Abstract
Free full text

Testisimmune privilege - Assumptions versus facts
Abstract
The testis has long enjoyed a reputation as an immunologically privileged site based on its ability to protect auto-antigenic germ cells and provide an optimal environment for the extended survival of transplanted allo- or xeno-grafts. Exploration of the role of anatomical, physiological, immunological and cellular components in testis immune privilege revealed that the tolerogenic environment of the testis is a result of the immunomodulatory factors expressed or secreted by testicular cells (mainly Sertoli cells, peritubular myoid cells, Leydig cells, and resident macrophages). The blood-testis barrier/Sertoli cell barrier, is also important to seclude advanced germ cells but its requirement in testis immune privilege needs further investigation. Testicular immune privilege is not permanent, as an effective immune response can be mounted against transplanted tissue, and bacterial/viral infections in the testis can be effectively eliminated. Overall, the cellular components control the fate of the immune response and can shift the response from immunodestructive to immunoprotective, resulting in immune privilege.
Immune privilege
Immune privileged sites are places in the body where foreign antigens are tolerated without evoking detrimental inflammatory immune responses and thus, foreign tissue grafted into an immune privileged site enjoys prolonged survival. Originally, immune privilege sites were considered ‘immunologically ignorant’. It was thought that the immune system was oblivious to the tissue transplanted into these sites due to the sequestering of cells behind blood tissue barriers and the lack of lymphatic drainage. In other words, the immune system was unable to evoke an active immune response at these sites (Streilein, 1995). This belief has since been challenged and it is now known that immune privilege is an active process governed by locally produced immunoregulatory factors that control the overall immune response. There are several immune privileged sites including the testis, anterior chamber of the eye, brain, central nervous system, maternal fetal interface of the placenta, hair follicles and some tumors (Forrester et al., 2008). This review will focus on immune privilege in the testis.
Testis as an immune privileged tissue
The testis is an organ that is highly specialized for the production of sperm and male sex hormones (Russell et al., 1990). Morphologically, it consists of two major compartments, the seminiferous tubules (site of spermatogenesis) surrounded by the interstitium (site of testosterone production). The interstitium contains Leydig cells, blood vessels, leukocytes, lymphatic vessels and fibroblasts. The seminiferous tubules are composed of Sertoli cells (SC) and germ cells surrounded by one (rodents) or more (large animals) layers of peritubular myoid cells (Dym and Fawcett, 1970; Dym, 1973; Fig. 1A and B).
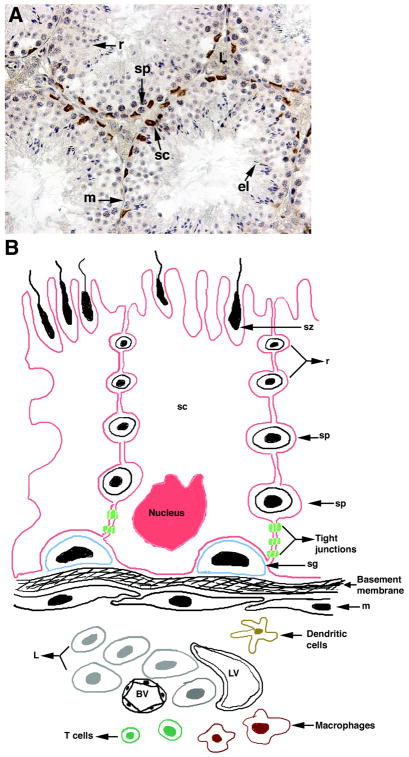
Testis histology and schematic diagram showing the BTB present between adjacent SC. A) Testes were collected from BALB/c mice (6–8 weeks old), paraffin embedded and sectioned. Tissue section was immunostained for Wilm’s tumor 1 to detect SC nuclei (brown color, A). Section was counterstained with hematoxylin to detect cell nuclei (blue color, A). B) BTB localization and cellular components present in the testicular interstitium and seminiferous tubules. Testis interstitium consists of Leydig cells, macrophages, dendritic cells, lymphocytes (mainly T cells), lymphatics and blood vessels. Peritubular myoid cells surround the seminiferous epithelium and form a primary barrier to substances penetrating from the interstitium to the seminiferous epithelium. The more effective and complete barrier is located within the seminiferous tubules and includes the body of SC and tight junctions between adjacent SC (BTB). The BTB delimits a basal compartment in the germinal epithelium containing the spermatogonia and early preleptotene spermatocytes, and an adluminal compartment containing the spermatocytes and spermatids. sc, Sertoli cell; m, myoid cell; sg, spermatogonia; sp, spermatocyte; r, round spermatid; el, elongated spermatid; sz, spermatozoa; L, Leydig cells; BV, blood vessel; LV, lymphatic vessel.
Advanced germ cells within the testis develop after the establishment of systemic self-tolerance. Despite the expression of new surface and intracellular proteins by germ cells (O’Rand and Romrell, 1977; Tung and Fritz, 1978), these immunogenic auto-antigens do not evoke an immunological attack, mainly due to the unique tolerogenic environment of the testis. Hence, testis immune privilege is important for preventing detrimental immune responses against auto-immunogenic germ cells.
The unique immunological privilege of the testis was first recognized over two centuries ago. In 1767, John Hunter performed the first described testis transplant. A testis from a rooster was transplanted into the abdominal cavity of a hen and histological examination showed that the testis was found to have a “perfectly normal structure” (Setchell, 1990). Later, the functionality of the transplanted testis was examined by performing a similar allotransplantation experiment in two castrated roosters and showed that normal male rooster characteristics returned in transplanted birds as compared to controls (reviewed in Setchell, 1990). Since then, several transplantation experiments, either involving transplantation of testicular tissue or transplantation of other tissues into the testis, have been performed with variable results (reviewed in Maddocks and Setchell, 1990). These early testis transplantation studies provided evidence that secretions from the testis are responsible for the maintenance of male sexual characteristics and the higher temperature of the abdominal cavity is detrimental to spermatogenesis. These studies also resulted in the use of testicular extracts as an elixir for their “rejuvenating effects”. From the 1910s to early 1940s, testicular tissue transplants from animals and humans into humans claimed to restore virility and improve psychological and mental health (Stanley, 1931). However, these claims were later questioned due to the lack of control groups or objective measurements. With the discovery of testosterone in 1935 and concerns about zoonosis (infections from animal sources), this procedure became unpopular (Bajic et al., 2012).
Transplantation of testicular germ cells originated in domestic birds, where an intravascular injection of primordial germ cells from turkeys into sterilized embryos of Rhode Island Red chickens led to the complete maturation of germ cells (Reynaud, 1976). Since then, numerous germ cell transplants have been performed in various species. While most of the rodent germ cell transplants used immune compromised recipients, a few were transplanted into immune competent recipients of the same species (syngeneic) or to genetically different hosts (allogeneic or xenogeneic). Success of the syngeneic grafts suggests the injection of immunogenic germ cells did not provoke an autoimmune response (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994). In rodent allogeneic (mouse to mouse or rat to rat) and xenogeneic (mouse to rat) experiments, germ cell colonization was limited without immune suppression and immune suppression was required to generate offspring (allograft model only). However, successful allogeneic germ cell colonization and sperm production in large animals has been achieved for pigs, goats, cattle, dogs and sheep without the use of immune suppression (reviewed in Honaramooz and Yang, 2010). Furthermore, live offspring have been generated in goats and sheep (Honaramooz et al., 2003; Herrid et al., 2009).
Recently, germ cell transplantation techniques have also been explored in chickens and fish. In the case of the domestic chicken, techniques to transplant spermatogonial stem cells or dispersed testis tissue into immune-competent juvenile or adult chickens have been developed (Lee et al., 2006; Trefil et al., 2006, 2010; Jung et al., 2010). The presence of donor-derived genes in the offspring indicated the successful colonization of donor germ cells in the recipient testis without immune rejection.
