Abstract
Objective
Invasion of uterine spiral arteries by extravillous trophoblasts in the first trimester of pregnancy results in loss of endothelial and musculoelastic layers. This remodeling is crucial for an adequate blood supply to the fetus with a failure to remodel implicated in the etiology of the hypertensive disorder preeclampsia. The mechanism by which trophoblasts induce this key process is unknown. This study gives the first insights into the potential mechanisms involved.Methods and results
Spiral arteries were dissected from nonplacental bed biopsies obtained at Caesarean section, and a novel model was used to mimic in vivo events. Arteries were cultured with trophoblasts in the lumen, and apoptotic changes in the endothelial layer were detected after 20 hours, leading to loss of endothelium by 96 hours. In vitro, coculture experiments showed that trophoblasts stimulated apoptosis of primary decidual endothelial cells and an endothelial cell line. This was blocked by caspase inhibition and NOK2, a FasL blocking antibody. NOK2 also abrogated trophoblast-induced endothelial apoptosis in the vessel model.Conclusions
Extravillous trophoblast induction of endothelial apoptosis is a possible mechanism by which the endothelium is removed, and vascular remodeling may occur in uterine spiral arteries. Fas/FasL interactions have an important role in trophoblast-induced endothelial apoptosis.Free full text

Uterine Spiral Artery Remodeling Involves Endothelial Apoptosis Induced by Extravillous Trophoblasts Through Fas/FasL Interactions
Associated Data
Abstract
Objective
Invasion of uterine spiral arteries by extravillous trophoblasts in the first trimester of pregnancy results in loss of endothelial and musculoelastic layers. This remodeling is crucial for an adequate blood supply to the fetus with a failure to remodel implicated in the etiology of the hypertensive disorder preeclampsia. The mechanism by which trophoblasts induce this key process is unknown. This study gives the first insights into the potential mechanisms involved.
Methods and Results
Spiral arteries were dissected from nonplacental bed biopsies obtained at Caesarean section, and a novel model was used to mimic in vivo events. Arteries were cultured with trophoblasts in the lumen, and apoptotic changes in the endothelial layer were detected after 20 hours, leading to loss of endothelium by 96 hours. In vitro, coculture experiments showed that trophoblasts stimulated apoptosis of primary decidual endothelial cells and an endothelial cell line. This was blocked by caspase inhibition and NOK2, a FasL blocking antibody. NOK2 also abrogated trophoblast-induced endothelial apoptosis in the vessel model.
Conclusions
Extravillous trophoblast induction of endothelial apoptosis is a possible mechanism by which the endothelium is removed, and vascular remodeling may occur in uterine spiral arteries. Fas/FasL interactions have an important role in trophoblast-induced endothelial apoptosis.
Remodeling of the uterine arteries is a key event in early pregnancy. In the first trimester of pregnancy, a sub-population of fetal trophoblast cells, the extravillous trophoblast, invade the uterine wall (interstitial invasion) and its blood vessels (endovascular invasion) as far as the myometrial segments. In the uterine spiral arteries, the trophoblasts interdigitate between the endothelial cells (ECs), replacing the endothelial lining and most of the musculoelastic tissue in the vessel walls. This creates a high-flow, low-resistance circulation that increases maternal blood flow to the placental villi at the maternal–fetal interface.
Data suggest that trophoblasts bind to and migrate along the luminal surfaces of the endothelium and transiently coexist on the walls of partially modified spiral arteries before replacing the endothelium.1,2 Little is known as to how these processes are regulated in normal pregnancies; however, their pivotal importance in the establishment and maintenance of a successful pregnancy is illustrated when they fail to occur or occur to a significantly reduced extent. Defective remodeling of the spiral arteries is associated with pregnancies complicated by preeclampsia and intrauterine growth restriction (IUGR)3 and is proposed to lead to an overall state of oxidative stress or fluctuations in oxygen concentrations analogous to hypoxia-reperfusion within the placental environment.4 Preeclampsia and IUGR are responsible for considerable perinatal mortality and morbidity and carry health implications in adult life, including increased risk of hypertension, heart disease, and diabetes.5 The importance of interactions between trophoblasts and the vascular cells of the spiral arteries, which may account for these differences in remodeling, have yet to be determined in normal or complicated pregnancies.
