Abstract
Free full text

Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies.
Abstract
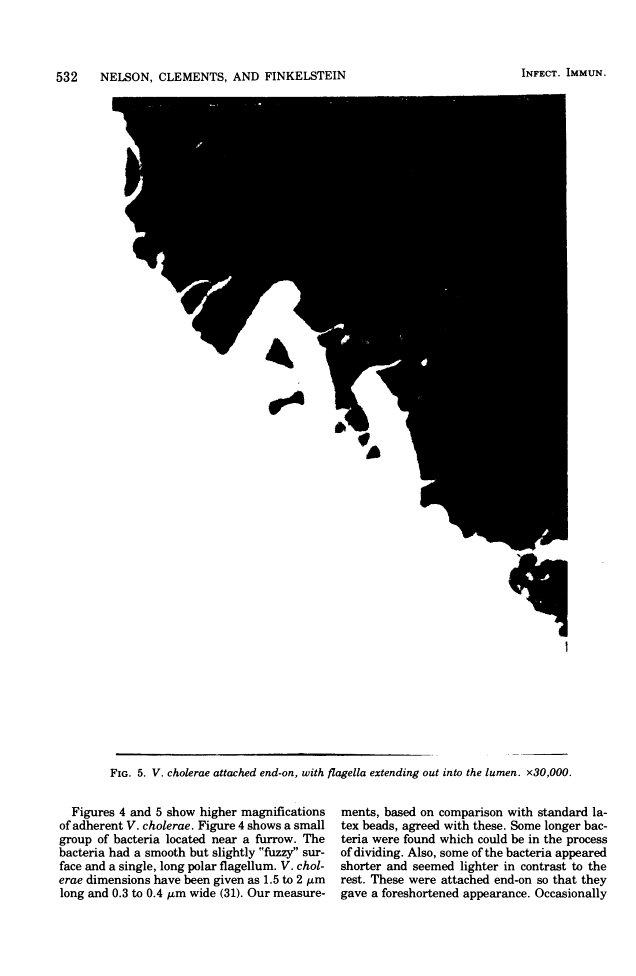
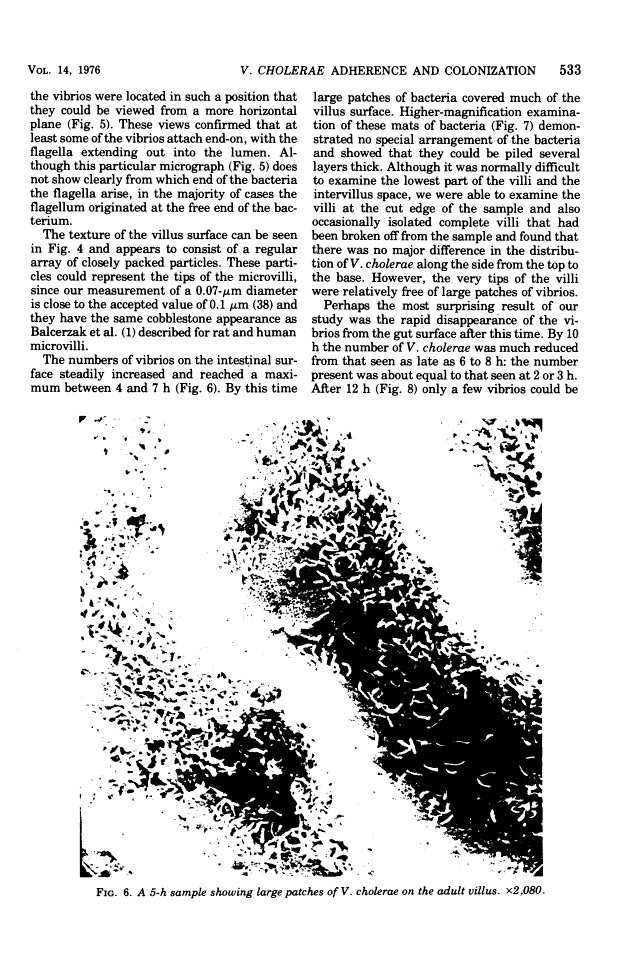
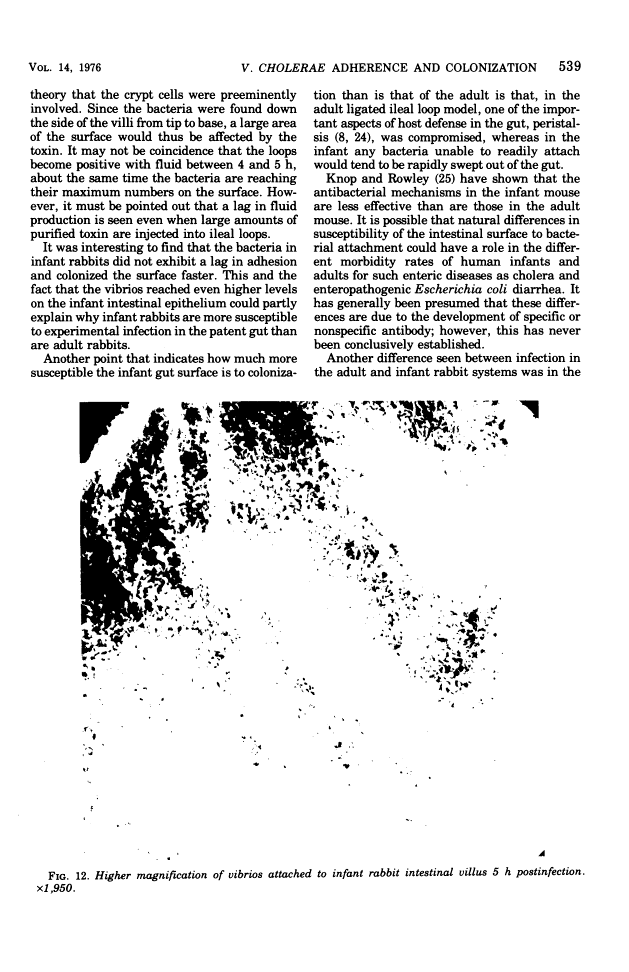
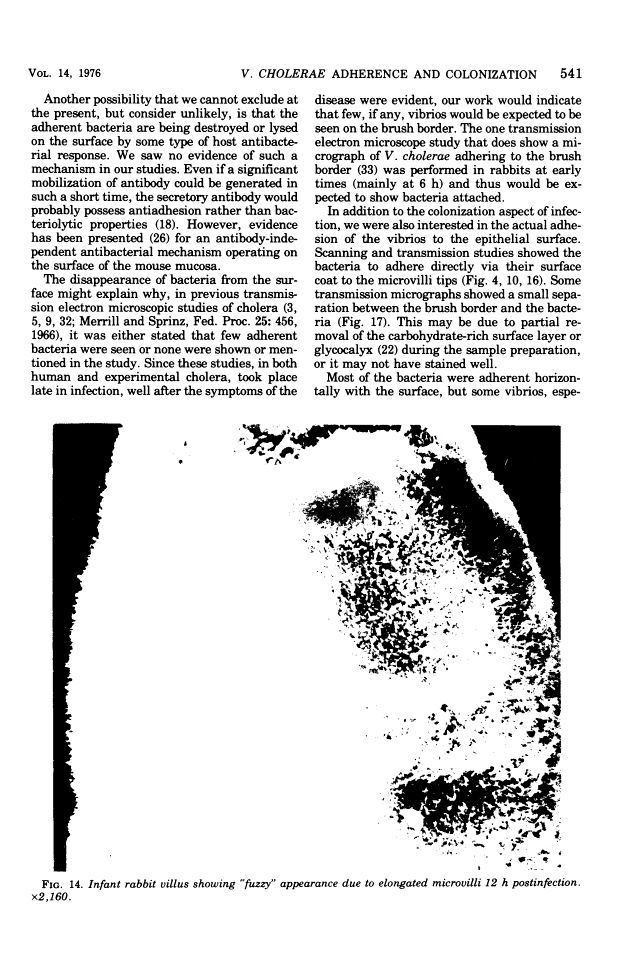
Colonization of the intestinal epithelium by Vibrio cholerae was examined in two model systems, in ligated ileal loops of adult rabbits and in the patent gut of infant rabbits, using both scanning and transmission electron microscopy. Time studies in the adult model showed a lag period of up to 1 h before the attachment of significant numbers of the vibrios. The bacteria appeared initially in small patches on the sides of the villi, predominantly along the transverse furrows. The number of adherent bacteria steadily increased, reaching a maximum between 4 and 7 h, when a dense mat of bacteria several layers thick covered much of the villi. After this time there was a rapid decline in the number of V. cholerae bound. By 12 to 16 h only a few bacteria could be seen on the surface of the villi, which had a rough, patchy appearance at these later times. Globular protrusions, with vibrios attached, may play a role in the clearance of bacteria. Colonization and clearance in the patent intestine of the infant rabbit occurred much as in the adult model. However, the bacteria adhered more uniformly and there was no lag in attachment. In both models the majority of bacteria were aligned horizontally with the epithelial surface, but some were attached in an end-on manner, with their flagella extending into the lumen. The bacteria adhered via their surface coats directly to the tips of the microvilli, except for a few vibrios that were partly embedded into the brush border. Some changes in the microvilli occurred as a consequence of the bacterial attachment.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (6.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcerzak SP, Lane WC, Bullard JW. Surface structure of intestinal epithelium. Gastroenterology. 1970 Jan;58(1):49–55. [Abstract] [Google Scholar]
