Abstract
Free full text

Development, differentiation and diversity of innate lymphoid cells
Abstract
Recent years have witnessed the discovery of an unprecedented complexity in innate lymphocyte lineages, now collectively referred to as innate lymphoid cells (ILC). ILC are preferentially located at barrier surfaces and are important for protection against pathogens and for the maintenance of organ homeostasis. Inappropriate activation of ILC has been linked to the pathogenesis of inflammatory and autoimmune disorders. Recent evidence suggests that ILC can be grouped into two separate lineages, cytotoxic ILC represented by conventional natural killer (cNK) cells and cytokine-producing helper-like ILC (i.e., ILC1, ILC2, ILC3). We will focus here on current work in humans and mice that has identified core transcriptional circuitry required for the commitment of lymphoid progenitors to the ILC lineage. The striking similarities in transcriptional control of ILC and T cell lineages reveal important insights into the evolution of transcriptional programs required to protect multicellular organisms against infections and to fortify barrier surfaces.
Introduction
The last years have witnessed an unprecedented change in our understanding of innate lymphocyte lineages. It was previously believed, that innate lymphocytes were represented by a single lymphoid lineage, namely natural killer (NK) cells, that, in many aspects, resemble cytotoxic T cells. However, it has become apparent that additional innate lymphocyte subsets exist that use transcriptional programs and display functions distinct from conventional NK (cNK) cells. All innate lymphocytes including cNK cells are now referred to as ILC. In addition to cNK cells, three additional groups of ILC are now being discriminated, ILC1, ILC2, and ILC3. Strikingly, the transcriptional and effector programs of the various ILC populations resemble those of T helper subsets, suggesting that the underlying transcriptional circuitry is evolutionarily more ancient than previously appreciated (Tanriver and Diefenbach, 2014). Here, we will discuss our current view of developmental and transcriptional programs common to all ILC lineages and those required for specification of distinct ILC populations. These recent data provide a framework for our current view of two principal ILC lineages, cytotoxic or killer ILC (i.e., cNK cells) and helper-like ILC (i.e., ILC1, ILC2, ILC3) (Figure 1). We will put a focus on recent progress in dissecting the ILC1 lineage and on common transcriptional programs controlling ILC specification.
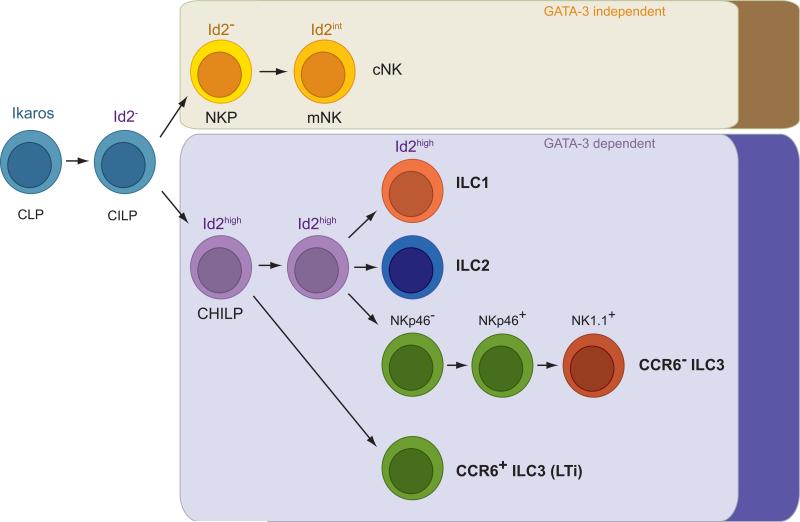
All lymphoid lineages are the progeny of the common lymphoid progenitor (CLP). After the branchpoint with the B and T lineages an ILC-restricted progenitor may exist (CILP). Downstream of the CILP, two main ILC lineages can be discriminated, killer ILC and helper-like ILC. Killer ILC are represented by cNK cells and helper-like ILC are composed of the various cytokine-producing ILC subsets (i.e., ILC1, ILC2, ILC3). While helper-like ILC express IL-7Rα and require GATA-3 for differentiation, killer ILC do not express IL-7Rα and are normally represented in GATA-3-deficient mice. All helper-like ILC (but not killer ILC) differentiate from the Id2+ CHILP. A PLZF+ CHILP population has been identified that has more restricted differentiation potential. A precursor/progeny relation between PLZF− and PLZF+ CHILP needs to be determined.
CLP: common lymphoid progenitor; CILP: common ILC progenitor; CHILP: comon helper-like ILC progenitor; NKP: cNK-restricted progenitor
Identification of ILC1: More than just NK cells?
ILC1 have only recently been better characterized and are now classified as an ILC group distinct of cNK cells that expresses and requires the transcription factor T-bet for lineage specification (Bernink et al., 2013; Daussy et al., 2014; Fuchs et al., 2013; Klose et al., 2014) (Figure 1, Tables 1--3).3). The identification of bona fide ILC1 in mice was obscured by the fact that ILC1 were found to express NK cell receptors such as natural killer cell p46-related protein (NKp46) and NK1.1 which have served as an operative definition of NK cells. Early on, Di Santo and colleagues noticed that thymic NK cells in mice have a distinct phenotype; they are less cytotoxic but secrete more interferon-γ (IFN-γ) than splenic NK cells do (Table 2) (Vosshenrich et al., 2006). They proposed that the dichotomy between splenic NK cells and thymic NK cells in mice may parallel the division of CD56low and CD56high NK cell subsets in human blood (Caligiuri, 2008) (Table 1). Recent data from organ-resident “NK cells” indicated that the population of NKp46+NK1.1+ cells may in fact be heterogeneous and composed of various ILC lineages (Daussy et al., 2014; Fuchs et al., 2013; Gordon et al., 2012; Klose et al., 2014; Vosshenrich et al., 2006). Indeed, liver-resident NKp46+NK1.1+ cells can be seperated into a VLA2 (CD49b)+ population expressing the T-box transcription factors Eomes and T-bet and into a VLA2−TRAIL+IL-7Rα+ population that expressed T-bet but not Eomes (Daussy et al., 2014; Gordon et al., 2012; Peng et al., 2013; Takeda et al., 2001). VLA2+TRAIL− cells likely represent cNK cells in that they are cytotoxic, require Eomes for development and express class I major histocompatibility complex (MHC)-specific inhibitory receptors (i.e., Ly49 receptors, NKG2A). VLA2−TRAIL+IL-7Rα+NKp46+NK1.1+ cells did not express Eomes but strictly required T-bet for their development (Gordon et al., 2012). It has been controversial if VLA2−TRAIL+IL-7Rα+ cells constitute immature cNK cells (Gordon et al., 2012; Takeda et al., 2005) or a distinct ILC lineage (Daussy et al., 2014). In the intestine, distinction between the various subsets of NKp46+NK1.1+ cells was even more complex because NKp46+ ILC3 were recognized as well (Cella et al., 2009; Cupedo et al., 2009; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008). Within the intraepithelial space of the intestine, an ILC1 subset was identified that was phenotypically distinct from cNK cells and required T-bet and nuclear factor, interleukin 3 regulated (NFIL3, also known as E4BP4) for differentiation (Table 2 and and3)3) (Fuchs et al., 2013). Genetic reporter systems for lineage-defining transcription factors allowed to identify intestinal ILC1 as an ILC lineage separate from cNK cells (expressing an Eomes reporter) and NKp46-expressing ILC3 (expressing a Rorc reporter). Intestinal ILC1 produced copious amounts of IFN-γ in response to interleukin-12 (IL-12) and provided innate protection against the intracellular parasite Toxoplasma gondii (Klose et al., 2014).
