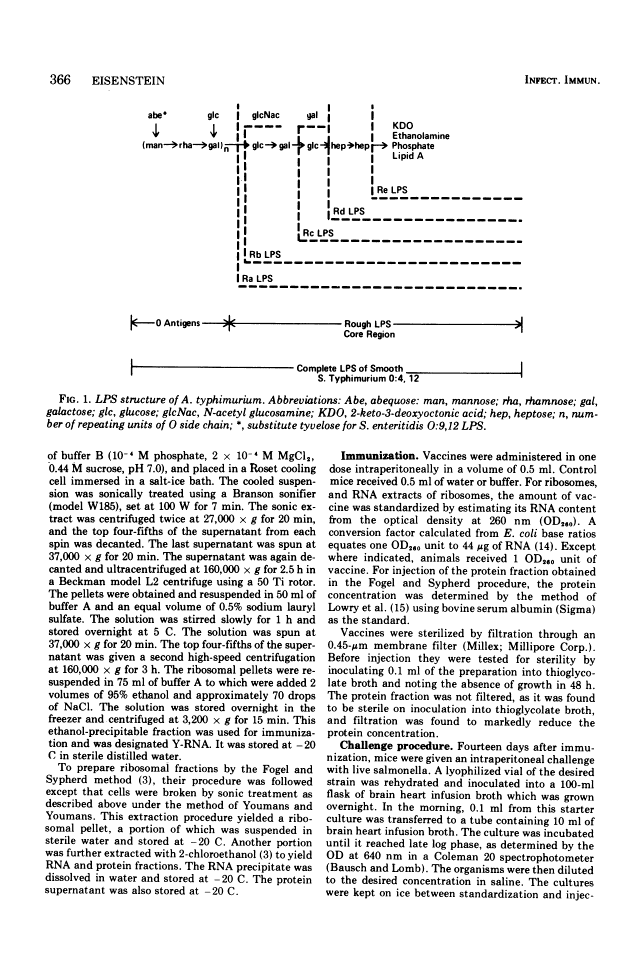
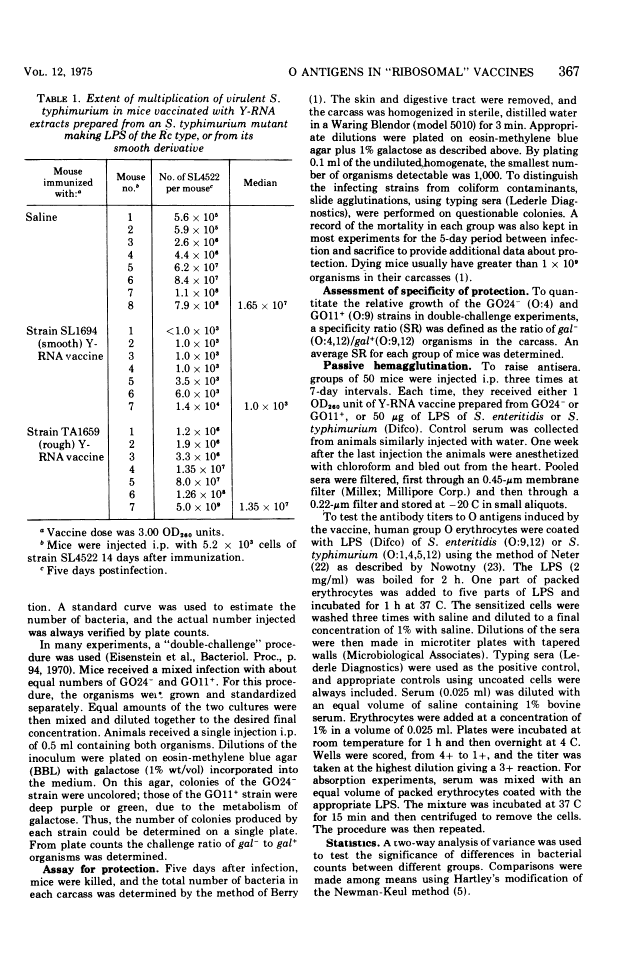
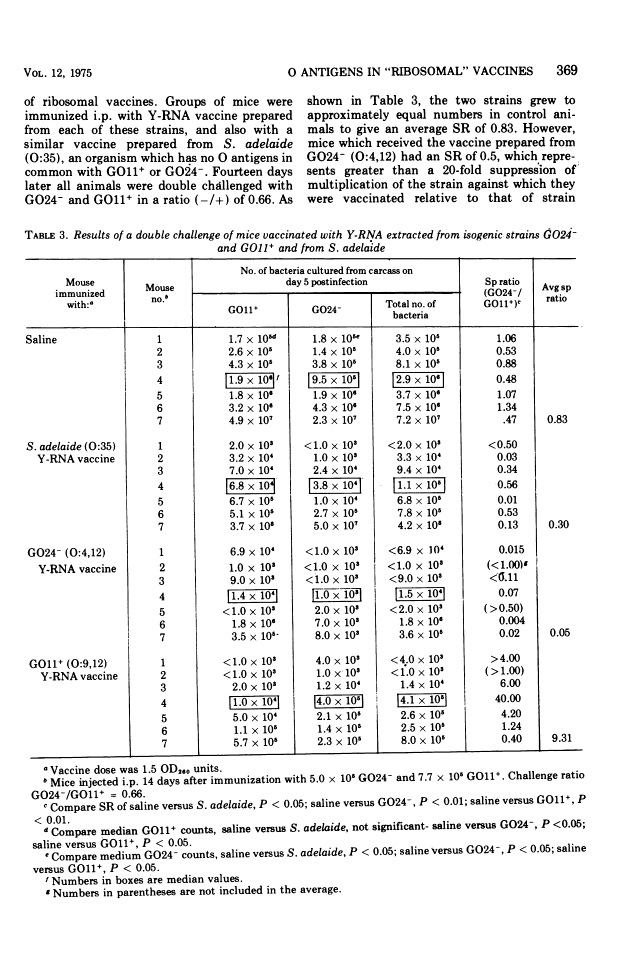