Studies of spiral arteries have been confined primarily to immunohistochemical analysis of placental bed biopsies,6 whereas in vitro studies have been hampered by the lack of suitable models to directly examine cellular interactions during invasion. To address these problems and to investigate the mechanisms responsible for these essential vascular changes, we have developed an in vitro model of spiral artery invasion and remodeling.7
It is well established that the balance between factors that promote and inhibit vascular cell apoptosis is important in the maintenance of vessel integrity.8 We postulate that the invasive extravillous trophoblast may play an active role in the vascular adaptations that occur in the uterine vessels, and that trophoblast induction of endothelial apoptosis may be an important mechanism in spiral artery remodeling.
Methods
Trophoblast and Endothelial Isolation and Culture
Informed consent was obtained and ethical committee approval was in place for all studies. Isolation of primary first-trimester cytotrophoblasts was performed as described previously.7 SGHPL-4 cells are a human first-trimester extravillous trophoblast-derived cell line cultured as described previously9 and used extensively as an extravillous trophoblast model.10 SGHPL-4 cells retain features of normal extravillous trophoblast including expression of human leukocyte antigen-G (HLA-G), cytokeratin-7, CD9, and human placental lactogen (hPL). SGHEC-7 cells are a human umbilical vein EC (HUVEC)-derived cell line and were cultured as described previously.11 Primary human first-trimester decidual ECs were isolated using a modification of the method described by Grimwood et al.12 Further details are available in an online supplement. Endothelial identity was confirmed by expression of CD31 and von Willebrand factor (vWF).
Spiral Artery Dissection and Culture With Trophoblasts
Endovascular perfusion of trophoblasts into isolated unmodified nonplacental bed spiral arteries was performed as described previously.7 In brief, decidual/myometrial biopsies were obtained from nonplacental bed areas from normal pregnant women undergoing elective Caesarean section at term for reasons such as breech presentation. Spiral arteries were dissected under sterile conditions and mounted on cannulae in a perfusion chamber (Living Systems). SGHPL-4 cells or primary first-trimester cytotrophoblasts were fluorescently labeled by incubation with 5 μmol/L CellTracker Orange probe (Molecular Probes) for 30 minutes at 37°C, followed by incubation with fresh medium for 30 minutes. The lumen of the artery was perfused with either labeled trophoblasts at 5×106 cells/mL (with ≈5×104 cells perfused into each artery) or culture media alone. Where indicated, anti-FasL blocking antibody (10 μg/mL NOK2;13 BD PharMingen) or control IgG2a,κ (10 μg/mL; Sigma) were added to the trophoblasts before perfusion. After a short perfusion, the ends of each spiral artery segment were tied (to prevent cells diffusing out), and the artery was supported in a fibrin gel9 with culture medium added on top. Vessels were incubated for up to 4 days, when arteries were cryosectioned. The presence of trophoblasts within the lumen was confirmed by fluorescence microscopy.
Immunohistochemistry
Sections were fixed in 4% (wt/vol) paraformaldehyde in PBS for 10 minutes, washed in PBS, and permeabilized in 0.2% (v/v) Triton X-100/PBS for 5 minutes. After 3 washes with PBS, blocking buffer (PBS/10% goat serum) was added for 20 minutes, then rabbit anti-human poly(ADP-ribose) polymerase (PARP) p85 fragment antibody (2.5 μg/mL, Promega), rabbit anti-human vWF (14.25 μg/mL; DAKO), rabbit immunoglobulin control, monoclonal anti-human Fas (25 μg/mL MAB142; R&D Systems), or isotype control immunoglobulin were added for 1 hour. After 3 washes, slides were incubated for 45 minutes with biotinylated goat anti-rabbit or anti-mouse antibodies (Vector Laboratories) at 7.5 μg/mL. After 3 further washes, slides were incubated with fluorescein-streptavidin at 15 μg/mL for 15 minutes, washed extensively, and Vectashield mounting medium added.