Germ cell transplantation has become a popular technique to increase the production of commercial fish such as tilapia, rainbow trout, salmon, blue fin tuna, yellow tail and zebra fish (extensively reviewed in Silva et al., 2012). Initially, germ cells were isolated from embryos or new born hatchlings and transplanted into embryos at different stages of development, or injected intraperitoneally into new-born hatchlings. Since the immune system of the fish is not established at that early age, there was very little risk of immune-rejection. However, spermatogonial stem cells have recently been transplanted in the testes of adult tilapia (allogeneic) or Patagonian pejerrey (xenogeneic; Lacerda et al., 2008, 2010; Majhi et al., 2009), which led to successful differentiation of germ cells into functional spermatozoa, and donor derived progeny in the case of tilapia. This shows that allo- or xeno-geneic fish germ cells were not rejected post-transplantation.
Not only can the germ cell transplantation technique be used to study germ cell development, this technique was recently employed to develop sperm of the endangered felid species, the ocelot, in the germ cell depleted testes of domestic cats. The transplanted spermatogonial stem cells were not only able to colonize and differentiate into elongated spermatids, no signs of immune rejection were observed, and after 13 weeks sperm were present in the recipient’s epididymis (Silva et al., 2012).
Tissue transplanted into the testis
In addition to transplantation of testis tissue/germ cells, the testis has been used as a transplantation site for studying the survival and functionality of non-testicular tissue. In 1919, Sand appears to have been the first person to transplant non-testicular tissue (ovary allografts) into rat or infantile guinea pig testes. Grafted tissue was collected at 3–4 months post-transplantation and survival was confirmed by the presence of mature follicles, corpora lutea and development of mammary glands that contained milk like secretions. This led to the conclusion that the “ovarium finds good condition for existence in the middle of the testis” (Sand, 1919). Besides the ovary, xenogeneic rabbit tumors and human fibrosarcoma tumors, allogeneic fetal testes, allogeneic fertilized eggs (morulae or blastocysts), allogeneic early embryos and trophoblast cells were also transplanted into the testis (reviewed in Setchell, 1990). However, the survival of the early transplants was highly variable (Setchell, 1990), which could be attributed to the lack of standardized transplantation procedures, along with limited knowledge on immune privilege.
Later in the 1970s, with a better knowledge of transplantation immunology and standardized surgical procedures, a series of studies supported the immune privilege status of the testis. A wide variety of tissues such as skin allografts (Whitmore and Gittes, 1975, 1977; Head et al., 1983a), pituitary allografts (Hill and Gardner, 1936), adrenal allografts (Medawar and Russell, 1958), parathyroid allo- and xenografts (Dib-Kuri et al., 1975; Naji and Barker, 1976; Head et al., 1983a; Whitmore et al., 1985), insulinoma allografts (Akimaru et al., 1981) and islet allo- and xeno-grafts (Ferguson and Scothorne, 1977; Bobzien et al., 1983; Selawry et al., 1985) survived long-term when transplanted intratesticularly. Besides extended survival, functionality of the grafted tissues was also reported. For instance, xenogeneic rat islets were transplanted into the testis, kidney, spleen and liver of diabetic mice without any immune suppression. The mean survival time (MST) of islets, determined by lowering of blood glucose levels, was significantly prolonged when transplanted intratesticularly as compared to the other transplantation sites. Normoglycemia was achieved in 100% of the transplanted animals and three of the animals (25%) remained normoglycemic for more than 2 months. Orchidectomy (removal of testis containing islet graft) led to hyperglycemia and histology of the collected tissue showed the presence of pancreatic α and β cells in the interstitium of the testis (Bobzien et al., 1983). Similarly, parathyroid allografts resumed normocalcemia in parathymectomized outbred Wistar rats for more than 3 months, and animals that received guinea pig or rabbit parathyroid xenografts remained normocalcemic for more than 25 days (Dib-Kuri et al., 1975).
In addition to small animals, the immune privilege status of the testis has been demonstrated in large animals by transplanting xenogeneic or allogeneic islets into the testes of dogs and diabetic Rhesus monkeys, respectively. Tissue obtained for histological examination at 100 days (dogs) and 5 years (monkeys) post-transplantation revealed the presence of insulin-positive islets. Normoglycemia was achieved in diabetic monkeys for 8, 54 and 60 months post-transplantation (Selawry, 1994; Gores et al., 2003). However, in a separate study, parathyroid allografts transplanted in the testes of rams and cynomolgous monkeys were rejected within 4 weeks (Maddocks and Setchell, 1988; Setchell et al., 1995). The variability in immune protection provided by the testis indicates that an immune response can be generated against tissue transplanted in the testis, suggesting that the testicular environment is not always tolerogenic or immunologically ignorant. This demonstrates the need to investigate the factors behind immune privilege status of the testis.
Exploration of factors behind testis immune privilege
In order to determine the mechanism(s) or factor(s) responsible for testis immune privilege, the contribution of various components such as anatomy (lymphatic drainage, blood testis barrier; BTB/SC barrier), physiology (lower temperature of the scrotum, higher zinc concentration), immunology (macrophages, dendritic cells, T cells and NK cells), and testicular cells (Leydig cells, myoid cells, SC and germ cells) were explored.
Anatomical components of the testis (lymphatic drainage and BTB)
Lymphatics
Originally, similar to other immune privileged sites (anterior chamber of the eye and brain), it was proposed that lymphatic drainage from the testis was impaired, preventing the activation of a normal immune response. The origin of this hypothesis was based on the notion that the distance between the testis and the nearest draining lymph node at the level of the kidney was unusually long. Additionally, it was suggested that lymph from the testis passes directly into the thoracic duct without passing through any lymph nodes (Engeset, 1959). These beliefs were challenged by the findings that skin allografts placed near the tip of the rat tail, where the distance to the nearest draining lymph node was longer (~19 cm), were rejected (Barker and Billingham, 1977). The lymphatic drainage was subsequently found to be efficient as dyes and cells injected into the testis travel to the draining lymph nodes within minutes (Fawcett et al., 1973; Head et al., 1983b). Furthermore, it was demonstrated that the afferent arm of the lymphatics was intact by injecting allogeneic cells into the testis and confirming the production of antibodies against the grafted cells (Head et al., 1983b). Infiltration of successful intratesticular islet grafts and parathyroid grafts with mononuclear cells and the rejection of transplanted cells in sensitized (transplanted with skin or insulinoma outside the testis) animals verified the existence of the efferent arm (Head et al., 1983b). The presence of leukocytes in normal testis interstitium (Hedger, 1997) further supports that the testis has efficient lymphatic drainage and thus, a lack of lymphatics is not responsible for testicular immune privilege.
Blood-testis barrier (BTB/SC barrier)
Similar to the brain, which has the blood brain barrier, the testis has the BTB. In the brain, the blood brain barrier consists of endothelial cells lining the blood vessels and tight junctions between these cells, which prevents the exit of molecules from the blood vessels (Brightman and Reese, 1969). In the testis, there is a high degree of permeability of the vascular endothelium and substances within the circulation diffuse freely into the interstitium (Maeda et al., 2007). Instead, the BTB/SC barrier is located within the seminiferous tubules and includes the body of SC and tight junctions formed between adjacent SC (Fawcett et al., 1970; Fig. 1B). The BTB separates the seminiferous epithelium into two compartments; basal, containing spermatogonia and preleptotene spermatocytes, and adluminal, containing meiotic and postmeiotic germ cells (Fig. 1B). This barrier prevents leukocytes and antibodies from entering the seminiferous tubules and sequesters the majority of the auto-antigenic germ cells (pachytene spermatocytes, spermatids and spermatozoa) from the immune system.