Table 1
Phenotype of human IFN-γ-producing ILC populations
| CD56 | NKp44 | CD94 | KIR | CD103 | CD160 | VLA1 CD49a | VLA2 CD49b | CD127 | |
|---|---|---|---|---|---|---|---|---|---|
| Blood CD56high NK cells | ++ | – | + | low/− | – | – | – | + | + |
| Blood CD56low NK cells | + | – | low/+ | + | – | – | – | + | – |
| Tonsil intraepithelial ILC1 | + | + | + | low/− | + | + | + | n.d. | – |
| Intestinal intraepithelial ILC1 | +/– | + | – | n.d. | + | + | + | n.d. | – |
| Tonsil and intestinal converted ILC3 | – | – | – | – | – | – | – | – | + |
n.d., not determined
Table 2
Phenotype of mouse cNK cells, ILC1 and other IFN-γ-producing NKp46+ ILC
| NKp46 | NK1.1 | VLA2 CD49b | VLA1 CD49a | TRAIL | CD160 | CD103 | CD127 | CD69 | Ly49 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Splenic cNK | + | + | + | – | – | – | – | subset | – | + |
| Liver cNK | + | + | + | – | – | – | – | – | – | + |
| Intestinal LP cNK | + | + | low | subset | low | + | n.d. | – | + | + |
| Salivary Gland NK | + | + | + | + | + | – | + | – | + | + |
| Uterine NK | + | + | – | + | + | – | ? | lo | + | + |
| Intraepithelial ILC1 | + | + | + | + | + | + | – | lo | + | ? |
| Intestinal LP ILC1 | + | + | – | + | (+) | + | ? | + | + | – |
| Liver ILC1 | + | + | – | + | + | + | – | subset | + | + |
| Intestinal LP ex-RORγt+ ILC3 | + | + | – | + | n.d. | + | n.d. | low/– | + | – |
| Thymic NK cells | + | + | low | n.d. | n.d. | n.d. | n.d. | hi | + | lo |
n.d., not determined
Table 3
Transcription factors and cytokines required for mouse NKp46+ ILC development and survival.
| Id2 | NFIL3 | GATA-3 (Vav-Cre or chimeras) | GATA-3 (Id2-CreERt2) | GATA-3 (Ncr1-Cre) | PLZF | Eomes | T-bet | RORγt | IL-7R | IL-15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Splenic cNK | ↓ ↓ ↓ | ↓ ↓ ↓ | → | n.d. | n.d. | n.d. | ↓ ↓ ↓ | → or↓↓ | → | ↑ ↑ | ↓ ↓ ↓ |
| Liver VLA2+ cNK | n.d. | ↓ ↓ ↓ | n.d. | n.d. | n.d. | → | ↓ ↓ ↓ | → | n.d. | → | ↓ ↓ ↓ |
| Liver VLA2− ILC1 | n.d. | →1 or ↓↓2 | n.d. | n.d. | n.d. | ↓ ↓ | → | ↓ ↓ ↓ | n.d. | n.d. | ↓ ↓ ↓ |
| Intestinal LP cNK | n.d. | ↓ ↓ ↓ | n.d. | n.d. | → | n.d. | ↓ ↓ ↓ | → | → | ↑ ↑ | ↓ ↓ ↓ |
| Intestinal LP ILC1 | n.d. | ↓ ↓ ↓ | n.d. | n.d. | ↓ ↓ ↓ | n.d. | → | ↓ ↓ ↓ | → | → | ↓ ↓ |
| Intestinal LP ex-RORD[unk]t+ ILC3 | n.d. | ↓ ↓ ↓ | n.d. | n.d. | → | n.d. | → | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ |
| Intraepithelial ILC1 | ↓ ↓ ↓ | ↓ ↓ ↓ | ↑ ↑ | n.d. | n.d. | → | n.d. | ↓ ↓ ↓ | → | n.d. | ↓ ↓ |
| Uterine VLA2+ cNK | n.d. | ↓ ↓ ↓ | n.d. | n.d. | n.d. | n.d. | ↓ ↓ ↓ | → | n.d. | n.d. | ↓ ↓ ↓ |
| Uterine VLA2− ILC1 | n.d. | → | n.d. | n.d. | n.d. | n.d. | → | → | n.d. | n.d. | ↓ ↓ ↓ |
| Salivary gland NKp46+ | n.d. | → | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ↓ ↓ ↓ |
| Thymic NKp46+ | → (?) | ↓ ↓ | ↓ ↓ ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ↓ ↓ ↓ | → |
| ILC2 (LP, visceral fat, lung) | ↓ ↓ ↓ | ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | n.d. | ↓ ↓ | n.d. | n.d. | → | ↓ ↓ ↓ | → |
| Intestinal LP CCR6+ ILC3 | ↓ ↓ ↓ | ↓ ↓ | ↓ ↓ ↓ | → | n.d. | → | → | → | ↓ ↓ ↓ | ↓ ↓ ↓ | → |
| Intestinal LP CCR6−/low ILC3 | ↓ ↓ ↓ | ↓ ↓ | ↓ ↓ ↓ | → | n.d. | → | → (NKp46+ subset ↓↓↓) | ↓ ↓ ↓ |
n.d., not determined; ↓↓↓, population strictly requires factor for development; ↓↓, population is reduced; →, no change in population size; ↑↑, population size is increased
Identification of ILC2
IL-25, IL-33 and thymic stromal lymphopoietin (TSLP), all of which are epithelial cell-derived cytokines, regulate type 2 innate immune responses against helminths and pathophysiology of airway allergens (Koyasu and Moro, 2012). The existence of lineage-negative innate immune cells producing type 2 cytokines was first reported by Fort et al. demonstrating that IL-25 administration induced production of IL-5 and IL-13 in Rag2−/− mice that lack all B and T cells (Fort et al., 2001). It was later shown that a non-B non-T c-Kit+ FcεR1− (non-mast cell) population, which appears during the initial stages of helminth infection, is capable of producing IL-4, IL-5 and IL-13 in response to IL-25 (Fallon et al., 2006; Humphreys et al., 2008; Hurst et al., 2002; Voehringer et al., 2006). The identity of such innate effector cells had been obscure until 2010 when natural helper cells (Moro et al., 2010) and nuocytes (Neill et al., 2010), which are now known as ILC2 were identified. ILC2, which produce large amounts of IL-5 and IL-13 in response to IL-25 or IL-33, were identified in mensenteric fat-associated lymphoid clusters (FALC) in naïve mice as natural helper cells (Moro et al., 2010) and in mesenteric lymph nodes of mice administered with IL-25 or IL-33 as nuocytes (Neill et al., 2010). ILC2 were later shown to be present in other tissues such as lung, intestinal lamina propria, bone marrow, liver and skin (Furusawa et al., 2013; Halim et al., 2012a; Hoyler et al., 2012; Kabata et al., 2013; McHedlidze et al., 2013; Roediger et al., 2013; Salimi et al., 2013). In addition to type 2 cytokines, ILC2 produce amphiregulin and support the recovery of epithelial barrier integrity after tissue damage (Monticelli et al., 2011). Recent studies have identified a role of ILC2 in the initiation of type 2 adaptive immune responses through class II MHC and cytokine-mediated activation of Th2 cells (Halim et al., 2014; Oliphant et al., 2014). Another type 2 innate cell population, MPPtype2 induced by IL-25 administration was also reported in 2010 (Saenz et al., 2010). MPPtype2 cells, however, differ from ILC2 in that they express neither IL-7 receptor (IL-7R) nor IL-33R and possess the potential to differentiate into myeloid cells (Saenz et al., 2013; Saenz et al., 2010). IL-5 and IL-13 produced by ILC2 are critical for innate protection against helminth and nematode infections (Koyasu et al., 2010).
ILC3: lymphoid tissue-inducer (LTi) cells and more
The first ILC subset to be characterized were retinoic acid-related orphan receptor γ t (RORγt)-expressing ILC3 in human tonsils (Cella et al., 2009; Cupedo et al., 2009) and the lamina propria of the intestine (in both human and mice) (Cella et al., 2009; Cupedo et al., 2009; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008), a subpopulation of which expressed cell surface markers also found on NK cells (e.g., NKp46, NKG2D, NKp44 or CD56). Intestinal ILC3 require RORγt for lineage specification and, consequently, mice genetically lacking RORγt have no ILC3 (Table 3). Previously, a fetal liver-derived innate lymphocyte subset has been identified in humans and mice that also depends on RORγt for development, termed lymphoid tissue (LTi) inducer cells (Adachi et al., 1997; Cupedo et al., 2009; Kurebayashi et al., 2000; Mebius et al., 1997; Sun et al., 2000). It is now believed that LTi cells constitute a subpopulation of ILC3 (Cupedo et al., 2009). While LTi cells are CCR6high ILC3, another ILC3 subset expresses only low amounts of CCR6 (Klose et al., 2013). Such CCR6−/low ILC3 rapidly proliferate after birth, express the transcription factor T-bet, and could upregulate NKp46 (Figure 1). One significant finding was that ILC3 express type 17 cytokines such as IL-22 and IL-17A (Cella et al., 2009; Cupedo et al., 2009; Sanos et al., 2009; Takatori et al., 2009). IL-22 produced by ILC3 is absolutely required for immunity to attaching-andeffacing bacterial infections such as those with Citrobacter rodentium (Sonnenberg et al., 2011; Zheng et al., 2008). These lines of findings have revealed unprecedented complexity in innate lymphocyte lineages. In the following, we will first review recent data that have revealed core transcriptional hubs required for the development of various groups of ILC. Then, we will highlight our current understanding of the molecular programs controlling the specification of distinct ILC lineages. Finally, we will discuss some of the future outstanding questions that are likely to move the field forward.