Time-Lapse Microscopy
SGHEC-7 ECs were fluorescently labeled by incubation with 5 μmol/L CellTracker Orange probe for 30 minutes at 37°C, followed by incubation with fresh medium for 30 minutes. SGHEC-7 cells were seeded at 1.32×105 cells/well in 6-well plates. After 6 hours, the medium was changed to phenol-red free RPMI medium 1640/M199 containing 0.5% (wt/vol) gelding serum (to remove the effect of estrogen in the culture medium). Decidual ECs were plated directly onto collagen-coated 6-well plates after isolation. After 24 to 48 hours, cells were washed to remove detached Dynabeads. After an additional 24 hours, cells were labeled with CellTracker probe as above, and medium was changed to McCoy’s medium supplemented with 25% (v/v) human serum. After 15 hours (for SGHEC-7) or 4 hours (for primary decidual ECs), SGHPL-4 extravillous trophoblast cells or ECs (not fluorescently labeled, to control for cell number) were added at 1.32×105 cells per well in the presence or absence of caspase inhibitor VI (zVAD-fmk; 50 μmol/L; Calbiochem), anti-FasL blocking antibody (10 μg/mL NOK2), or control IgG2a,κ (10 μg/mL). Once the additional cells had adhered, the plate was transferred to an Olympus IX70 inverted fluorescence microscope with motorized stage and cooled charge-coupled device camera and enclosed in a heated, humidified chamber at 37°C with 5% CO2 in air. Images were taken every 15 minutes for 36 hours, and time-lapse sequences were analyzed using ImagePro Plus (Media Cybernetics). In each field of view, 40 ECs (identified by fluorescence) were randomly chosen, with 4 fields of view examined. Experiments were repeated at least 3×. Apoptotic cells were scored according to the time at which clear apoptotic morphology was first observed. Apoptotic morphology was considered as cytoplasmic and nuclear shrinkage, a change to a phase-bright appearance and the formation of membrane blebs and blisters. Use of time-lapse microscopy to determine apoptotic morphological changes over time has been well established and allows the kinetics of the apoptotic response to be determined.14,15
Western Blot Analysis of Cleaved PARP Expression
SGHEC-7 ECs were seeded in culture plates. SGHPL-4 extravillous trophoblast cells or SGHEC-7 (to control for cell number) were seeded at the same concentration as the SGHEC-7 cells in tissue culture inserts (Nalge Nunc; 0.4 μm membrane). After 16 hours, the medium was changed to phenol-red free RPMI medium 1640/M199 containing 0.5% (wt/vol) gelding serum, and the inserts were added to the plates (3 25-mm inserts/9-cm plate). After the stated time, ECs on the plate were lysed in RIPA buffer, and Western blot analysis was performed. Samples were separated by SDS-PAGE and transferred to a nitrocellulose membrane. After incubation in blocking buffer (10 mmol/L Tris, pH 8, 150 mmol/L NaCl, 0.05% Tween 20, and 10% [wt/vol] milk powder) for 1 hour at room temperature, the membrane was incubated with rabbit polyclonal anti-human cleaved PARP (1:1000; Promega) or anti-actin (1:1000; Sigma) for 1 hour. Anti-rabbit IgG peroxidase (1:6000; A6154; Sigma) was added for 1 hour. Detection of membrane-bound antibodies was performed by chemiluminescence (ECLPlus; Amersham).
Western Blot Analysis of Endothelial Fas and c-FLIP Expression
ECs were analyzed for expression of Fas, and experiments were performed to determine the effect of coculture with trophoblasts on endothelial c-FLICE/caspase 8-inhibitory protein (FLIP) expression, as detailed in the online supplement.
Statistical Analysis
All data were analyzed using a repeated-measures ANOVA with Tukey’s multiple-comparison post-test unless stated. Statistical significance was assumed at P<0.05.