The importance of the BTB in immune privilege and spermatogenesis was demonstrated by mice with SC specific deletion of the androgen receptor (androgen knock out, Ar-KO; Meng et al., 2011). In these Ar-KO mice, integrity of the tight junctions was compromised as evidenced by an increased permeability to biotin. Furthermore, a humoral immune response, demonstrated by the deposition of antibodies (which normally do not cross the BTB), was mounted against spermatocytes and spermatids within the adluminal compartment. An arrest in spermatogenesis was observed in these Ar-KO mice, which could be due to increased permeability of the BTB along with the generation of a humoral immune response against germ cells. Despite the increased numbers of leukocytes, macrophages, neutrophils and eosinophils detected in the testis interstitium of Ar-KO mice as compared to the control, their infiltration was not evident within the seminiferous tubules. Even though the BTB was compromised in these animals and an immune response was mounted against the developing germ cells, the knock down of the androgen receptor could be affecting many different androgen regulated genes and pathways in the SC. Hence, the resultant immune response might not just be attributable to the disrupted BTB.
The BTB being the sole criteria for testis immune privilege was discredited by several studies. To begin with, not all the germ cells with auto-immunogenic antigens are sequestered within the BTB, as shown by the presence of spermatogonia and preleptotene spermatocytes in the basal compartment (Yule et al., 1988) and yet, an immune response is not normally generated against these germ cells. Similarly, allo- or xeno-geneic tissue (skin fragments, islets or parathyroid tissue) transplanted into the testis interstitium (outside the BTB) enjoys prolonged survival (Barker and Billingham, 1977; Setchell, 1990; Selawry, 1994) as compared to tissue transplanted into non-immune privileged sites. Also, routine fine needle biopsies (causing local injury to the seminiferous epithelium) in humans does not lead to autoimmune orchitis (Mallidis and Baker, 1994). Moreover, in seasonal breeders, the BTB is cyclically disrupted during the nonbreeding period and the development of meiotic spermatocytes is possible even in the absence of a complete, impermeable BTB (Pelletier, 1986). Furthermore, the permeability of the SC barrier was altered in mice by knockout of the tight junction protein claudin 11 or treatment with a mutant occludin peptide. Despite the increased permeability of the barrier, an autoimmune reaction was not generated (Gow et al., 1999; Wong et al., 2007). Therefore, other local mechanisms or factors along with BTB are required to create a tolerogenic environment in the testis.
Physiological components of the testis (lower temperature and higher zinc concentration)
In mammals, the scrotal temperature is 2–8°C below the core body temperature. It was speculated that this could be important for testis immune privilege because exposure to low temperature (20°C) significantly decreases or sometimes eliminates both cellular and humoral immune responses in cold-blooded vertebrates (Cone and Marchalonis, 1972). To test this, parathyroid allografts were placed into scrotal testes, cryptorchid testes, subcutaneously in the ear (hypothermic) and in the renal subcapsular space (Head and Billingham, 1985). Grafts placed subcutaneously in the ear rejected even faster than the intrarenal grafts, whereas parathyroid allografts transplanted in cryptorchid testes enjoyed prolonged survival. Similarly, no adverse effects on islet allo- and xeno-graft survival when transplanted into cryptorchid testes suggests that temperature plays no major role in extending graft survival in the testis (Selawry and Whittington, 1984).
Zinc is a key component of some enzymes and is vital for many biological functions. Zinc plays a crucial role in the immune response. For instance, the administration of zinc at therapeutic levels resulted in selective suppression of allogeneic immune responses (Faber et al., 2004). The testis contains a high zinc concentration as compared to other organs, and therefore it was thought that high zinc levels or zinc dependent enzymes in the testis could be important in abolishing the immune reaction (Whitmore and Gittes, 1978). To test this criterion, parathyroid allografts were transplanted in testis and prostate. In comparison to the testis, grafted tissue did not survive in the prostate even though it contains the highest zinc concentration of all organs (Whitmore and Gittes, 1978), supporting the notion that a higher zinc concentration is not responsible for testicular immune privilege. Furthermore, islets, which contain a high zinc concentration (Jindal et al., 1992), are rejected when transplanted as allo- or xeno-grafts at other ectopic sites (e.g. kidney capsule).
Immunological components of the testis
To elicit an effective immune response, antigen presenting cells (macrophages and/or dendritic cells (DC)) are required to process and present antigen to T cells. It is now known that immune cells (antigen presenting cells (APC), T cells and NK cells) involved in mounting an effective immune response are present in the testis. However, a shortage of APC (DC and macrophages) was historically thought to be a reason behind testicular immune privilege. This was negated by showing that substantial numbers of Ia+/MHC-class II+ cells were present in the testis interstitium. Most of these cells were macrophages as Ia+ cells were also positive for esterase (Head and Billingham, 1985). It is now known that the majority of the immune cell population of the testis is comprised of macrophages (Hedger, 1997, 2002). In fact, rodent testicular macrophages exist in direct contact with Leydig cells and form specialized contact sites known as digitations (microvillus-like Leydig cell processes inserted within the coated pits of the macrophages; Hutson, 1992, 2006). Leydig cells and macrophages are functionally coupled, as depletion of one cell type affects the number and function of the other (Hedger, 2002; Hutson, 2006). Although testicular macrophages resemble traditional macrophages histologically, they have a reduced capacity to produce pro-inflammatory cytokines compared to macrophages from other tissues and exhibit immunosuppressive characteristics in vitro (Hedger, 2002). The importance of macrophages in testicular immune privilege was further supported by the fact that the ram testis, which contains very few resident macrophages, was unable to provide prolonged protection to parathyroid allografts (Maddocks and Setchell, 1988; Pollanen and Maddocks, 1988).
Testicular DC, on the other hand, have received very little attention despite their well-recognized reputation as antigen presenting cells that can activate immune responses and induce tolerance (Fijak and Meinhardt, 2006). Previously, cells that possess DC-like morphology were detected in the testis interstitium of rodents and humans (Haas et al., 1988; Derrick et al., 1993; Itoh et al., 1995; Hoek et al., 1997). However, the markers used to identify DC in these studies also cross-reacted with macrophages, therefore it was inconclusive whether DC were present in the testis. Thereafter, by employing DC specific markers (OX-62 and CD11c), it was shown that the normal rat testis interstitium contains DC. Recently, co-stimulatory molecules (CD80 and CD86) were also detected on DC in normal and experimental autoimmune orchitis rat testes, suggesting that local activation of T cells by DC is possible in the testis (Rival et al., 2006). Under normal conditions, an immune response is not initiated in the testis, suggesting testicular DC could be functionally immature and involved in immune privilege, but when exposed to inflammation they can overcome tolerance and become functionally mature and immunogenic (Fijak and Meinhardt, 2006). However, more thorough studies depicting the phenotype and role of DC in testis immune privilege need to be carried out to draw any strong conclusions.
T cells (CD4 and CD8) comprise ~15% of the total leukocyte population of the rodent testis interstitium (Hedger, 1997; Hedger and Meinhardt, 2000), while in humans, the data is controversial. Some studies reported the presence of T cells within the testis interstitium of normal males (Ritchie et al., 1984; Pollanen and Niemi, 1987), while others have reported that T cells were only detected in the testes of subfertile patients and not in normal males (El-Demiry et al., 1987). Similarly, a substantial population of NK cells has been detected in the rat testis (Tompkins et al., 1998), but their presence in the human testis is debatable (Pollanen and Niemi, 1987). Under normal conditions, B cells are rarely detected in rat and human testes (Pollanen and Niemi, 1987; Wang et al., 1994). Rodent testes also contain significant numbers of immunoregulatory cells, mainly NKT cells and CD4+CD25+ regulatory T cells (T regs), although their role in maintaining testis immune privilege needs further study (Hedger, 2011a).