Common developmental programs for ILC fate
Given that ILC and adaptive immune system lymphocytes all derive from the common lymphoid progenitor (CLP) (Cherrier et al., 2012; Klose et al., 2014; Moro et al., 2010; Possot et al., 2011; Yang et al., 2011), the question arose if an ILC lineage-restricted progenitor for some or all ILC populations exists that is downstream of the CLP. The existence of such common innate lymphoid progenitor (CILP) or ILC progenitor (ILCP) was predicted because mice lacking expression of the transcriptional regulator inhibitor of DNA binding 2 (Id2) lack all ILC lineages whereas B and T cell development was largely unperturbed (Cherrier et al., 2012; Moro et al., 2010; Satoh-Takayama et al., 2010; Yokota et al., 1999). Id2 is a member of the helix-loop-helix (HLH) family of proteins that have important functions in immune cell fate decisions (Kee, 2009; Murre, 2005). Id2 heterodimerizes with E proteins that function as transcriptional activators or repressors. Four mammalian E proteins have been characterized (E2A, HEB, E12 and E47) that bind to specific DNA sequences called E-box sites. While Id2 cannot bind to chromatin itself (due to the lack of a basic HLH domain), it heterodimerizes with E proteins and sequesters them away from chromatin, thereby controlling the pool of E proteins available for DNA binding (Benezra et al., 1990). Id2 is expressed in high amounts in all ILC lineages (Carotta et al., 2011; Hoyler et al., 2012) whereas naïve T cells and B cells either express only very low amounts of Id2 or are Id2-negative. Interestingly, B cell lineage specification requires the transcription factor early B cell factor 1 (EBF1) (Lin and Grosschedl, 1995) which is a potent repressor of Id2 (Treiber et al., 2010). Current concepts indicate that repression of Id2 is required to establish appropriate concentrations of E2A, which are permissive for the development of B cells (O'Riordan and Grosschedl, 1999). Deletion of EBF1 in B cell progenitors (late pro-B cells) led to de-repression of Id2 and Notch and reprogrammed committed B cell progenitors into ILC and T cells (Nechanitzky et al., 2013). These data suggest that high level Id2 expression is required to establish ILC fate. While Id2 is now recognized as an important knot within the transcriptional network establishing ILC fate, the stage-specific Id2 controlled transcriptional programs on ILC specification and/or maintenance are an important goal for future research.
Id2 and ILC fate
Recently, Id2 reporter mice (Rawlins et al., 2009) were employed to identify Id2+ lymphoid progenitors in fetal liver and in the bone marrow of adult mice that were characterized by the expression of IL-7Rα and intermediate levels of c-Kit and Sca-1. While these cells expressed markers also found on the CLP such as 2B4 and CD27, they were distinct from the CLP as they expressed Id2 and integrin α4β7 (not expressed by CLP) but did not express Flt3 and CD93 (expressed by CLP). In addition, Lin−Id2+IL-7Rα+α4β7+Flt3−CD25− cells did not express any of the lineage-defining transcription factors of ILC populations (i.e., RORγt, Eomes, T-bet) suggesting that this population is not specified to any of the ILC lineages (Klose et al., 2014). After transfer into alymphoid mice or on the clonal level in vitro, a single Lin−Id2+IL-7Rα+α4β7+Flt3−CD25− cell had the potential to differentiate into ILC2, ILC3 (including CCR6+ LTi-like cells), and a peculiar non-NK (i.e., Eomes−T-bet+) ILC1 population. Thus, on the clonal level, Lin-Id2+IL-7Rα+α4β7+Flt3−CD25− cells gave rise to all ILC lineages but not to cNK cells, suggesting that all cytokine-producing, helper-like ILC share a common progenitor and that cNK cells branch off earlier from a putative CILP (Figure 1). Given its restricted helper-like ILC potential, the Id2+ common progenitor has been dubbed CHILP, common helper-like ILC progenitor (CHILP).
Promyelocytic leukaemia zinc finger (PLZF) is transiently expressed in helper-like ILC progenitors
Bendelac and colleagues found that ILC do not express the transcription factor PLZF, which controls the formation of an innate-like program in CD1d-restricted T cells (also referred to as iNKT cells) (Kovalovsky et al., 2008; Savage et al., 2008). However, lineage tracing for expression of Zbtb16, the gene encoding PLZF, revealed that all helper-like ILC were prominently labelled whereas cNK cells and LTi cells did not express PLZF during lineage specification (Constantinides et al., 2014). Accordingly, Zbtb16-deficient mice showed normal development of cNK cells and LTi cells whereas ILC2 and liver ILC1 were mildly reduced (Table 3). While NKp46+ ILC3 were positive in a Zbtb16 fate map, they did not require PLZF for differentiation and/or maintenance. These data suggest that PLZF is not strictly required for ILC development. Interestingly, within Lin−IL-7Rα+c-Kit+α4β7+ cells in fetal liver and bone marrow a population of PLZF+ progenitors could be discerned. Adoptive transfer experiments and clonal differentiation assays revealed, that PLZF+ Lin−IL-7Rα+c-Kit+α4β7+ cells gave rise to ILC1, ILC2 and CCR6−/low ILC3 but not to cNK cells or CD4+ ILC3 (i.e., LTi cells). The more restricted differentiation potential of PLZF+ Lin−IL-7Rα+c-Kit+α4β7+ cells as compared to the CHILP may be explained by the fact that only a subpopulation of CHILP expressed PLZF (Klose et al., 2014), suggesting that PLZF+ CHILP are the progeny of PLZF− CHILP and have a more restricted differentiation potential. Furthermore, clonal analyses showed that only less than 10% of the PLZF+ progenitor population had potential to generate three ILC subsets and more than 50% of clones generated only a single ILC type, raising the possibility that these progenitor populations are a mixture of bona fide CHILP and already committed cells (Constantinides et al., 2014). Future work will need to reveal how PLZF and Id2 restrict the lymphoid differentiation program to the ILC lineage.
Helper-like ILC require the transcription factor GATA-3 for development
Both of the above reports (Constantinides et al., 2014; Klose et al., 2014) may indicate that cNK cells develop independently of CHILP populations, suggesting that NK cell progenitors branch off earlier during ILC development (Figure 1). Indeed, cNK cells seem to up-regulate Id2 only after lineage specification because the recently identified refined NK cell progenitor (Fathman et al., 2011) is largely Id2− (Klose et al., 2014), which is consistent with previous reports (Boos et al., 2007). The view that ILC may be subdivided into two major lineages (i.e., killer ILC and helper-like ILC) is supported by another set of data revealing that GATA binding protein-3 (GATA-3) plays important roles for the development of helper-like ILC but not for that of cNK cells. Genetic deletion of Gata3 in all hematopoietic cells resulted in a lack of all IL-7Rα-expressing helper-like ILC whereas the differentiation of cNK cells was largely normal (Samson et al., 2003; Vosshenrich et al., 2006; Yagi et al., 2014). Very similar data were obtained using fetal liver chimeras generated with Gata3-deficient hematopoietic progenitors (Serafini et al., 2014). It is interesting that helper-like ILC but not cytotoxic cNK cells are Gata3-dependent, revealing another interesting analogy to T cell lineages. Previous data demonstrated that deletion of Gata3 in developing T cells diminished helper T cell development but not that of cytotoxic CD8 T cells (Zhu et al., 2004). It should be noted that the roles of GATA-3 for ILC development and function are complex and highly stage-specific. Early deletion of Gata3 in all hematopoietic cells perturbs development of all helper-like ILC lineages, whereas deletion of Gata3 downstream of the CHILP such as in all Id2-expressing cells or in Ncr1-expressing cells leads to a selective block in ILC2 (Hoyler et al., 2012; Yagi et al., 2014) or ILC1 development (Klose et al., 2014), respectively, whereas the differentiation of other ILC subsets was rather normal (Table 3).