Results
Trophoblasts Induce Apoptosis and Loss of ECs in a Spiral Artery Explant Model
Spiral arteries were cultured with trophoblast cells or medium alone in the lumen of the vessel for 20 to 96 hours before cryosectioning. The presence of trophoblasts in the lumen of vessels perfused with cells was confirmed by fluorescence microscopy. When vessels were cultured for 96 hours, those with medium in the lumen had an intact endothelium, as determined by vWF staining (Figure 1A), whereas arteries with trophoblasts in the lumen showed significant loss of vWF-positive ECs. This effect was observed in vessels perfused with primary cytotrophoblasts and the SGHPL-4 extravillous trophoblast cell line. Cells staining positive for the p85 caspase-cleaved fragment of PARP (a marker of apoptosis) could be detected lining the lumen of the arteries that had been cultured with trophoblasts for 20 hours (Figure 1B), whereas no cleaved PARP-positive cells could be detected in vessels cultured with medium for the same length of time. The presence of trophoblasts within the lumen of the perfused vessels is shown in Figure I. The same effect was observed using TUNEL staining (data not shown). To confirm that the cleaved PARP staining was in the endothelium, consecutive sections were stained for vWF and cleaved PARP. Cleaved PARP-positive cells were in the same position lining the lumen of the vessel as the vWF-positive cells, confirming that apoptosis was associated with the endothelium (Figure 1C).
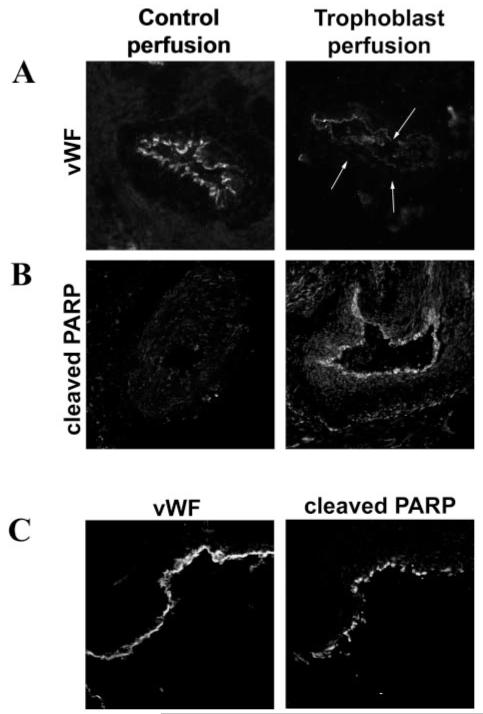
Unmodified, nonplacental bed uterine spiral arteries were perfused with CellTracker-fluorescently labeled SGHPL-4 cells or primary first-trimester cytotrophoblasts or media (control). Vessels were incubated for periods of 20 to 96 hours before sectioning. A, Expression of vWF indicating the presence of endothelium in the control perfusion and absence of endothelium in vessels cultured with primary trophoblasts for 96 hours (the position of the lumen is indicated by the arrows). B, Expression of p85-cleaved PARP in the endothelium of trophoblast-perfused vessels, incubated for 20 hours. C, Colocalization of cleaved PARP with vWF staining in consecutive sections from vessels cultured with SGHPL-4 cells, confirming apoptosis in the endothelium. Original magnification ×150.