Contrary to previous belief, it is now known that immune cells and lymphatics are present in the testis and are capable of evoking an effective immune response. For instance, despite the immune privileged status of the testis, there are still incidences of bacterial and viral infections in the testes (Masarani et al., 2006; Bhushan et al., 2009), but in most cases the infection and inflammation are cleared-up, indicating that an efficient immune response can be mounted. Rarely, these infections can lead to autoimmune orchitis (Masarani et al., 2006; Bhushan et al., 2009). Toll-like receptors (TLRs) recognize specific bacterial and viral components and activate a downstream signaling cascade, which ultimately results in the induction of inflammation. Nishimura and Naito (2005) were the first to demonstrate that TLRs are expressed in the human testis. Subsequent studies have shown TLR expression in mouse and rat testes. TLRs (most commonly 2, 3 and 4) are expressed on somatic (SC, Leydig cells, testicular macrophages) and germ cells of the testis (Palladino et al., 2007; Bhushan et al., 2008; Wang et al., 2012). When the appropriate stimulus is provided, these TLRs are capable of being activated, inducing inflammation and oxidative stress (reviewed in Hedger, 2011b). Interestingly, some recent reports suggest that SC and Leydig cells can negatively regulate TLRs by Tyro3, Axl and Mer (TAM) receptors and hence, prevent prolonged inflammation and damage to the testis (Sun et al., 2010; Shang et al., 2011).
Collectively, these data suggest that testicular immune privilege is not permanent and the balance can be tipped, leading to autoimmune orchitis or destruction of the allo- or xeno-grafts transplanted in the testis (Fig. 2). The local factors and cellular components of the testis determine the immune response. For instance, extended survival of intratesticularly transplanted islet allografts was a result of eliminating memory CD8 T cells by apoptosis and inducing CD4+CD25+ T regs in the testis (Dai et al., 2005; Nasr et al., 2005). Therefore, it is imperative to examine the contribution of factors expressed or secreted by different testicular cells in order to better understand the immune privileged status of the testis.
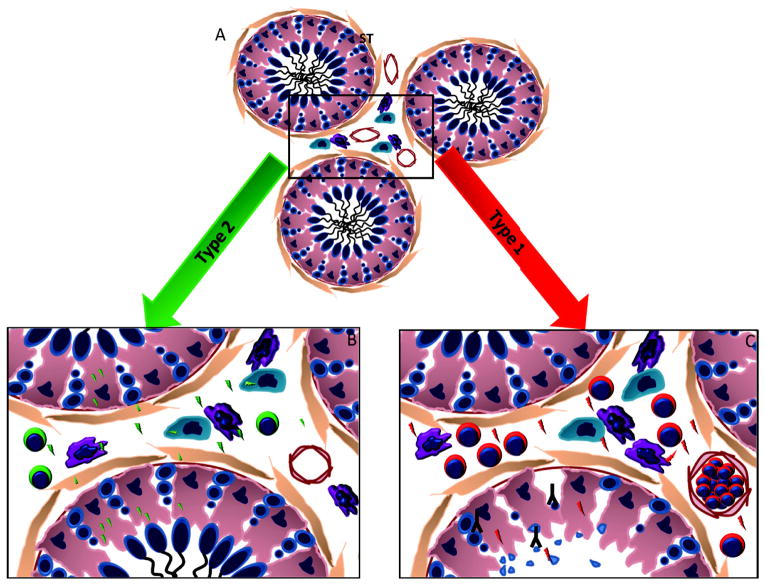
Immune environment in the testis. A) Testis under normal circumstances. The seminiferous tubules (ST) with intact germ cells and interstitium with the Leydig cells (jade), macrophages and dentritic cells (purple), depicts a balanced state in the testis. B) Type 2 immune response in the testis. In the case of a bacterial/viral infection or a transplant in the testis which does not disrupt normal testicular function, a type 2 immune response is initiated. In this type of response, most of the cells in the testis secrete immunoregulatory factors (green bolts) which favor the presence of regulatory T cells and the testis maintains a milieu supporting germ cell and transplant survival. C) Type 1 immune response in the testis. Rarely, when the bacterial/viral infections or transplanted tissue outbalance the normal environment of the testis, a type 1 immune response can be initiated that results in the secretion of inflammatory factors by the different cells in the testis (red bolts), resulting in the recruitment of cytotoxic T cells. This further increases inflammation in the testis which leads to a disruption of the BTB/SC tight junctions, binding of antibody (inverted Y) within the ST and eventually a loss of germ cell populations via apoptosis.
Somatic and germ cell role in testis immune privilege
Testicular immune privilege is an active phenomenon where immunomodulatory factors expressed or secreted locally by different testicular cells control the overall immune response. The presence of immunosuppressive activity in testicular fluid has been evidenced by several studies. For example, fluid collected from the interstitium and seminiferous tubule segments II-VIII exhibited maximum immunosuppressive activity as shown by a reduced proliferation of concanavalin A stimulated peripheral blood lymphocytes (Pollanen et al., 1988, 1992). Furthermore, culture media collected from isolated testicular cells (e.g. SC) inhibited activated T and B cell proliferation in vitro (Wyatt et al., 1988; Selawry et al., 1991; De Cesaris et al., 1992). Recently, Foulds et al. (2008) characterized specific lyso-glycerophosphocholines as T cell inhibitory molecules of gonadal fluids (bovine ovarian follicular fluid and rat testicular interstitial fluid). These molecules suppressed T cell proliferation and inhibited T cell IL-2 production, thus inducing T cell apoptosis in vitro.
Prostaglandins (PG), particularly PGE2 and PGE2α, predominate in the adult testis. The rate limiting enzyme for prostaglandin production, prostaglandin synthase (PTGS2), is constitutively produced by somatic (Leydig and SC) and spermatogenic cells. Testicular macrophages constitutively produce low levels of PTGS2 and PGE2, but their expression can be increased by inflammatory stimuli. PGE2 acts as an inhibitor of the type 1 response (pro-inflammatory environment) by suppressing T cell proliferation (Goodwin et al., 1978) and NK cell function (Spaggiari et al., 2008), skewing the response toward the type 2 response (anti-inflammatory and protective environment; Snijdewint et al., 1993) and modulating the immune properties of antigen presenting cells (Baratelli et al., 2005). Recently, it was shown that PGE2 induces indoleamine-pyrrole 2,3-dioxygenase (IDO) mRNA expression in DC (Braun et al., 2005), T reg specific transcription factor (FOXP3) in CD4+CD25-T cells and immunosuppressive function of CD4+CD25+ T regs in vitro (Baratelli et al., 2005).
Furthermore, galectin-1, a highly conserved β-galactosidase-binding protein that can induce apoptosis of lymphocytes (T and B cells), skew the immune response towards the type 2 response, inhibit pro-inflammatory cytokine secretion and induce the production of CD4+CD25+ T regs (Rabinovich and Toscano, 2009), was detected in SC, peritubular myoid cells and late spermatids of rat and human testes (Wollina et al., 1999; Dettin et al., 2003; Chui et al., 2011). Additionally, testicular cells produce or express anti-inflammatory cytokines, complement inhibitors, apoptosis inhibitors and other immunoregulatory factors (Hedger and Meinhardt, 2003; Guazzone et al., 2009; Meinhardt and Hedger, 2011), demonstrating the importance of exploring the role of testicular cells in immune privilege (Fig. 2).