NFIL3, a central transcription factor for all ILC?
Very recently the transcription factor NFIL3 (also known as E4BP4) has been implicated in the differentiation of various ILC lineages. Previous work had identified NFIL3 to be required for the differentiation of cNK cells (Gascoyne et al., 2009; Kamizono et al., 2009). Now a broader role of NFIL3 for the differentiation of various ILC lineages is becoming apparent (Geiger et al., 2014; Seillet et al., 2014b). NFIL3-deficient mice have reduced numbers of ILC2 and ILC3 (including LTi cells) leading to profound defects in immunity to C. rodentium infection (requiring IL-22-producing ILC3) or in papain-induced allergies (requiring ILC2) (Seillet et al., 2014b). NFIL3-deficient mice lacked the CHILP population indicating that Nfil3 may be already needed for the generation of early ILC progenitors most likely at the stage of CILP given that both cytotoxic and helper-like ILC are affected by Nfil3 deficiency (Figure 1, Table 3). However, this model needs to be revisited in the light of previous data showing that the NK precursor is unaffected in Nfil3−/− mice and that mice lacking NFIL3 still have lymph nodes indicating that ILC3 development cannot be entirely blocked (Gascoyne et al., 2009; Kamizono et al., 2009). Future studies will need to define on a molecular level how NFIL3 controls or contributes to ILC fate.
Notch and ILC development
Notch signals play a complex role in ILC development, and it has recently become apparent that they are a major common denominator for ILC lineage differentiation. The CHILP gives rise to ILC1, ILC2, and ILC3 in in vitro cultures with OP9 feeder cells expressing the Notch ligand delta-like 1 (DL1) (OP9-DL1) (Klose et al., 2014). In line with these findings, ILC1 as well as NKp46+ ILC3 were severely reduced in mice lacking the Notch signaling adaptor RBP/J (Lee et al., 2012). Moreover, ILC2 development in vitro and in vivo required Notch signals (Wong et al., 2012; Yang et al., 2013). ILC3 also require Notch for development, although its necessity seems to differ depending on the origin of the precursor used (fetal vs. adult) (Cherrier et al., 2012; Possot et al., 2011). It was suggested that ILC3 differentiation is supported by intermittent provision of Notch ligands, while it is hindered in constant presence of Notch signaling, adding an additional layer of complexity to Notch signaling in developing ILC (Cherrier et al., 2012). Beyond the initial development, ILC3 differentiation and acquisition of T-bet and NKp46 requires Notch signals, corroborating the previous findings in RBP/J-deficient animals (Rankin et al., 2013). Notch signaling drives the development of both ILC and T cells, however in humans, the signal strength through Notch regulates the fate of progenitor cells and directs lineage commitment (Gentek et al., 2013). Moreover, the Notch signaling pathway probably differs in ILC and T cells since Hes-1, a target of the Notch signaling pathway, is dispensable for ILC2 but not for T cell differentiation (Yang et al., 2013). Downstream of Notch, TCF-1 encoded by Tcf7 is induced and directly trigger upregulation of Il7ra gene expression in ILC2 (Mielke et al., 2013; Yang et al., 2013). The role of Notch also extends to ILC function since the Notch-TCF-1 axis positively regulates the expression of receptors for IL-2, IL-25 and IL-33 through induction of GATA-3 and is required for IL-22 production by ILC3 (Mielke et al., 2013; Yang et al., 2013). However, spatio-temporal control of Notch signals for ILC fate is not well understood at the moment. The work summarized has provided a great deal of information on transcriptional programs affecting common ILC fate in the mouse model. Analogous precursors in humans still remain to be identified.
Differentiation of ILC1
The general challenge when dealing with ILC1 subsets was their phenotypic resemblance (e.g., NKp46+, NK1.1 +, T-bet+) making it difficult to discriminate subsets and to assign them to separate lineages. Only recently, through the use of genetic reporter systems faithfully reporting the expression of lineage-specifying transcription factors, some progress has been made in separating ILC1 populations from cNK cells. Differentiation of cNK cells depends on sequential steps controlled by the transcription factors Id2, NFIL3 and Eomes. The development and maintenance of cNK cells require IL-15 signaling through IL-2Rβ (CD122) and the cytokine receptor common γ chain (γc). However, thymic NK cells develop through a distinct developmental pathway that involves the transcription factor GATA-3 and signaling through the IL-7 receptor (CD127) and produce IFN-γ and granulocyte macrophage colony-stimulating factor (GM-CSF) in much higher amounts than cNK cells (Ribeiro et al., 2010; Vosshenrich et al., 2006) (Table 3). IFN-γ-producing ILC in the oral and intestinal epithelium of both humans and mice (intraepithelial ILC1) are equipped with a unique set of integrins and adhesion molecules such as VLA-1, CD160 and CD103 (in humans), manifest strong transforming growth factor beta (TGF-β) imprinting and do not entirely rely on IL-15 signaling (Fuchs et al., 2013) (Tables 1 and and22).
The human oral and intestinal mucosa were found to harbor yet another subset of IFN-γ-producing ILC that express the receptor for IL-7 and lack NK cell markers and lytic enzymes (Bernink et al., 2013). These cells were also called ILC1 and were proposed to arise from the conversion of RORγt+ ILC3 under the influence of IL-12 during inflammation. Supporting this, human ILC3 can differentiate into RORγt− IFN-γ-producing cells in vitro when cultured in IL-2 (Cella et al., 2010; Hughes et al., 2010; Hughes et al., 2009). Moreover, fate-mapping experiments using Rorc-Cre mice and ILC3 transfer experiments showed that some IFN-γ-producing ILC do, indeed, originate from RORγt+ ILC3 (Klose et al., 2014; Vonarbourg et al., 2010). Together, these studies have suggested that a certain percentage of IFN-γ-producing ILC may stem from ILC3.
Because the development of cNK cells required Eomes and T-bet was required for functional maturation and bone marrow egress (Gordon et al., 2012; Jenne et al., 2009; Townsend et al., 2004), attention has focused on these two transcription factors. Interestingly, TRAIL+VLA-1(CD49a)+VLA-2− liver NK cells are T-bet and IL-15-dependent, but Eomes-independent (Daussy et al., 2014; Sojka et al., 2014), consistent with a distinct developmental pathway. Indeed, TRAIL+VLA-2− liver NK cells did not express Eomes but strictly required T-bet for their development (Daussy et al., 2014; Gordon et al., 2012). Uterine NK cells are both T-bet and Eomes independent (Sojka et al., 2014), perhaps reflecting redundancy of these transcription factors. This may also be the case for salivary gland NK cells, as they express high amounts of both T-bet and Eomes (Cortez et al., 2014).
NFIL3 promotes the development of bone marrow-derived NK cells from CLP in the steady state (Gascoyne et al., 2009; Kamizono et al., 2009; Male et al., 2012). Recent studies have shown that Nfil3 is required for the formation of splenic cNK cells as well as thymic NK cells, possibly by directly regulating the expression of downstream transcription factors such as Eomes and Id2 (Male et al., 2014; Seillet et al., 2014a). In contrast, liver VLA-2–Eomes– NK cells (Sojka et al., 2014) and salivary gland VLA-2+Eomes+ NK cells (Cortez et al., 2014) seem to develop independently of NFIL3. It should be noted, however, that the impact of NFIL3 seems to be affected by the environment and may vary due to infection, inflammation, and perhaps housing conditions. For example, while NFIL3 is required for the development of conventional NK cells in the steady-state, NFIL3 is dispensable for NK cells that express the MCMV-specific receptor Ly49H during MCMV infection (Firth et al., 2013). This variability may explain seemingly contradictory results regarding the impact of NFIL3 on the development of thymic NK cells (Crotta et al., 2014; Seillet et al., 2014a) and liver VLA- 1+ NK cells (Sojka et al., 2014). Therefore, dependence on NFIL3 is most likely insufficient to unequivocally define a cell lineage (Table 3).
While cNK cells develop from Id2– CLP, intestinal ILC1 and VLA-1+ liver NK cells develop from Id2+PLZF+ CHILP (Constantinides et al., 2014; Klose et al., 2014). Thus, these studies establish liver VLA-1+ NK cells and intestinal ILC1 as a separate ILC1 lineage, distinct from cNK cells. It will be interesting to see whether uterine and salivary gland NK cells also arise from CHILP or are more related to cNK cells. In addition to originating from discrete precursors, cNK cells and ILC1 may also differ in the timing and site of development. For example, liver ILC1 may develop early on during embryogenesis from the fetal liver and may replicate in situ to maintain stable numbers.