Extravillous Trophoblasts Induce Endothelial Apoptosis When Cocultured In Vitro
SGHPL-4 extravillous trophoblasts induced morphological changes characteristic of apoptosis in SGHEC-7 ECs after coculture, including cytoplasmic retraction, a phase-bright appearance, and membrane blebs and blisters (Figure 2A). Because HUVEC-derived ECs may not respond in the same way as ECs of the placental bed, experiments were repeated with primary decidual ECs, and the same effect was seen (Figure 2A). Analysis of the kinetics of these changes using time-lapse microscopy showed that the level of SGHEC-7 and primary decidual EC apoptosis was significantly increased when cocultured with trophoblasts, reaching statistical significance after 10 hours (P=0.04; n=9; paired Student t test) for SGHEC-7 cells and after 21 hours (P=0.04; n=5; paired Student t test) for decidual ECs (Figure 2B). This is consistent with detection of apoptotic changes in the explant model at 20 hours. Apoptosis was confirmed by use of the broad-spectrum caspase inhibitor zVAD-fmk (Figure 2B), which significantly decreased the response after 36 hours for decidual ECs (P<0.001; n=5) and decreased the SGHEC-7 cell apoptosis to levels seen in the absence of trophoblasts (P<0.05; n=3). Although the percentage of apoptotic cells appears high, in reality, it represents a much smaller proportion of the total cell population. The cell population also proliferates, and the results shown only relate to the percentage of apoptotic cells detected within the 40 cells chosen for analysis at the start of the experiment. The levels of apoptosis in trophoblasts after coculture with ECs was no higher than the level of basal trophoblast apoptosis observed in the absence of ECs (data not shown). Apoptosis was additionally confirmed by transwell coculture experiments and Western blot analysis of EC expression of the p85 fragment of cleaved PARP. During coculture with trophoblasts, endothelial-cleaved PARP expression was increased slightly after 24 hours and further increased after 60 hours (Figure 3).
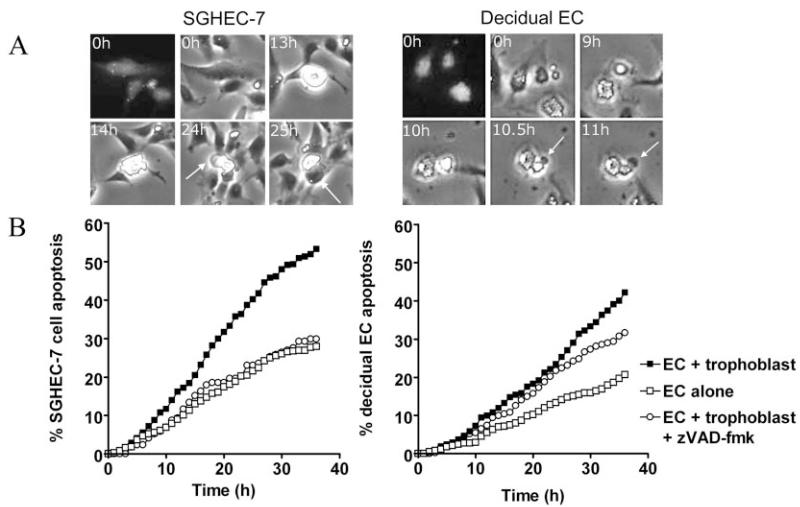
A, SGHPL-4 cells were cocultured with fluorescently labeled SGHEC-7 cells or decidual ECs shown at 0 hours as a fluorescent and phase-contrast image. Apoptotic morphology could be detected in ECs over time, evidenced by cytoplasmic retraction and a phase-bright appearance, and membrane blebbing and blistering (indicated by arrows). Apoptosis in an individual EC is shown to illustrate the morphological changes occurring over time. B, Apoptosis of SGHEC-7 and primary decidual ECs cocultured with trophoblasts was monitored by time-lapse microscopy. Cells were scored by the time point at which clear apoptotic morphology was observed. At least 3 separate experiments were performed, and 40 cells were assessed per well in each experiment. Pooled mean data are shown. Error bars have been omitted for clarity because of the number of time points. Apoptotic morphological changes could be detected throughout the experiment; however, the difference between EC apoptosis in the presence of trophoblasts compared with ECs alone reached statistical significance after 10 hours (P=0.04; n=9) for SGHEC-7 cells and after 21 hours (P=0.04; n=5) for decidual ECs. zVAD-fmk was added at the same time as trophoblasts.