Germ cells
Germ cells are immunogenic, yet an immune response is normally not generated against them. Additionally, culture media collected from germ cells inhibits lymphocyte proliferation in vitro (Hurtenbach and Shearer, 1982). It has also been demonstrated that syngeneic germ cells injected into rodents had immunosuppressive effects, as shown by reduced NK cell activity and a cytotoxic T lymphocyte response (Hurtenbach and Shearer, 1982). However, in this study the inoculum used for injection contained other somatic cells (potentially SC) as contaminants; thus it is difficult to conclude that germ cells possess immunosuppressive properties, as the contribution from other cells towards this immune suppression cannot be ruled out. Furthermore, to evaluate the role of germ cells or spermatogenesis in testis immune privilege, islets and parathyroid cells were transplanted in the cryptorchid or irradiated testis, where abrogation of spermatogenesis and a loss of germ cells occured (Selawry and Whittington, 1984; Head and Billingham, 1985; Whitmore et al., 1985). Grafted tissue experienced prolonged survival, even in the absence of spermatogenesis or germ cells. Germ cell depletion by cold testicular ischemia also had no detrimental effect on transplanted tissue survival (Cameron et al., 1990). Collectively, these data suggest that spermatogenesis and germ cells are not the key components to testicular immune privilege.
Leydig cells
Steroid (testosterone and progesterone) and nonsteroid (activin and inhibin) products of Leydig cells have been shown to inhibit local immune responses (Born and Wekerle, 1982; Grossman, 1985; Head and Billingham, 1985; Hedger, 1989). Leydig cell conditioned media inhibits the proliferation of adult rat lymphocytes (Hedger et al., 1990), suggesting that Leydig cells contribute to testicular immune privilege. Nevertheless, Selawry and Whittington (1988) showed that the administration of leuprolide (GnRH analog, inhibits testosterone production) had no effect on the prolonged survival of islet allografts despite a significant reduction in serum and intratesticular testosterone levels. Later, the same group reported that killing Leydig cells using ethane dimethanesulphonate and preventing their regeneration by β-estradiol treatment (given at the time of transplantation) had no effect on functional islet allograft survival (Cameron et al., 1990). It is possible, although it was not examined, that the macrophage numbers were also reduced in this study since the Leydig cell and macrophage populations are dependent upon each other (Hutson, 2006).
Although the above mentioned studies indicate that Leydig cells and androgens are not required for testis immune privilege, Head and Billingham (1985) showed that estrogen (to decrease testosterone) administered prior to transplantation resulted in graft rejection, while estrogen given at the time of transplantation had no adverse effect on graft survival. The incongruity arising between these two studies could be explained by differences in the timing of estrogen treatment (i.e. prior to or at the time of transplant), indicating that the status of local hormone synthesis in the testis prior to graft transplantation could be important in supporting or rejecting the transplanted graft. Selawry and Whittington (1988) only measured testosterone levels 30 days after treatment, so it is hard to conclude the level and effect of testosterone at the time of transplantation. As discussed previously, SC specific deletion of the androgen receptor resulted in increased permeability of the BTB, generation of germ cell antigen specific antibodies and increased infiltration of leukocytes in the interstitium and thus, compromised testicular immune privilege (Meng et al., 2011). Furthermore, testosterone supplementation exerts a protective effect against experimental autoimmune orchitis by inhibiting the synthesis of Th1 specific pro-inflammatory cytokines and macrophages and CD4 T cell infiltration, while simultaneously inducing CD4+CD25+ T regs both in vivo and in vitro (Fijak et al., 2011).
Peritubular myoid cells
The involvement of peritubular myoid cells in testicular immune privilege has not been studied extensively and needs further investigation. Interaction between peritubular myoid cells and SC up-regulates the expression and/or secretion of several factors by SC (Skinner and Fritz, 1985a, b). For example, the co-culture of peritubular myoid cells and SC supports the basal-apical orientation of SC and significantly increases clusterin (complement inhibitor) secretion by SC (Zwain et al., 1993). Peritubular myoid cells also express several immunomodulatory factors (e.g. TGF-β, B7-H1; Fijak and Meinhardt, 2006) and can provide systemic tolerance as splenocytes from mice injected (i.v.) with peritubular myoid cells showed a reduced response to allogenic or xenogenic cells as compared to the controls (Shamekh et al., 2006), suggesting these cells might contribute to testicular immune privilege. Further evidence that myoid cells could be playing a role in testis immune privilege comes from SC transplantation studies (discussed in next section). Immune privileged SC transplanted outside the testis survive as allo- or xeno-grafts. However, myoid cells are a common contaminant of isolated SC populations. Increasing the SC purity to 100% led to reduced graft protection and a short course of cyclosporine was required in order to increase graft survival (Selawry and Cameron, 1993), suggesting that myoid cells could be involved in creating an immune privileged site outside of the testis.
Sertoli cells
Evidence that SC are important for testis immune privilege initially came from SC co-transplantation studies where SC protected non-testicular cellular grafts when transplanted allo- or xeno-geneically outside the testis. Selawry and Cameron (1993) were the first to demonstrate that SC survive when transplanted as allografts and protect co-transplanted islets. Later, several studies verified the immune privilege status of SC and their ability to provide protection to co-transplanted allogeneic and xenogeneic islets, xenogeneic adrenal chromaffin cells, xenogeneic hepatocytes, xenogeneic neurons, allogeneic or xenogeneic skin and heart grafts (reviewed in Mital et al., 2010). Thus, SC have a critical role in testis immune privilege as they can mimic the immune privilege environment outside of the testis. As mentioned earlier, peritubular myoid cells could be providing an additive effect to this immune protection by secreting immunosuppressive factors or enhancing the production of these factors by SC.
SC secrete and express several factors that inhibit innate (complement inhibitors), humoral and cell-mediated immune responses (B and T cell proliferation inhibitors, apoptosis inhibitors). These factors likely contribute to their survival and the protection of co-grafted cells when transplanted across immunological barriers (reviewed in Mital et al., 2010). Besides inhibiting these main pathways of graft rejection, SC express immunomodulatory factors (TGF-β and IDO) and chemokines (CCL27), which could lead to either the production or recruitment of T regs or modulation of the phenotype of immune cells at the graft site, thus protecting the grafted cells from immune rejection. In other words, an intricate interplay between several immunomodulatory factors expressed by SC to prevent and modify the innate and adaptive immune responses could be responsible for providing an ideal environment for germ cell protection in the testis and the protection of co-transplanted cells at ectopic sites.
Conclusions
Testicular immune privilege is a complex phenomenon which is the result of the combined contribution of testis cellular components rather than a single cell type acting alone (Fig. 2). The immunoregulatory molecules expressed and/or produced by testicular cells have the potential to deviate the pro-inflammatory and destructive immune response (type 1) to immunoprotective (type 2) by inducing tolerogenic macrophages, DC and T regs, and therefore could be the key mechanism/factor(s) making the testis an immune privileged site.
Acknowledgments
This work was supported in part by NIH grant HD067400 (to JMD) from the Eunice Kennedy Shriver NICHD.