Differentiation of ILC2
Beyond Id2, IL-7 and the cytokine receptor common γ chain (Cao et al., 1995; Moro et al., 2010; Satoh-Takayama et al., 2010; Yokota et al., 1999), differentiation of ILC2 requires the transcription factors GATA-3, RORα, and TCF-1 as well as Notch signaling (Furusawa et al., 2013; Gentek et al., 2013; Hoyler et al., 2012; Klein Wolterink et al., 2013; Mielke et al., 2013; Moro et al., 2010; Wong et al., 2012; Yang et al., 2013). GATA-3 has broader roles in ILC development as deletion of Gata3 in all hematopoietic cells using Vav-Cre (Yagi et al., 2014) or in fetal liver chimeras (Serafini et al., 2014) revealed that GATA-3 is critical for the differentiation of all helper-like ILC subsets but not for cNK cells. Deletion of the Gata3 geneat a later time point using Id2CreERt2/+ mice resulted in the impaired differentiation of ILC2 but not ILC3 (Hoyler et al., 2012). Similarly, when Rosa26CreERt2/+ × Gata3flox/flox bone marrow cells were transplanted into lethally irradiated mice and 4-hydroxytamoxifen was administered into the mice 1 day after the bone marrow transplantation, differentiation of ILC2 but not of ILC3 was strongly suppressed (Furusawa et al., 2013; Yagi et al., 2014). Transgenic overexpression of GATA-3 increased ILC2 numbers concomitant with higher expression of IL-33R (Klein Wolterink et al., 2013). These results suggest that GATA-3 plays important roles in multiple steps of ILC differentiation and/or survival but high level GATA-3 expression in lineage-specified ILC is specifically required for ILC2 maintenance (Table 3). GATA-3 is also required for the differentiation of human ILC2 (Mjosberg et al., 2012). Future research needs to provide a molecular understanding of the GATA-3-directed gene expression networks in the various ILC subsets and in progenitor populations.
RORα, a member of retinoic-acid-receptor-related orphan nuclear receptor, is highly expressed in ILC2 (Moro et al., 2010; Wong et al., 2012). ILC2 differentiation was severely impaired in a natural Rora mutant (Rorasg/sg mouse) without affecting other ILC subsets (Halim et al., 2012b; Wong et al., 2012), indicating that RORα is a transcription factor specifically required for ILC2 differentiation. Small numbers of ILC2 cells are present in the mesentery of Rorasg/sg mice and those residual cells are able to produce IL-5 and IL-13 in response to IL-33 (Furusawa et al., 2013), indicating that RORα is dispensable for cytokine production induced by IL-33 signals. Unlike RORα, GATA-3 is essential for the expression of IL-5 and IL-13 in mature ILC2 (Furusawa et al., 2013; Hoyler et al., 2012; Klein Wolterink et al., 2013; Liang et al., 2012; Yagi et al., 2014).
In addition to factors involved in the differentiation of ILC2, other factors play roles in differentiation and functional maturation of ILC2. Vitamin A and its metabolites derived from food control the balance between ILC2 and ILC3 in the intestine as discussed later (Spencer et al., 2014; van de Pavert et al., 2014). Functional specification of ILC2 requires Gfi1 because the lack of the Gfi1 gene resulted in the loss of GATA-3expression and coexpression of IL-13 and IL-17 (Spooner et al., 2013). Gfi1 directly activated the expression of Il1rl1 and Il17rb genes encoding receptors for IL-33 and IL-25, respectively. On the other hand, Gfi1 suppressed the characteristics of ILC3 through the suppression of Sox4-Rorc axis required for the expression of Il17a gene (Spooner et al., 2013). Interestingly, work with human ILC3 had indicated that they can become producers of IL-5 and IL-13 when stimulated with TLR2 ligands (Crellin et al., 2010). These results suggest potential plasticity between ILC2 and ILC3 under certain environmental conditions, which remains to be elucidated. Other outstanding questions are whether class II MHC expression is limited to ILC2 (Oliphant et al., 2014) and ILC3 (Hepworth et al., 2013) and how such class II MHC expression fits with our current understanding of transcriptional regulation of ILC and of ILC being part of the lymphoid branch of hematopoietic cells.
Differentiation of ILC3
Group 3 ILC contain various populations of RORγt-expressing ILC that play important roles in lymphoid organogenesis and that are a substantial innate source of “type 17 cytokines” involved in protecting mucosal barriers against extracellular bacterial and fungal infections (Diefenbach, 2013). The common denominator of the various ILC3 populations is their dependency on the transcription factor RORγt which specifies and defines the ILC3 lineage. RORγt expression has not been reported in other ILC lineages. Rorc(γt), an alternative transcript of the Rorc gene, was initially found to be expressed in all CD4+CD8+ thymocytes and overexpression of RORγt protected such DP thymocytes against activation-induced cell death (He et al., 1998). Surprisingly, genetic deletion of the Rorc gene had a mild T cell phenotype but these mice lacked all peripheral lymph nodes and Peyer's patches (Eberl et al., 2004; Kurebayashi et al., 2000; Sun et al., 2000). Lack of lymphoid organ development in RORγt-deficient animals was linked to the absence of LTi cells. LTi cells were first identified as innate lymphocytes in lymph node and Peyer's patch anlagen of E13.5-15.5 mice (Adachi et al., 1998; Adachi et al., 1997; Mebius et al., 1997; Yoshida et al., 2002). Interestingly, a phenotypically related and RORγt-dependent lymphoid population was found to be required in the small intestine to coordinate formation of dispersed lymphoid clusters found in the lamina propria of the intestine known as cryptopatches and isolated lymphoid follicles (Eberl, 2005; Eberl and Littman, 2004; Hamada et al., 2002; Kanamori et al., 1996). ILC3 can be differentiated from Lin−IL-7Rα+Flt3−γ4β7+ fetal liver progenitors that sequentially acquired Id2, CXCR6, and RORγt expression (Cherrier et al., 2012; Possot et al., 2011).
While a role of LTi cells as organizers of lymphoid organ development is well documented, only recently it became clear that they are substantially represented in the intestinal lamina propria of adult mice where they produce copious amounts of IL-22 and IL-17A and contribute to immunity to infections and the pathogenesis of inflammatory diseases (Buonocore et al., 2010; Cella et al., 2009; Cupedo et al., 2009; Luci et al., 2009; Powell et al., 2012; Sanos et al., 2009; Satoh-Takayama et al., 2008; Takatori et al., 2009; Vonarbourg et al., 2010). More recently, it was shown that ILC3 also produce GM-CSF, which established important crosstalk with GM-CSF-responsive mononuclear phagocytes that regulate oral tolerance (Mortha et al., 2014). These intestinal “LTi-like cells” expressed surface receptors often found on NK cells such as NKp46, NKp44, and CD56, raising questions about the relationship between NKp46+ ILC3 and NK cells. Compelling evidence from fate-map studies has been provided showing that NKp46+ ILC3 develop independent of the NK cell lineage and are the progeny of NKp46− ILC3 (Crellin et al., 2010; Klose et al., 2014; Satoh-Takayama et al., 2010; Sawa et al., 2010; Vonarbourg et al., 2010).