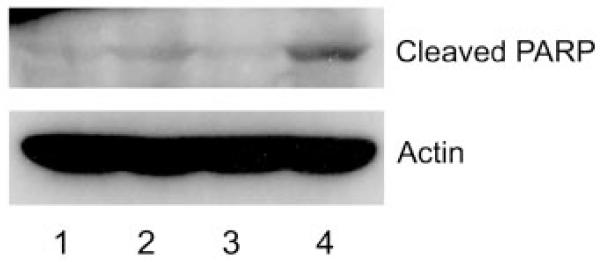
SGHEC-7 cells were cultured for 24 hours or 60 hours with SGHPL-4 trophoblast cells or SGHEC-7 (to control for cell number) in tissue culture inserts before lysis of the ECs. Western blot analysis with rabbit polyclonal anti-human cleaved PARP showed a band at 85 kDa. Actin loading control is shown with a band at 42 kDa. Lane 1, 24-hour endothelial culture; lane 2, 24-hour endothelial+trophoblast coculture; lane 3, 60-hour endothelial culture; lane 4, 60-hour endothelial+trophoblast coculture.
Fas/FasL Interactions Are Involved in Trophoblast Induction of Endothelial Apoptosis
Fas expression was detected on spiral artery endothelial and smooth muscle cells by immunohistochemistry (Figure 4A) and on SGHEC-7 cells and decidual ECs by immunoprecipitation and Western blot analysis (Figure 4B). To determine whether FasL was responsible for the induction of endothelial apoptosis, coculture time-lapse experiments were repeated in the presence of a blocking antibody to the membrane-boundand soluble forms of FasL, NOK2 (Figure 5). The presence of trophoblasts led to a 1.91-fold increase in SGHEC-7 apoptosis (P<0.01; n=9) and a 2.04-fold increase in decidual EC apoptosis (P<0.05; n=5) after 36-hour coculture. NOK2 abrogated the trophoblast induction of SGHEC-7 apoptosis (P<0.05) and decidual EC apoptosis (P<0.05). The isotype control immunoglobulin for the NOK2 antibody had no significant effect on the level of trophoblast-induced apoptosis. To investigate whether trophoblasts cause a decrease in endothelial expression of the antiapoptotic protein FLIP, coculture experiments were performed and FLIP expression was assessed. Western blot analysis showed no differences in expression of the long form of FLIP, and no consistent effect on any of the shorter forms of FLIP were detected on culture with trophoblasts compared with culture with ECs (to control for cell number), after 8, 24, or 60 hours (data not shown). To determine whether FasL was responsible for the induction of endothelial apoptosis observed in the vessel model, labeled trophoblasts were perfused in the presence of the FasL blocking antibody or immunoglobulin control (Figure 6), vessels were incubated for 20 hours, and cleaved PARP was detected. Vessels cultured with trophoblasts and control immunoglobulin showed apoptosis in the endothelium. In contrast, vessels cultured with trophoblasts in the presence of the FasL blocking antibody showed no detectable apoptosis.
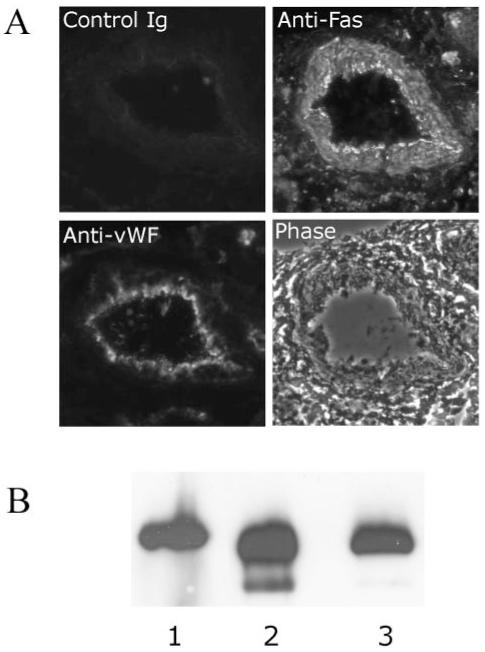
A, Immunohistochemical analysis of Fas expression in spiral arteries. Sections were labeled with anti-Fas, anti-vWF, or control immunoglobulin in consecutive sections. Original magnification ×150. B, Western blot analysis of Fas expression (45 KDa) after immunoprecipitation with polyclonal anti-Fas and detection with monoclonal anti-Fas. Lane 1, Decidual ECs; lanes 2 and 3, SGHEC-7 cells.