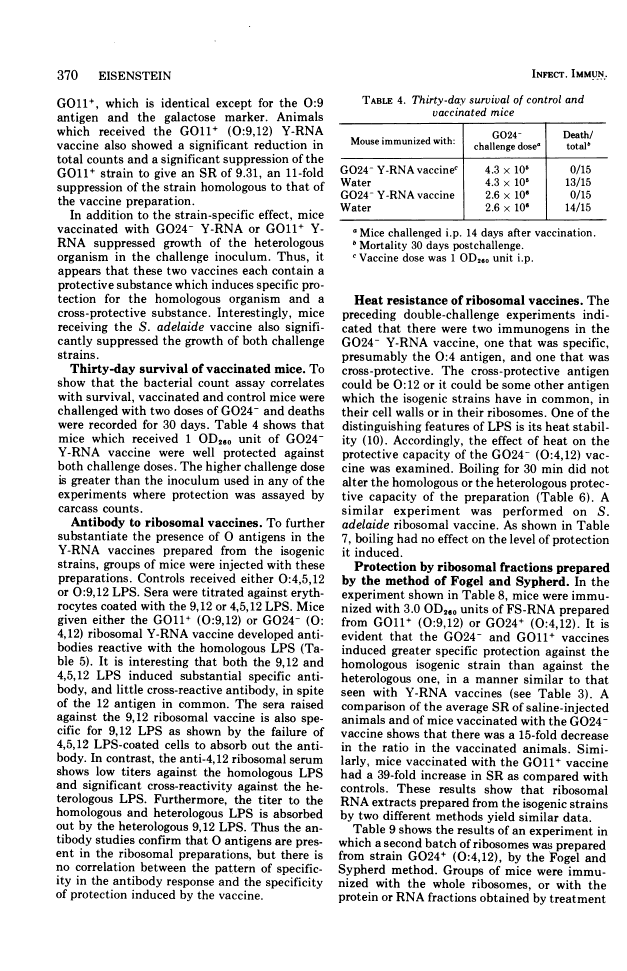
Abstract
Free full text

Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella.