- Chen HC, Reyes V, Fresh JW. An electron microscopic study of the small intestine in human cholera. Virchows Arch B Cell Pathol. 1971;7(2):236–259. [Abstract] [Google Scholar]
- DE SN, CHATTERJE DN. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. [Abstract] [Google Scholar]
- De Jonge HR. The response of small intestinal villous and crypt epithelium to choleratoxin in rat and guinea pig. Evidence against a specific role of the crypt cells in choleragen-induced secretion. Biochim Biophys Acta. 1975 Jan 13;381(1):128–143. [Abstract] [Google Scholar]
- DIXON JM. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. [Abstract] [Google Scholar]
- Elliott HL, Carpenter CC, Sack RB, Yardley JH. Small bowel morphology in experimental canine cholera. A light and electron microscopic study. Lab Invest. 1970 Feb;22(2):112–120. [Abstract] [Google Scholar]
- Finkelstein RA, Sobocinski PZ, Atthasampunna P, Charunmethee P. Pathogenesis of experimental cholera: identification of choleragen (procholeragen A) by disc immunoelectrophoresis and its differentiation from cholera mucinase. J Immunol. 1966 Jul;97(1):25–33. [Abstract] [Google Scholar]
- Finkelstein RA, Vasil ML, Holmes RK. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. [Abstract] [Google Scholar]
- Freter R. Interactions between mechanisms controlling the intestinal microflora. Am J Clin Nutr. 1974 Dec;27(12):1409–1416. [Abstract] [Google Scholar]
- FRETER R, SMITH HL, Jr, SWEENEY FJ., Jr An evaluation of intestinal fluids in the pathogenesis of cholera. J Infect Dis. 1961 Jul-Aug;109:35–42. [Abstract] [Google Scholar]
- Gibbons RJ. Bacterial adherence to mucosal surfaces and its inhibition by secretory antibodies. Adv Exp Med Biol. 1974;45(0):315–325. [Abstract] [Google Scholar]
- Goldstein HB, Merrill TG, Sprinz H. Experimental cholera. Morphologic evidence of cytotoxicity. Arch Pathol. 1966 Jul;82(1):54–59. [Abstract] [Google Scholar]
- Hohmann A, Wilson MR. Adherence of enteropathogenic Escherichia coli to intestinal epithelium in vivo. Infect Immun. 1975 Oct;12(4):866–880. [Europe PMC free article] [Abstract] [Google Scholar]
- HOWARD MB, LANKFORD CE. Minimum nutritional requirements for mucinase synthesis by Vibrio cholerae. Tex Rep Biol Med. 1960;18:612–619. [Abstract] [Google Scholar]