The existence of NKp46− and NKp46+ ILC3 raised the question if these are independent ILC lineages or rather differentiation or activation products of the same lineage. Two reports have found that ILC3 can be subdivided into CCR6+IL-7RαhighKithigh and CCR6−/lowIL-7RαlowKitlow cells that may constitute separate ILC3 lineages, both of which require RORγt for development (Klose et al., 2013; Sawa et al., 2010). It has now become clear that CCR6+ and CCR6−/low ILC3 follow distinct transcriptional programs. CCR6high ILC3 seed the intestine early during fetal development and seem to be long-lived and slowly cycling cells (Eberl and Littman, 2004; Hanash et al., 2012; Sawa et al., 2010). In contrast, CCR6−/low ILC3 account only for a small fraction of ILC3 at birth but vigorously expand during the first four weeks after birth (Klose et al., 2013). Expansion of CCR6−/low ILC3 was dependent on the expression of the ligand activated transcription factor aryl hydrocarbon receptor (AhR). Mice genetically lacking AhR expression in all cells or in ILC3 had a largely unaltered compartment of CCR6+ ILC3 whereas CCR6−/low ILC3 were diminished (Kiss et al., 2011; Klose et al., 2013; Lee et al., 2012; Qiu et al., 2011). AhR-independent development of CCR6+ ILC3 with LTi function was also documented by the fact that fetally forming lymphoid organs (i.e., lymph nodes, Peyer's patches) were normal in AhR-deficient mice. Curiously, cryptopatches and ILF were absent in AhR-deficient mice, suggesting that CCR6−/low ILC3 have either non-redundant LTi function or that AhR regulates crucial LTi effector molecules in CCR6+ ILC3. The reduced maintenance of CCR6−/low ILC3 in AhR-deficient mice may be explained by a role of AhR in regulating expression of the receptor tyrosine kinase c-Kit because AhR directly bound to the c-Kit promoter and controlled transcription of the Kit gene (Kiss et al., 2011). AhR is a sensor of various small molecules which confer transcriptional activity to the AhR (McIntosh et al., 2010). Interestingly, nutrient-derived AhR ligands such as glucobrassicins contained in vegetables of the Brassicaceae family (e.g., broccoli, Brussel sprouts) were recently identified to have an important role in driving postnatal expansion of CCR6−/low ILC3 (Kiss et al., 2011) and intraepithelial γδ T cells (Li et al., 2011) demonstrating a broad role of plant-derived phytochemicals in controlling development and maintenance of immune system components at barrier surfaces.
The majority of NKp46−CCR6−/low ILC3 co-expressed T-bet (Klose et al., 2013; Rankin et al., 2013; Sciume et al., 2012). While plastic (‘ex-RORγt+’) ILC3 can be found in humans (Bernink et al., 2013), it remains to be seen if T-bet-expressing CCR6−/low ILC3 exist in humans. Co-expression of T-bet and RORγt was a remarkable finding because it was believed that lineage-specifying transcription factors such as T-bet and RORγt may be mutually exclusive in expression. T-bet was not required for the differentiation or maintenance of NKp46−CCR6−/low ILC3 but controlled differentiation of NKp46−CCR6−/low ILC3 to NKp46+CCR6−/low ILC3 (Rankin et al., 2013). In addition, an increasing T-bet gradient in CCR6−/low ILC3 directed a transcriptional program that allowed for functional and phenotypic plasticity (Figure 1). For example, T-bet controlled IFN-γ production in NKp46+CCR6−/low ILC3 that contributed to early immunity to Salmonella enterica infection. When inappropriately stimulated, NKp46+CCR6−/low ILC3 contributed to intestinal inflammatory diseases in mice and humans (Bernink et al., 2013; Vonarbourg et al., 2010). T-bet expression in CCR6−/low ILC3 was independent of IL-12 signaling but was promoted by IL-23 (Klose et al., 2013), revealing an interesting parallel to the signals driving plasticity of Th17 cells in a mouse model of multiple sclerosis (Hirota et al., 2011).
Recent data revealed an important role for maternal vitamin A metabolites in the differentiation and maintenance of fetal CCR6+ ILC3 with LTi function. Mice deficient of retinoic acid receptor (RAR)α expression in hematopoietic cells had reduced numbers of fetal CCR6+ ILC3 due to inefficient upregulation of the Rorc(γt) transcript of the Rorc gene (van de Pavert et al., 2014). Reduced ILC3 numbers led to the formation of substantially smaller lymphoid organs, ultimately reducing the fitness of adult mice to virus infections (van de Pavert et al., 2014). Similar findings were reported for adult mice under conditions of vitamin A malnutrition that presented with reduced numbers of IL-22-producing ILC3 (Spencer et al., 2014). Vitamin A depravation of adult mice led to impaired immunity to attaching-and-effacing infections with C. rodentium. While ILC3 numbers and function were reduced under conditions of retinoic acid deficiency, ILC2 were increased in a compensatory manner resulting in enhanced immunity to nematode infection (Spencer et al., 2014). These data and the data from Ahr-deficient mice establish an important trajectory by which nutrients instruct differentiation and maintenance of mucosal ILC subsets.
Conclusions and perspectives
The discovery of new ILC lineages has entirely redefined our hematopoietic lineage maps (Figure 1). It is now clear that the diversity of ILC is much higher than previously appreciated. The description of ILC-restricted progenitors (i.e., CHILP) has now opened opportunities to examine the cues that drive differentiation of the various helper-like ILC populations. While the similarities between helper-like ILC and T helper cell subsets are striking, it is already clear that the cytokine signals required for T helper fate decisions are dispensable for the differentiation into the various helper-like ILC populations (Guo et al., 2014; Klose et al., 2013; Liang et al., 2012). Thus, one of the important future goals will be to identify the signals that drive differentiation of the CHILP. While a naïve CD4 T cell can be differentiated into any Th effector state depending on the cytokine environment provided, it should be considered that ILC fate may be determined through a timed release of progenitors during ontogeny into an environment that allows for the preferential differentiation into a certain ILC fate. Precedent for such temporal organization of lymphoid fate decisions are the various waves of distinct γδ T cell subsets leaving the thymus before birth. Such future lines of research may also reveal an evolutionary perspective on how such transcriptional programs controlling lymphoid fate have emerged. In addition, although ILC2 seem a relatively stable subset compared to ILC1 and ILC3, there is still a possibility of plasticity between ILC2 and other ILC subsets (Crellin et al., 2010; Spooner et al., 2013). Given the emerging roles of transcriptional and functional plasticity of lymphocyte subsets for the pathogenesis of inflammatory diseases (Hirota et al., 2011; Vonarbourg et al., 2010), the molecular cues stabilizing ILC fates and those driving plasticity should be identified because they may reveal therapeutic targets for such debilitating diseases. Thus, future research needs to identify the signals and the spatiotemporal organization of helper-like ILC development.
Research into ILC lineages has also revealed that the population of NKp46+ cells, previously addressed as “NK cells”, is more complex than appreciated. For example, the population of NKp46+ cells in the lamina propria of the intestine is composed of at least three distinct ILC populations (Klose et al., 2014). The unique contributions of these various NKp46+ ILC subsets to immune responses remain to be determined. In that context, it is interesting to note that cNK cells may be a lineage distinct from helper-like ILC. It will be informative to position the branching point of the cytotoxic and helper-like ILC lineages on hematopoietic lineage maps. This will be complemented by analyzing the core transcriptional program determining ILC fate. Id2 has already been discovered as one central hub for ILC fate and NFIL3 and TOX may be other candidates. The DNA binding factor TOX is an interesting candidate because Tox−/− mice lack NK cell and ILC3 development (Aliahmad et al., 2012; Geiger et al., 2014).
Finally, lymphoid cells use immune recognition receptors for development and to interact with infected or damaged cells. It remains unclear if immunoreceptors expressed by ILC are required for their function and/or development. Mice lacking the Ncr1 gene and consequently not expressing NKp46 showed normal development and function of ILC3 documented by their resistance to C. rodentium infection (Satoh-Takayama et al., 2009). Consecutive work documented a role for activating immunoreceptors for ILC function. Human ILC3 from tonsils and colon could be activated for TNF release when triggered by the NKp44 receptor (Glatzer et al., 2013). In mice, liver-resident ILC1 responded to engagement of various activating immunoreceptors (i.e., NKp46, NKG2D, NK1.1) with the production of TNF, IFN-γ and CCL3 (Daussy et al., 2014). Interestingly, liver-resident ILC1 with an “NK cell phenotype” display antigen-specific memory that does not require recombining receptors (O'Leary et al., 2006; Paust et al., 2010). The phenotype of such memory “NK cells” may indicate that they are an ILC1 subset. It will be highly informative to gain a more molecular understanding of how ILC discriminate between “self” and “non-self” and how previous activation may introduce a memory-like program into innate lymphocytes.
Acknowledgements
We are grateful to C.S.N.Klose and M.Flach for comments on the manuscript. Work in the Diefenbach laboratory is supported by grants from the ERC (NutrImmune) and the DFG (Di764/3-1 and SPP1636). The Koyasu laboratory is supported by a Grant-in-Aid for Scientific Research (S) (22229004) from the Japan Society for the Promotion of Science (JSPS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
S.K. is a consultant for Medical and Biological Laboratories, Co. Ltd.