References
- Akimaru K, Stuhlmiller GM, Seigler HF. Allotransplantation of insulinoma into the testis of diabetic rats. Transplantation. 1981;32:227–232. [Abstract] [Google Scholar]
- Bajic P, Selman SH, Rees MA. Voronoff to virion: 1920s testis transplantation and AIDS. Xenotransplantation. 2012;19:337–341. [Abstract] [Google Scholar]
- Baratelli F, Krysan K, Heuze-Vourc’h N, Zhu L, Escuadro B, Sharma S, Reckamp K, Dohadwala M, Dubinett SM. PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. J Leukoc Biol. 2005;78:555–564. [Abstract] [Google Scholar]
- Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [Abstract] [Google Scholar]
- Bhushan S, Tchatalbachev S, Klug J, Fijak M, Pineau C, Chakraborty T, Meinhardt A. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independet signalling pathways in rat testicular cells. J Immunol. 2008;180:5537–5547. [Abstract] [Google Scholar]
- Bhushan S, Schuppe HC, Fijak M, Meinhardt A. Testicular infection: microorganisms, clinical implications and host-pathogen interaction. J Reprod Immunol. 2009;83:164–167. [Abstract] [Google Scholar]
- Bobzien B, Yasunami Y, Majercik M, Lacy PE, Davie JM. Intratesticular transplants of islet xenografts (rat to mouse) Diabetes. 1983;32:213–216. [Abstract] [Google Scholar]
- Born W, Wekerle H. Leydig cells nonspecifically suppress lymphoproliferation in vitro: implications for the testis as an immunologically privileged site. Am J Reprod Immunol. 1982;2:291–295. [Abstract] [Google Scholar]
- Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. [Europe PMC free article] [Abstract] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. [Europe PMC free article] [Abstract] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. [Europe PMC free article] [Abstract] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. [Europe PMC free article] [Abstract] [Google Scholar]
- Cameron DF, Whittington K, Schultz RE, Selawry HP. Successful islet/abdominal testis transplantation does not require Leydig cells. Transplantation. 1990;50:649–653. [Abstract] [Google Scholar]
- Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh AL, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20:619–635. [Europe PMC free article] [Abstract] [Google Scholar]
- Cone RE, Marchalonis JJ. Cellular and humoral aspects of the influence of environmental temperature on the immune response of poikilothermic vertebrates. J Immunol. 1972;108:952–957. [Abstract] [Google Scholar]
- Dai Z, Nasr IW, Reel M, Deng S, Diggs L, Larsen CP, Rothstein DM, Lakkis FG. Impaired recall of CD8 memory T cells in immunologically privileged tissue. J Immunol. 2005;174:1165–1170. [Abstract] [Google Scholar]
- De Cesaris P, Filippini A, Cervelli C, Riccioli A, Muci S, Starace G, Stefanini M, Ziparo E. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: biological effects on lymphocytes. Biochem Biophys Res. 1992;186:1639–1646. [Abstract] [Google Scholar]
- Derrick EK, Barker JN, Khan A, Price ML, Macdonald DM. The tissue distribution of factor XIIIa positive cells. Histopathology. 1993;22:157–162. [Abstract] [Google Scholar]
- Dettin L, Rubinstein N, Aoki A, Rabinovich GA, Maldonado CA. Regulated expression and ultrastructural localization of galectin-1, a proapoptotic beta-galactoside-binding lectin, during spermatogenesis in rat testis. Biol Reprod. 2003;68:51–59. [Abstract] [Google Scholar]
- Dib-Kuri A, Revilla A, Chavex-Peon F. Successful rat parathyroid allografts and xenografts to the testis without immunosuppression. In: Schlesinger M, Billingham R, editors. Transplantation Today. New York, NY: Grune and Stratton; 1975. pp. 753–756. [Google Scholar]
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. [Abstract] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973;175:639–656. [Abstract] [Google Scholar]
- El-Demiry MI, Hargreave TB, Busuttil A, Elton R, James K, Chisholm GD. Immunocompetent cells in human testis in health and disease. Fertil Steril. 1987;48:470–479. [Abstract] [Google Scholar]
- Engeset A. The route of peripheral lymph to the blood stream; an x-ray study of the barrier theory. J Anat. 1959;93:96–100. [Europe PMC free article] [Abstract] [Google Scholar]
- Faber C, Gabriel P, Ibs KH, Rink L. Zinc in pharmacological doses suppresses allogeneic reaction without affecting the antigenic response. Bone Marrow Transplant. 2004;33:1241–1246. [Abstract] [Google Scholar]
- Fawcett DW, Leak LV, Heidger PM., Jr Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–122. [Abstract] [Google Scholar]
- Fawcett DW, Neaves WB, Flores MN. Comparative observations on intertubular lymphatics and the organization of the interstitial tissue of the mammalian testis. Biol Reprod. 1973;9:500–532. [Abstract] [Google Scholar]
- Ferguson J, Scothorne RJ. Extended survival of pancreatic islet allografts in the testis of guinea-pigs. J Anat. 1977;124:1–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. [Abstract] [Google Scholar]
- Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–5172. [Abstract] [Google Scholar]
- Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372–381. [Abstract] [Google Scholar]
- Foulds LM, Boysen RI, Crane M, Yang Y, Muir JA, Smith AI, de Kretser DM, Hearn MT, Hedger MP. Molecular identification of lyso-glycerophosphocholines as endogenous immunosuppressives in bovine and rat gonadal fluids. Biol Reprod. 2008;79:525–536. [Abstract] [Google Scholar]
- Goodwin JS, Messner RP, Peake GT. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978;62:753–760. [Europe PMC free article] [Abstract] [Google Scholar]
- Gores PF, Hayes DH, Copeland MJ, Korbutt GS, Halberstadt C, Kirkpatrick SA, Rajotte RV. Long-term survival of intratesticular porcine islets in nonimmunosuppressed beagles. Transplantation. 2003;75:613–618. [Abstract] [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. [Abstract] [Google Scholar]
- Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–261. [Abstract] [Google Scholar]
- Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Micros Res Tech. 2009;72:620–628. [Abstract] [Google Scholar]
- Haas GG, Jr, D’Cruz OJ, De Bault LE. Distribution of human leukocyte antigen-ABC and -D/DR antigens in the unfixed human testis. Am J Reprod Immunol Microbiol. 1988;8:47–51. [Abstract] [Google Scholar]
- Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983a;36:423–431. [Abstract] [Google Scholar]
- Head JR, Neaves WB, Billingham RE. Reconsideration of the lymphatic drainage of the rat testis. Transplantation. 1983b;35:91–95. [Abstract] [Google Scholar]
- Head JR, Billingham RE. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation. 1985;40:269–275. [Abstract] [Google Scholar]
- Hedger MP. The testis: an ‘immunologically suppressed’ tissue? Reprod Fertil Dev. 1989;1:75–79. [Abstract] [Google Scholar]
- Hedger MP, Qin JX, Robertson DM, de Kretser DM. Intragonadal regulation of immune system functions. Reprod Fertil Dev. 1990;2:263–280. [Abstract] [Google Scholar]
- Hedger MP. Testicular leukocytes: what are they doing? Rev Reprod. 1997;2:38–47. [Abstract] [Google Scholar]
- Hedger MP, Meinhardt A. Local regulation of T cell numbers and lymphocyte-inhibiting activity in the interstitial tissue of the adult rat testis. J Reprod Immunol. 2000;48:69–80. [Abstract] [Google Scholar]
- Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol. 2002;57:19–34. [Abstract] [Google Scholar]
- Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58:1–26. [Abstract] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. 2011a;32:625–640. [Europe PMC free article] [Abstract] [Google Scholar]