- Ito S. Structure and function of the glycocalyx. Fed Proc. 1969 Jan-Feb;28(1):12–25. [Abstract] [Google Scholar]
- Knop J, Rowley D. Antibacterial mechanisms in the intestine. Elimination of V. cholerae from the gastrointestinal tract of adult mice. Aust J Exp Biol Med Sci. 1975 Apr;53(2):137–146. [Abstract] [Google Scholar]
- Knop J, Rowley D. Antibacterial mechanisms in the intestine. Elimination of V. cholerae from the intestines of infant mice and the role of antibody. Aust J Exp Biol Med Sci. 1975 Apr;53(2):147–154. [Abstract] [Google Scholar]
- Knop J, Rowley D. Protection against cholera. A bactericidal mechanism on the mucosal surface of the small intestine of mice. Aust J Exp Biol Med Sci. 1975 Apr;53(2):155–165. [Abstract] [Google Scholar]
- LANKFORD CE. Factors of virulence of Vibrio cholerae. Ann N Y Acad Sci. 1960 Nov 21;88:1203–1212. [Abstract] [Google Scholar]
- Norris HT, Majno G. On the role of the ileal epithelium in the pathogenesis of experimental cholera. Am J Pathol. 1968 Aug;53(2):263–279. [Europe PMC free article] [Abstract] [Google Scholar]
- Savage DC, Blumershine RV. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974 Jul;10(1):240–250. [Europe PMC free article] [Abstract] [Google Scholar]
- Schrank GD, Verwey WF. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. [Europe PMC free article] [Abstract] [Google Scholar]
- Schwartz CJ, Kimberg DV, Ware P. Adenylate cyclase in intestinal crypt and villus cells: stimulation by cholera enterotoxin and prostaglandin E1. Gastroenterology. 1975 Jan;68(1):94–104. [Abstract] [Google Scholar]
- Trier JS, Rubin CE. Electron microscopy of the small intestine: a review. Gastroenterology. 1965 Nov;49(5):574–603. [Abstract] [Google Scholar]
- Weiser MM, Quill H. Intestinal villus and crypt cell responses to cholera toxin. Gastroenterology. 1975 Aug;69(2):479–482. [Abstract] [Google Scholar]
- Yardley JH, Bayless TM, Luebbers EH, Halsted CH, Hendrix TR. Goblet cell mucus in the small intestine. Findings after net fluid production due to cholera toxin and hypertonic solutions. Johns Hopkins Med J. 1972 Jul;131(1):1–10. [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.14.2.527-547.1976