References
- Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor alpha in the formation of Peyer's patch anlage. Int Immunol. 1998;10:1–6. [Abstract] [Google Scholar]
- Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol. 1997;9:507–514. [Abstract] [Google Scholar]
- Aliahmad P, Seksenyan A, Kaye J. The many roles of TOX in the immune system. Current opinion in immunology. 2012;24:173–177. [Europe PMC free article] [Abstract] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. [Abstract] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology. 2013;14:221–229. [Abstract] [Google Scholar]
- Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. The Journal of experimental medicine. 2007;204:1119–1130. [Europe PMC free article] [Abstract] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. [Europe PMC free article] [Abstract] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. [Europe PMC free article] [Abstract] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. [Abstract] [Google Scholar]
- Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–5452. [Abstract] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. [Europe PMC free article] [Abstract] [Google Scholar]
- Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10961–10966. [Europe PMC free article] [Abstract] [Google Scholar]
- Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. The Journal of experimental medicine. 2012;209:729–740. [Europe PMC free article] [Abstract] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. [Europe PMC free article] [Abstract] [Google Scholar]
- Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. 2014;192:4487–4491. [Abstract] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. [Abstract] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. The Journal of experimental medicine. 2010;207:281–290. [Europe PMC free article] [Abstract] [Google Scholar]
- Crotta S, Gkioka A, Male V, Duarte JH, Davidson S, Nisoli I, Brady HJ, Wack A. The transcription factor E4BP4 is not required for extramedullary pathways of NK cell development. J Immunol. 2014;192:2677–2688. [Europe PMC free article] [Abstract] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. [Abstract] [Google Scholar]
- Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. The Journal of experimental medicine. 2014;211:563–577. [Europe PMC free article] [Abstract] [Google Scholar]
- Diefenbach A. Innate lymphoid cells in the defense against infections. European journal of microbiology & immunology. 2013;3:143–151. [Europe PMC free article] [Abstract] [Google Scholar]
- Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nature reviews. Immunology. 2005;5:413–420. [Abstract] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. [Abstract] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. [Abstract] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. [Europe PMC free article] [Abstract] [Google Scholar]
- Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118:5439–5447. [Europe PMC free article] [Abstract] [Google Scholar]
- Firth MA, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, Kubo M, Rothman PB, Vivier E, Sun JC. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J Exp Med. 2013;210:2981–2990. [Europe PMC free article] [Abstract] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. [Abstract] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial Type 1 Innate Lymphoid Cells Are a Unique Subset of IL-12-and IL-15-Responsive IFN-gamma-Producing Cells. Immunity. 2013;38:769–781. [Europe PMC free article] [Abstract] [Google Scholar]
- Furusawa J, Moro K, Motomura Y, Okamoto K, Zhu J, Takayanagi H, Kubo M, Koyasu S. Critical role of p38 and GATA3 in natural helper cell function. J Immunol. 2013;191:1818–1826. [Europe PMC free article] [Abstract] [Google Scholar]
- Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nature immunology. 2009;10:1118–1124. [Abstract] [Google Scholar]
- Geiger TL, Abt MC, Gasteiger G, Firth MA, O'Connor MH, Geary CD, O'Sullivan TE, van den Brink MR, Pamer EG, Hanash AM, Sun JC. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. The Journal of experimental medicine. 2014 [Europe PMC free article] [Abstract] [Google Scholar]
- Gentek R, Munneke JM, Helbig C, Blom B, Hazenberg MD, Spits H, Amsen D. Modulation of Signal Strength Switches Notch from an Inducer of T Cells to an Inducer of ILC2. Frontiers in immunology. 2013;4:334. [Europe PMC free article] [Abstract] [Google Scholar]
- Glatzer T, Killig M, Meisig J, Ommert I, Luetke-Eversloh M, Babic M, Paclik D, Bluthgen N, Seidl R, Seifarth C, et al. RORgammat(+) innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity. 2013;38:1223–1235. [Abstract] [Google Scholar]
- Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. [Europe PMC free article] [Abstract] [Google Scholar]
- Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, Zhou L, Fu YX. Induction of Innate Lymphoid Cell-Derived Interleukin-22 by the Transcription Factor STAT3 Mediates Protection against Intestinal Infection. Immunity. 2014;40:25–39. [Europe PMC free article] [Abstract] [Google Scholar]
- Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012a;36:451–463. [Abstract] [Google Scholar]
- Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012b;37:463–474. [Abstract] [Google Scholar]
- Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. [Abstract] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. [Europe PMC free article] [Abstract] [Google Scholar]
- He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. [Europe PMC free article] [Abstract] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. [Europe PMC free article] [Abstract] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology. 2011;12:255–263. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The Transcription Factor GATA-3 Controls Cell Fate and Maintenance of Type 2 Innate Lymphoid Cells. Immunity. 2012;37:634–648. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. [Europe PMC free article] [Abstract] [Google Scholar]
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. [Abstract] [Google Scholar]
- Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. [Abstract] [Google Scholar]
- Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. The Journal of experimental medicine. 2009;206:2469–2481. [Europe PMC free article] [Abstract] [Google Scholar]
- Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, Betsuyaku T, Koyasu S, Asano K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. [Abstract] [Google Scholar]
- Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, Akashi K, Lind EF, Haight JP, Ohashi PS, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. [Europe PMC free article] [Abstract] [Google Scholar]
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. [Europe PMC free article] [Abstract] [Google Scholar]
- Kee BL. E and ID proteins branch out. Nature reviews. Immunology. 2009;9:175–184. [Abstract] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. [Abstract] [Google Scholar]
- Klein Wolterink RG, Serafini N, van Nimwegen M, Vosshenrich CA, de Bruijn MJ, Fonseca Pereira D, Veiga Fernandes H, Hendriks RW, Di Santo JP. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10240–10245. [Europe PMC free article] [Abstract] [Google Scholar]
- Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell. 2014;157:340–356. [Abstract] [Google Scholar]
- Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, Goppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. [Abstract] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature immunology. 2008;9:1055–1064. [Europe PMC free article] [Abstract] [Google Scholar]
- Koyasu S, Moro K. Role of innate lymphocytes in infection and inflammation. Frontiers in immunology. 2012;3:101. [Europe PMC free article] [Abstract] [Google Scholar]
- Koyasu S, Moro K, Tanabe M, Takeuchi T. Natural helper cells: a new player in the innate immune response against helminth infection. Advances in immunology. 2010;108:21–44. [Abstract] [Google Scholar]
- Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10132–10137. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nature immunology. 2012;13:144–151. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous Stimuli Maintain Intraepithelial Lymphocytes via Aryl Hydrocarbon Receptor Activation. Cell. 2011;147:629–640. [Abstract] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nature immunology. 2012;13:58–66. [Europe PMC free article] [Abstract] [Google Scholar]
- Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. [Abstract] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nature immunology. 2009;10:75–82. [Abstract] [Google Scholar]
- Male V, Nisoli I, Gascoyne DM, Brady HJ. E4BP4: an unexpected player in the immune response. Trends in immunology. 2012;33:98–102. [Abstract] [Google Scholar]
- Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM, Wack A, Brady HJ. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. The Journal of experimental medicine. 2014;211:635–642. [Europe PMC free article] [Abstract] [Google Scholar]
- McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S, Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. [Europe PMC free article] [Abstract] [Google Scholar]
- McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–645. [Abstract] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3-LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. [Abstract] [Google Scholar]
- Mielke LA, Groom JR, Rankin LC, Seillet C, Masson F, Putoczki T, Belz GT. TCF-1 controls ILC2 and NKp46+RORgammat+ innate lymphocyte differentiation and protection in intestinal inflammation. J Immunol. 2013;191:4383–4391. [Abstract] [Google Scholar]
- Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, Te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37:649–659. [Abstract] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology. 2011;12:1045–1054. [Europe PMC free article] [Abstract] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. [Abstract] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. [Europe PMC free article] [Abstract] [Google Scholar]