Abstract
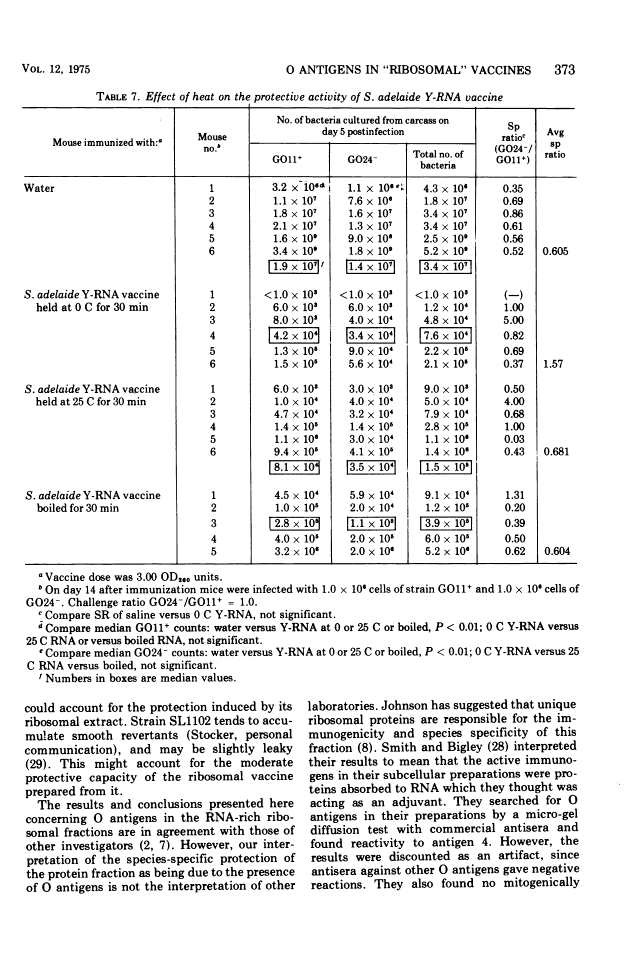
The nature of the protective substance in ribosomal ribonucleic acid and protein extracts of Salmonella has been investigated. The results of experiments in which vaccines were prepared from isogenic strains and strains with defects in lipopolysaccharide synthesis show that O antigens contaminate both ribonucleic acid and protein ribosomal extracts, and are responsible for at least part of their strain-specific protective activity. In addition, it was observed that a ribosomal ribonucleic acid preparation from S. adelaide contains a heat-stable immunogen which is not an O antigen or that gives cross-protection across species lines. The contribution of ribosomes to the immunity induced by "ribosomal vaccines" is discussed.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fogel S, Sypherd PS. Extraction and isolation of individual ribosomal proteins from Escherichia coli. J Bacteriol. 1968 Aug;96(2):358–364. [Europe PMC free article] [Abstract] [Google Scholar]
- Gorzynski EA, Ambrus JL, Neter E. Effect of common enterobacterial antiserum on experimental Salmonella typhimurium infection of mice. Proc Soc Exp Biol Med. 1971 Sep;137(4):1209–1212. [Abstract] [Google Scholar]
- Jensen R, Gregory B, Naylor J, Actor P. Isolation of protective somatic antigen from Vibrio cholerae (Ogawa) ribosomal preparations. Infect Immun. 1972 Aug;6(2):156–161. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. [Europe PMC free article] [Abstract] [Google Scholar]
- Kasai N, Nowotny A. Endotoxic glycolipid from a heptoseless mutant of Salmonella minnesota. J Bacteriol. 1967 Dec;94(6):1824–1836. [Europe PMC free article] [Abstract] [Google Scholar]
- Krishnapillai V. Uridinediphosphogalactose-4-epimerase deficiency in Salmonella typhimurium and its correction by plasmoid-borne galactose genes of Escherichia coli K-12: effects on mouse virulence, phagocytosis, and serum sensitivity. Infect Immun. 1971 Sep;4(3):177–188. [Europe PMC free article] [Abstract] [Google Scholar]
- Krishnapillai V, MacPhee DG, Stocker BA. Properties of a Salmonella typhimurium mutant with an incomplete deficiency of uridinediphosphogalactose-4-epimerase. J Bacteriol. 1971 Jul;107(1):155–161. [Europe PMC free article] [Abstract] [Google Scholar]
- KUNIN CM. SEPARATION, CHARACTERIZATION, AND BIOLOGICAL SIGNIFICANCE OF A COMMON ANTIGEN IN ENTEROBACTERIACEAE. J Exp Med. 1963 Oct 1;118:565–586. [Europe PMC free article] [Abstract] [Google Scholar]
- Kurland CG. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Lüderitz O, Galanos C, Lehmann V, Nurminen M, Rietschel ET, Rosenfelder G, Simon M, Westphal O. Lipid A: chemical structure and biological activity. J Infect Dis. 1973 Jul;128(Suppl):17–29. [Abstract] [Google Scholar]
- McCabe WR. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [Abstract] [Google Scholar]
- McCabe WR, Kreger BE, Johns M. Type-specific and cross-reactive antibodies in gram-negative bacteremia. N Engl J Med. 1972 Aug 10;287(6):261–267. [Abstract] [Google Scholar]
- MacPhee DG, Stocker BA. Suppression of amber and ochre mutants in Salmonella typhimurium by a mutant F'-1-gal factor carrying an ochre suppressor gene. J Bacteriol. 1969 Oct;100(1):240–246. [Europe PMC free article] [Abstract] [Google Scholar]
- Morgenroth A, Duguid JP. Demonstration of different mutational sites controlling rhamnose fermentation in FIRN and non-FIRN rha-strains of Salmonella typhimurium: an essay in bacterial archaeology. Genet Res. 1968 Apr;11(2):151–169. [Abstract] [Google Scholar]
- Mullan NA, Newsome PM, Cunnington PG, Palmer GH, Wilson ME. Protection against gram-negative infections with antiserum to lipid A from Salmonella minnesota R595. Infect Immun. 1974 Dec;10(6):1195–1201. [Europe PMC free article] [Abstract] [Google Scholar]