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/14/2/527.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/14/2/527
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/14/2/527
Citations & impact
Impact metrics
Article citations
The Interface of Vibrio cholerae and the Gut Microbiome.
Gut Microbes, 13(1):1937015, 01 Jan 2021
Cited by: 19 articles | PMID: 34180341 | PMCID: PMC8244777
Review Free full text in Europe PMC
Host-Microbe-Pathogen Interactions: A Review of Vibrio cholerae Pathogenesis in Drosophila.
Front Immunol, 10:3128, 24 Jan 2020
Cited by: 8 articles | PMID: 32038640 | PMCID: PMC6993214
Review Free full text in Europe PMC
Anti-biofilm Properties of the Fecal Probiotic Lactobacilli Against Vibrio spp.
Front Cell Infect Microbiol, 8:120, 24 Apr 2018
Cited by: 57 articles | PMID: 29740541 | PMCID: PMC5928150
Vibrio cholerae Biofilms and Cholera Pathogenesis.
PLoS Negl Trop Dis, 10(2):e0004330, 04 Feb 2016
Cited by: 100 articles | PMID: 26845681 | PMCID: PMC4741415
Review Free full text in Europe PMC
Bacterial community associated with the intestinal tract of Chinese mitten crab (Eriocheir sinensis) farmed in Lake Tai, China.
PLoS One, 10(4):e0123990, 13 Apr 2015
Cited by: 29 articles | PMID: 25875449 | PMCID: PMC4395229
Go to all (68) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
In vivo adherence and colonization of Vibrio cholerae strains that differ in hemagglutinating activity and motility.
Infect Immun, 55(9):2093-2102, 01 Sep 1987
Cited by: 19 articles | PMID: 3623694 | PMCID: PMC260662
Interaction of vibrio cholerae El Tor and gut mucosa in ligated rabbit ileal loop experiment.
Med Biol, 55(3):130-140, 01 Jun 1977
Cited by: 6 articles | PMID: 895212
Localization of Vibrio cholerae O1 in the intestinal tissue.
Asian Pac J Allergy Immunol, 11(2):155-165, 01 Dec 1993
Cited by: 0 articles | PMID: 8080608
Expression of Vibrio cholerae virulence genes in response to environmental signals.
Curr Issues Intest Microbiol, 3(2):29-38, 01 Sep 2002
Cited by: 25 articles | PMID: 12400636
Review