 106 articles | PMID: 23786688 | PMCID: PMC4363139
Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes.
Am J Psychiatry, 170(10):1114-1133, 01 Oct 2013
Cited by: 390 articles | PMID: 23982148 | PMCID: PMC3928064
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIDA NIH HHS (3)
Grant ID: R01 DA012903
Grant ID: R01 DA12903
Grant ID: R01-DA12903