- NETER E, BERTRAM LF, ZAK DA, MURDOCK MR, ARBESMAN CE. Studies on hemagglutination and hemolysis by escherichia coli antisera. J Exp Med. 1952 Jul;96(1):1–15. [Europe PMC free article] [Abstract] [Google Scholar]
- O'NEILL GJ, TODD JP. Extraction of nucleic acid-free lipopolysaccharides from gram-negative bacteria. Nature. 1961 Apr 22;190:344–345. [Abstract] [Google Scholar]
- Pace NR. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. [Europe PMC free article] [Abstract] [Google Scholar]
- Rudbach JA, Anacker RL, Haskins WT, Milner KC, Ribi E. Physical structure of a native protoplasmic polysaccharide from Escherichia coli. J Immunol. 1967 Jan;98(1):1–7. [Abstract] [Google Scholar]
- Sanderson KE. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith RA, Bigley NJ. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. [Europe PMC free article] [Abstract] [Google Scholar]
- Tripodi D, Nowotny A. Relation of structure to function in bacterial O-antigens. V. Nature of active sites in endotoxic lipopolysaccharides of Serratia marcescens. Ann N Y Acad Sci. 1966 Jun 30;133(2):604–621. [Abstract] [Google Scholar]
- Venneman MR. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. [Europe PMC free article] [Abstract] [Google Scholar]
- Venneman MR, Bigley NJ. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilkinson RG, Gemski P, Jr, Stocker BA. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. [Abstract] [Google Scholar]
- YOUMANS AS, YOUMANS GP. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. [Europe PMC free article] [Abstract] [Google Scholar]
- Youmans AS, Youmans GP. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J Bacteriol. 1966 Jun;91(6):2139–2145. [Europe PMC free article] [Abstract] [Google Scholar]
- Ziegler EJ, Douglas H, Sherman JE, Davis CE, Braude AI. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.12.2.364-377.1975
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/12/2/364.full.pdf
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/12/2/364
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/12/2/364
Citations & impact
Impact metrics
Citations of article over time
Article citations
Salmonella serotype determination utilizing high-throughput genome sequencing data.
J Clin Microbiol, 53(5):1685-1692, 11 Mar 2015
Cited by: 227 articles | PMID: 25762776 | PMCID: PMC4400759
A broadly applicable approach to T cell epitope identification: application to improving tumor associated epitopes and identifying epitopes in complex pathogens.
J Immunol Methods, 373(1-2):111-126, 18 Aug 2011
Cited by: 5 articles | PMID: 21872603 | PMCID: PMC3195951
Immunization of mice against Salmonella typhimurium using different DNA preparations.
Immunopharmacol Immunotoxicol, 23(4):519-530, 01 Nov 2001
Cited by: 0 articles | PMID: 11792011
Protection against local Shigella sonnei infection in mice by parenteral immunization with a nucleoprotein subcellular vaccine.
Infect Immun, 63(7):2762-2765, 01 Jul 1995
Cited by: 8 articles | PMID: 7790095 | PMCID: PMC173369
Protective ribosomal preparation from Shigella sonnei as a parenteral candidate vaccine.
Infect Immun, 59(10):3610-3618, 01 Oct 1991
Cited by: 14 articles | PMID: 1716612 | PMCID: PMC258928
Go to all (51) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium.
Infect Immun, 24(3):808-816, 01 Jun 1979
Cited by: 16 articles | PMID: 381202 | PMCID: PMC414379
Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium.
Infect Immun, 8(3):395-400, 01 Sep 1973
Cited by: 34 articles | PMID: 4199718 | PMCID: PMC422861
Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis.
Infect Immun, 5(6):947-952, 01 Jun 1972
Cited by: 38 articles | PMID: 4564407 | PMCID: PMC422469
Microbial ribosomal vaccines.
Rev Infect Dis, 8(2):208-217, 01 Mar 1986
Cited by: 35 articles | PMID: 3518022
Review