- Murre C. Helix-loop-helix proteins and lymphocyte development. Nature immunology. 2005;6:1079–1086. [Abstract] [Google Scholar]
- Nechanitzky R, Akbas D, Scherer S, Gyory I, Hoyler T, Ramamoorthy S, Diefenbach A, Grosschedl R. Transcription factor EBF1 is essential for the maintenance of B cell identity and prevention of alternative fates in committed cells. Nature immunology. 2013;14:867–875. [Abstract] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell-and B cell-independent adaptive immunity mediated by natural killer cells. Nature immunology. 2006;7:507–516. [Abstract] [Google Scholar]
- Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, Fallon PG, McKenzie AN. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21–31. [Abstract] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature immunology. 2010;11:1127–1135. [Europe PMC free article] [Abstract] [Google Scholar]
- Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. The Journal of clinical investigation. 2013;123:1444–1456. [Europe PMC free article] [Abstract] [Google Scholar]
- Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, Golub R. Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nature immunology. 2011;12:949–958. [Abstract] [Google Scholar]
- Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG, et al. The Transcription Factor T-bet Regulates Intestinal Inflammation Mediated by Interleukin-7 Receptor(+) Innate Lymphoid Cells. Immunity. 2012;37:674–684. [Europe PMC free article] [Abstract] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity. 2011;36:92–104. [Europe PMC free article] [Abstract] [Google Scholar]
- Rankin LC, Groom JR, Chopin M, Herold MJ, Walker JA, Mielke LA, McKenzie AN, Carotta S, Nutt SL, Belz GT. The transcription factor T-bet is essential for the development of NKp46(+) innate lymphocytes via the Notch pathway. Nature immunology. 2013;14:389–395. [Europe PMC free article] [Abstract] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. [Europe PMC free article] [Abstract] [Google Scholar]
- Ribeiro VS, Hasan M, Wilson A, Boucontet L, Pereira P, Lesjean-Pottier S, Satoh-Takayama N, Di Santo JP, Vosshenrich CA. Cutting edge: Thymic NK cells develop independently from T cell precursors. J Immunol. 2010;185:4993–4997. [Abstract] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature immunology. 2013;14:564–573. [Europe PMC free article] [Abstract] [Google Scholar]
- Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A, Artis D. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. The Journal of experimental medicine. 2013;210:1823–1837. [Europe PMC free article] [Abstract] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. [Europe PMC free article] [Abstract] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–2950. [Europe PMC free article] [Abstract] [Google Scholar]
- Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CA, Colucci F, Buer J, Grosveld F, Godin I, Di Santo JP. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. [Abstract] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nature immunology. 2009;10:83–91. [Europe PMC free article] [Abstract] [Google Scholar]
- Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S, Ribeiro VS, Mandelboim O, Renauld JC, Vosshenrich CA, Di Santo JP. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J Immunol. 2009;183:6579–6587. [Abstract] [Google Scholar]
- Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. The Journal of experimental medicine. 2010;207:273–280. [Europe PMC free article] [Abstract] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. [Abstract] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. [Abstract] [Google Scholar]
- Sciume G, Hirahara K, Takahashi H, Laurence A, Villarino AV, Singleton KL, Spencer SP, Wilhelm C, Poholek AC, Vahedi G, et al. Distinct requirements for T-bet in gut innate lymphoid cells. The Journal of experimental medicine. 2012;209:2331–2338. [Europe PMC free article] [Abstract] [Google Scholar]
- Seillet C, Huntington ND, Gangatirkar P, Axelsson E, Minnich M, Brady HJ, Busslinger M, Smyth MJ, Belz GT, Carotta S. Differential requirement for Nfil3 during NK cell development. J Immunol. 2014a;192:2667–2676. [Abstract] [Google Scholar]
- Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M, Huntington ND, Belz GT, Carotta S. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014b [Europe PMC free article] [Abstract] [Google Scholar]
- Serafini N, Klein Wolterink RG, Satoh-Takayama N, Xu W, Vosshenrich CA, Hendriks RW, Di Santo JP. Gata3 drives development of RORgammat+ group 3 innate lymphoid cells. The Journal of experimental medicine. 2014;211:199–208. [Europe PMC free article] [Abstract] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. [Europe PMC free article] [Abstract] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. [Europe PMC free article] [Abstract] [Google Scholar]
- Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr., Wang J, Ramalingam TR, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. [Europe PMC free article] [Abstract] [Google Scholar]
- Spooner CJ, Lesch J, Yan D, Khan AA, Abbas A, Ramirez-Carrozzi V, Zhou M, Soriano R, Eastham-Anderson J, Diehl L, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nature immunology. 2013;14:1229–1236. [Abstract] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. [Abstract] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. The Journal of experimental medicine. 2009;206:35–41. [Europe PMC free article] [Abstract] [Google Scholar]
- Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, Yagita H, Kinoshita K, Okumura K, Smyth MJ. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. [Abstract] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nature medicine. 2001;7:94–100. [Abstract] [Google Scholar]
- Tanriver Y, Diefenbach A. Transcription factors controlling development and function of innate lymphoid cells. Int Immunol. 2014;26:119–128. [Abstract] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. [Abstract] [Google Scholar]
- Treiber T, Mandel EM, Pott S, Gyory I, Firner S, Liu ET, Grosschedl R. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription-independent poising of chromatin. Immunity. 2010;32:714–725. [Abstract] [Google Scholar]
- van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. [Europe PMC free article] [Abstract] [Google Scholar]
- Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. The Journal of experimental medicine. 2006;203:1435–1446. [Europe PMC free article] [Abstract] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, et al. Regulated Expression of Nuclear Receptor RORgammat Confers Distinct Functional Fates to NK Cell Receptor-Expressing RORgammat(+) Innate Lymphocytes. Immunity. 2010;33:736–751. [Europe PMC free article] [Abstract] [Google Scholar]
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nature immunology. 2006;7:1217–1224. [Abstract] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORalpha is critical for nuocyte development. Nature immunology. 2012;13:229–236. [Europe PMC free article] [Abstract] [Google Scholar]
- Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, Hu G, Barron L, Sharma S, Nakayama T, et al. The Transcription Factor GATA3 Is Critical for the Development of All IL-7Ralpha-Expressing Innate Lymphoid Cells. Immunity. 2014;40:378–388. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, De Obaldia ME, Bailis W, Bryson JL, Toscano K, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. Journal of immunology. 2011;187:5505–5509. [Europe PMC free article] [Abstract] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. [Abstract] [Google Scholar]
- Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–833. [Abstract] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. [Abstract] [Google Scholar]
- Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr., Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nature immunology. 2004;5:1157–1165. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.immuni.2014.09.005
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1074761314003161/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.immuni.2014.09.005
Article citations
NKp46<sup>+</sup> ILC3s promote early neutrophil defense against <i>Clostridioides difficile</i> infection through GM-CSF secretion.
Proc Natl Acad Sci U S A, 121(45):e2416182121, 30 Oct 2024
Cited by: 1 article | PMID: 39475653
Natural killer cell biology and therapy in multiple myeloma: challenges and opportunities.
Exp Hematol Oncol, 13(1):114, 13 Nov 2024
Cited by: 0 articles | PMID: 39538354 | PMCID: PMC11562869
Review Free full text in Europe PMC
Vaccine induced mucosal and systemic memory NK/ILCs elicit decreased risk of SIV/SHIV acquisition.
Front Immunol, 15:1441793, 05 Sep 2024
Cited by: 1 article | PMID: 39301032 | PMCID: PMC11410642
Type II innate lymphoid cell plasticity contributes to impaired reconstitution after allogeneic hematopoietic stem cell transplantation.
Nat Commun, 15(1):6000, 17 Jul 2024
Cited by: 0 articles | PMID: 39019846 | PMCID: PMC11255294
Dietary Fiber-Derived Microbial Butyrate Suppresses ILC2-Dependent Airway Inflammation in COPD.
Mediators Inflamm, 2024:6263447, 09 Jul 2024
Cited by: 1 article | PMID: 39015676 | PMCID: PMC11251798
Go to all (357) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Lymphoid tissue inducer-A divergent member of the ILC family.
Cytokine Growth Factor Rev, 42:5-12, 13 Feb 2018
Cited by: 32 articles | PMID: 29454785 | PMCID: PMC6089680
Review Free full text in Europe PMC
Heterogeneity of NK Cells and Other Innate Lymphoid Cells in Human and Murine Decidua.
Front Immunol, 10:170, 08 Feb 2019
Cited by: 28 articles | PMID: 30800126 | PMCID: PMC6375891
Review Free full text in Europe PMC
Innate lymphoid cells in inflammation and immunity.
Immunity, 41(3):366-374, 01 Sep 2014
Cited by: 222 articles | PMID: 25238094
Review
Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice.
Eur J Immunol, 45(8):2171-2182, 18 Jun 2015
Cited by: 119 articles | PMID: 26031799
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R21 CA167192
Grant ID: R21CA16719
NIAID NIH HHS (2)
Grant ID: U01 AI095542
Grant ID: 1U01AI095542
NIDCR NIH HHS (2)
Grant ID: R01 DE021255
Grant ID: R01DE021255