- Hedger MP. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation--a perspective. J Reprod Immunol. 2011b;88:130–141. [Europe PMC free article] [Abstract] [Google Scholar]
- Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, Hutton K, Hope S, Hill JR. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81:898–905. [Abstract] [Google Scholar]
- Hill RT, Gardner WU. Function of pituitary grafts in mice. Proc Soc Exp Biol Med. 1936;34:78–79. [Google Scholar]
- Hoek A, Allaerts W, Leenen PJ, Schoemaker J, Drexhage HA. Dendritic cells and macrophages in the pituitary and the gonads. Evidence for their role in the fine regulation of the reproductive endocrine response. Eur J Endocrinol. 1997;136:8–24. [Abstract] [Google Scholar]
- Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. [Abstract] [Google Scholar]
- Honaramooz A, Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int. 2010:657860. pii. [Europe PMC free article] [Abstract] [Google Scholar]
- Hurtenbach U, Shearer GM. Germ cell-induced immune suppression in mice. Effect of inoculation of syngeneic spermatozoa on cell-mediated immune responses. J Exp Med. 1982;155:1719–1729. [Europe PMC free article] [Abstract] [Google Scholar]
- Hutson JC. Development of cytoplasmic digitations between Leydig cells and testicular macrophages of the rat. Cell Tissue Res. 1992;267:385–389. [Abstract] [Google Scholar]
- Hutson JC. Physiologic interactions between macrophages and Leydig cells. Exp Biol Med (Maywood) 2006;231:1–7. [Abstract] [Google Scholar]
- Itoh M, De Rooij DG, Jansen A, Drexhage HA. Phenotypical heterogeneity of testicular macrophages/dendritic cells in normal adult mice: an immunohistochemical study. J Reprod Immunol. 1995;28:217–232. [Abstract] [Google Scholar]
- Jindal RM, Taylor RP, Gray DW, Esmeraldo R, Morris PJ. A new method for quantification of islets by measurement of zinc content. Diabetes. 1992;41:1056–1062. [Abstract] [Google Scholar]
- Jung JG, Lee YM, Kim JN, Kim TM, Shin JH, Kim TH, Lim JM, Han JY. The reversible developmental unipotency of germ cells in chicken. Reproduction. 2010;139:113–119. [Abstract] [Google Scholar]
- Lacerda SM, Batlouni SR, Assis LH, Resende FM, Campos-Silva SM, Campos-Silva R, Segatelli TM, Franca LR. Germ cell transplantation in tilapia (Oreochromis niloticus) Cybium. 2008;32:115–118. [Google Scholar]
- Lacerda SM, Batlouni SR, Costa GM, Segatelli TM, Quirino BR, Queiroz BM, Kalapothakis E, Franca LR. A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PloS one. 2010;5:e10740. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee YM, Jung JG, Kim JN, Park TS, Kim TM, Shin SS, Kang DK, Lim JM, Han JY. A testis-mediated germline chimera production based on transfer of chicken testicular cells directly into heterologous testes. Biol Reprod. 2006;75:380–386. [Abstract] [Google Scholar]
- Maddocks S, Setchell BP. The rejection of thyroid allografts in the ovine testis. Immunol Cell Biol. 1988;66(pt 1):1–8. [Abstract] [Google Scholar]
- Maddocks S, Setchell BP. Recent evidence for immune privilege in the testis. J Reprod Immunol. 1990;18:9–18. [Abstract] [Google Scholar]
- Maeda T, Goto A, Kobayashi D, Tamai I. Transport of organic cations across the blood-testis barrier. Mol Pharm. 2007;4:600–607. [Abstract] [Google Scholar]
- Majhi SK, Hattori RS, Yokota M, Watanabe S, Strussmann CA. Germ cell transplantation using sexually competent fish: an approach for rapid propagation of endangered and valuable germlines. PloS one. 2009;4:e6132. [Europe PMC free article] [Abstract] [Google Scholar]
- Mallidis C, Baker HW. Fine needle tissue aspiration biopsy of the testis. Fertil Steril. 1994;61:367–375. [Abstract] [Google Scholar]
- Masarani M, Wazait H, Dinneen M. Mumps orchitis. J R Soc Med. 2006;99:573–575. [Europe PMC free article] [Abstract] [Google Scholar]
- Medawar PB, Russell PS. Adrenal homografts in mice, with special reference to immunological adrenalectomy. Immunology. 1958;1:1–12. [Abstract] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cellular Endocrinol. 2011;335:60–68. [Abstract] [Google Scholar]
- Meng J, Greenlee AR, Taub CJ, Braun RE. Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol Reprod. 2011;85:254–260. [Europe PMC free article] [Abstract] [Google Scholar]
- Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:95–504. [Abstract] [Google Scholar]
- Naji A, Barker CF. The influence of histocompatibility and transplant site on parathyroid allograft survival. J Surg Res. 1976;20:261–267. [Abstract] [Google Scholar]
- Nasr IW, Wang Y, Gao G, Deng S, Diggs L, Rothstein DM, Tellides G, Lakkis FG, Dai Z. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol. 2005;174:6161–6168. [Abstract] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. [Abstract] [Google Scholar]
- O’Rand MG, Romrell LJ. Appearance of cell surface auto- and isoantigens during spermatogenesis in the rabbit. Dev Biol. 1977;55:347–358. [Abstract] [Google Scholar]
- Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male reproductive tract. Biol Reprod. 2007;76:958–964. [Abstract] [Google Scholar]
- Pelletier RM. Cyclic formation and decay of the blood-testis barrier in the mink (Mustela vison), a seasonal breeder. Am J Anat. 1986;175:91–117. [Abstract] [Google Scholar]
- Pollanen P, Niemi M. Immunohistochemical identification of macrophages, lymphoid cells and HLA antigens in the human testis. Int J Androl. 1987;10:37–42. [Abstract] [Google Scholar]
- Pollanen P, Maddocks S. Macrophages, lymphocytes and MHC II antigen in the ram and the rat testis. J Reprod Fertil. 1988;82:437–445. [Abstract] [Google Scholar]
- Pollanen P, Soder O, Uksila J. Testicular immunosuppressive protein. J Reprod Immunol. 1988;14:125–138. [Abstract] [Google Scholar]
- Pollanen P, von Euler M, Sainio-Pollanen S, Jahnukainen K, Hakovirta H, Soder O, Parvinen M. Immunosuppressive activity in the rat seminiferous tubules. J Reprod Immunol. 1992;22:117–126. [Abstract] [Google Scholar]
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. [Abstract] [Google Scholar]
- Reynaud G. Capacités reproductrices et descendance de poulets ayant subi un transfert de cellules germinales primordiales durant la vie embryonnarie. Wilhelm Roux’s Arch Dev Biol. 1976;179:85–110. [Abstract] [Google Scholar]
- Ritchie AW, Hargreave TB, James K, Chisholm GD. Intra-epithelial lymphocytes in the normal epididymis. A mechanism for tolerance to sperm auto-antigens? Br J Urol. 1984;56:79–83. [Abstract] [Google Scholar]
- Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A, Fijak M. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006;324:311–318. [Abstract] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Clearwater FL: Cache River Press; 1990. p. 286. [Google Scholar]
- Sand K. Experiments on the internal secretion of the sexual glands, especially on experimental hermaphroditism. J Physiol. 1919;53:257–263. [Abstract] [Google Scholar]
- Selawry HP, Whittington K. Extended allograft survival of islets grafted into intra-abdominally placed testis. Diabetes. 1984;33:405–406. [Abstract] [Google Scholar]
- Selawry HP, Fajaco R, Whittington K. Intratesticular islet allografts in the spontaneously diabetic BB/W rat. Diabetes. 1985;34:1019–1024. [Abstract] [Google Scholar]
- Selawry HP, Whittington KB. Prolonged intratesticular islet allograft survival is not dependent on local steroidogenesis. Horm Metab Res. 1988;20:562–565. [Abstract] [Google Scholar]
- Selawry HP, Kotb M, Herrod HG, Lu ZN. Production of a factor, or factors, suppressing IL-2 production and T cell proliferation by Sertoli cell-enriched preparations. A potential role for islet transplantation in an immunologically privileged site. Transplantation. 1991;52:846–850. [Abstract] [Google Scholar]
- Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–129. [Abstract] [Google Scholar]
- Selawry HP. Islet transplantation to immuneprivilged sites. In: Lanza RP, Chick WL, editors. Pancreatic islet transplantation: immunomodulation of pancreatic islets. Austin, TX: Landes/CRC Press; 1994. pp. 75–86. [Google Scholar]
- Setchell BP. The testis and tissue transplantation: historical aspects. J Reprod Immunol. 1990;18:1–8. [Abstract] [Google Scholar]
- Setchell BP, Granholm T, Ritzen EM. Failure of thyroid allografts to function in the testes of cynomolgous monkeys. J Reprod Immunol. 1995;28:75–80. [Abstract] [Google Scholar]
- Shamekh R, El-Badri NS, Saporta S, Pascual C, Sanberg PR, Cameron DF. Sertoli cells induce systemic donor-specific tolerance in xenogenic transplantation model. Cell Transplant. 2006;15:45–53. [Abstract] [Google Scholar]
- Shang T, Zhang X, Wang T, Sun B, Deng T, Han D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology. 2011;152:2827–2836. [Abstract] [Google Scholar]
- Silva RC, Costa GM, Lacerda SM, Batlouni SR, Soares JM, Avelar GF, Bottger KB, Silva SF, Jr, Nogueira MS, Andrade LM, Franca LR. Germ cell transplantation in felids: a potential approach to preserving endangered species. J Androl. 2012;33:264–276. [Abstract] [Google Scholar]
- Skinner MK, Fritz IB. Androgen stimulation of Sertoli cell function is enhanced by peritubular cells. Mol Cellular Endocrinol. 1985a;40:115–122. [Abstract] [Google Scholar]
- Skinner MK, Fritz IB. Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell functions. Proc Natl Acad Sci USA. 1985b;82:114–118. [Europe PMC free article] [Abstract] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [Abstract] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. [Abstract] [Google Scholar]
- Stanley LL. Testicular substance implantation: comments on some six thousand implantations. Cal West Med. 1931;35:411–415. [Europe PMC free article] [Abstract] [Google Scholar]
- Streilein JW. Unraveling immune privilege. Science. 1995;270:1158–1159. [Abstract] [Google Scholar]
- Sun B, Qi N, Shang T, Wu H, Deng T, Han D. Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010;151:2886–2897. [Abstract] [Google Scholar]
- Tompkins AB, Hutchinson P, de Kretser DM, Hedger MP. Characterization of lymphocytes in the adult rat testis by flow cytometry: effects of activin and transforming growth factor beta on lymphocyte subsets in vitro. Biol Reprod. 1998;58:943–951. [Abstract] [Google Scholar]
- Trefil P, Micakova A, Mucksova J, Hejnar J, Poplstein M, Bakst MR, Kalina J, Brillard JP. Restoration of spermatogenesis and male fertility by transplantation of dispersed testicular cells in the chicken. Biol Reprod. 2006;75:575–581. [Abstract] [Google Scholar]
- Trefil P, Bakst MR, Yan H, Hejnar J, Kalina J, Mucksova J. Restoration of spermatogenesis after transplantation of c-Kit positive testicular cells in the fowl. Theriogenology. 2010;74:1670–1676. [Abstract] [Google Scholar]
- Tung PS, Fritz IB. Specific surface antigens on rat pachytene spermatocytes and successive classes of germinal cells. Dev Biol. 1978;64:297–315. [Abstract] [Google Scholar]
- Wang J, Wreford NG, Lan HY, Atkins R, Hedger MP. Leukocyte populations of the adult rat testis following removal of the Leydig cells by treatment with ethane dimethane sulfonate and subcutaneous testosterone implants. Biol Reprod. 1994;51:551–561. [Abstract] [Google Scholar]
- Wang T, Zhang X, Chen Q, Deng T, Zhang Y, Li N, Shang T, Chen Y, Han D. Toll-like receptor 3-initiated antiviral responses in mouse male germ cells in vitro. Biol Reprod. 2012;86:106. [Abstract] [Google Scholar]
- Whitmore WF, Gittes RF. Studies on the prostate and testis as immunologically privileged sites. Cancer Treat Rep. 1977;61:217–222. [Abstract] [Google Scholar]
- Whitmore WF, Gittes RF. Intratesticular grafts: The testis as an exceptional immunologically privileged site. Trans Am Assoc Genitourin Surg. 1978;70:76–80. [Abstract] [Google Scholar]
- Whitmore WF, 3rd, Gittes RF. Afferent lymphatics: their importance to immunologically privileged sites. Surg Forum. 1975;26:338–340. [Abstract] [Google Scholar]
- Whitmore WF, 3rd, Karsh L, Gittes RF. The role of germinal epithelium and spermatogenesis in the privileged survival of intratesticular grafts. J Urol. 1985;134:782–786. [Abstract] [Google Scholar]
- Wollina U, Schreiber G, Gornig M, Feldrappe S, Burchert M, Gabius HJ. Sertoli cell expression of galectin-1 and -3 and accessible binding sites in normal human testis and Sertoli cell only-syndrome. Histol Histopathol. 1999;14:779–784. [Abstract] [Google Scholar]
- Wong CH, Mruk DD, Lee WM, Cheng CY. Targeted and reversible disruption of the blood-testis barrier by an FSH mutant-occludin peptide conjugate. FASEB J. 2007;21:438–448. [Europe PMC free article] [Abstract] [Google Scholar]
- Wyatt CR, Law L, Magnuson JA, Griswold MD, Magnuson NS. Suppression of lymphocyte proliferation by proteins secreted by cultured Sertoli cells. J Reprod Immunol. 1988;14:27–40. [Abstract] [Google Scholar]
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. [Abstract] [Google Scholar]
- Zwain IH, Grima J, Stahler MS, Saso L, Cailleau J, Verhoeven G, Bardin CW, Cheng CY. Regulation of Sertoli cell alpha 2-macroglobulin and clusterin (SGP-2) secretion by peritubular myoid cells. Biol Reprod. 1993;48:180–187. [Abstract] [Google Scholar]
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113997046
Article citations
Temporal maturation of Sertoli cells during the establishment of the cycle of the seminiferous epithelium†.
Biol Reprod, 111(4):959-974, 01 Oct 2024
Cited by: 0 articles | PMID: 39077996
9-cis-retinoic acid signaling in Sertoli cells regulates their immunomodulatory function to control lymphocyte physiology and Treg differentiation.
Reprod Biol Endocrinol, 22(1):75, 26 Jun 2024
Cited by: 1 article | PMID: 38926848
Impact of endocrine disrupting chemicals and pharmaceuticals on Sertoli cell development and functions.
Front Endocrinol (Lausanne), 14:1095894, 30 Jan 2023
Cited by: 5 articles | PMID: 36793282 | PMCID: PMC9922725
Review Free full text in Europe PMC
Complementing Testicular Immune Regulation: The Relationship between Sertoli Cells, Complement, and the Immune Response.
Int J Mol Sci, 24(4):3371, 08 Feb 2023
Cited by: 4 articles | PMID: 36834786 | PMCID: PMC9965741
Review Free full text in Europe PMC
Bibliometric and visual analysis of blood-testis barrier research.
Front Pharmacol, 13:969257, 22 Aug 2022
Cited by: 3 articles | PMID: 36071829 | PMCID: PMC9441755
Go to all (31) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An overview of a Sertoli cell transplantation model to study testis morphogenesis and the role of the Sertoli cells in immune privilege.
Environ Epigenet, 3(3):dvx012, 01 Jul 2017
Cited by: 9 articles | PMID: 29492314 | PMCID: PMC5804552
Review Free full text in Europe PMC
Structural, cellular and molecular aspects of immune privilege in the testis.
Front Immunol, 3:152, 11 Jun 2012
Cited by: 106 articles | PMID: 22701457 | PMCID: PMC3371599
Somatic-Immune Cells Crosstalk In-The-Making of Testicular Immune Privilege.
Reprod Sci, 29(10):2707-2718, 27 Sep 2021
Cited by: 5 articles | PMID: 34580844
Review
Sertoli cells--immunological sentinels of spermatogenesis.
Semin Cell Dev Biol, 30:36-44, 03 Mar 2014
Cited by: 109 articles | PMID: 24603046 | PMCID: PMC4043859
Review Free full text in Europe PMC