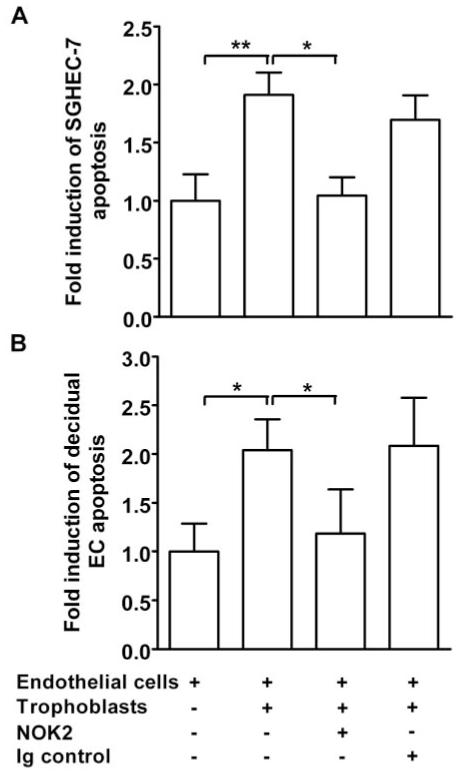
Apoptosis of SGHEC-7 cells (A) or decidual ECs (B) cocultured with trophoblasts (SGHPL-4) was monitored by time-lapse microscopy. NOK2 (FasL blocking antibody) or control immunoglobulin (Ig) were added at the same time as the SGHPL-4 cells. Cells were scored by the time point at which clear apoptotic morphology was observed. Forty cells were assessed per well in each experiment, and the results represent the mean fold induction of apoptosis over control levels after 36-hour coculture+SEM of data pooled from at least 4 separate experiments. *P<0.05; **P<0.01.
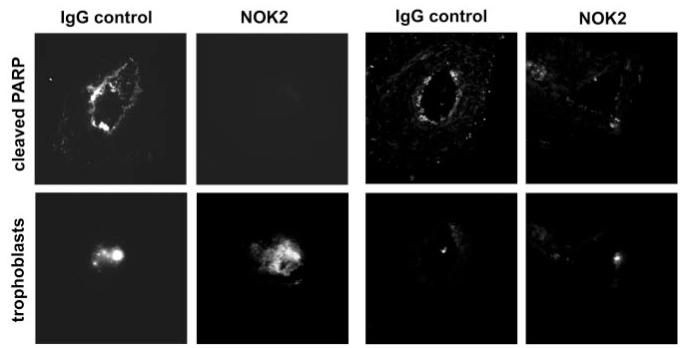
Unmodified spiral arteries were perfused with CellTracker-fluorescently labeled SGHPL-4 cells in the presence of either NOK2 (FasL blocking antibody) or control immunoglobulin. Vessels were incubated for 20 hours before sectioning. Top panels, p85-cleaved PARP was expressed in the endothelium of vessels perfused with trophoblasts+immunoglobulin control but was not detected in vessels perfused with trophoblasts+NOK2. Bottom panels, Presence of fluorescently labeled trophoblasts in the lumen. The same number of trophoblast cells were perfused in each experiment; however, the number detectable in an individual section varied along the length of the vessel. The same result was evident when a considerable number of trophoblasts could be detected in that section and when fewer trophoblasts were present in that section. Original magnification ×150.
Discussion
Loss of ECs from the lumen of the maternal uterine spiral arteries is a prerequisite for a successful pregnancy. To date, our knowledge is derived from immunohistochemical analysis of placental bed biopsies. From these studies, it is clear that extravillous trophoblasts detach from the anchoring villi and invade the uterine wall and its blood vessels as far as the inner myometrial segments. This occurs during the first 20 weeks of pregnancy, during which time the trophoblasts, endothelial, and vascular smooth muscle cells transiently coexist in partially modified spiral arteries.3 Although endothelial and smooth muscle cells are subsequently lost from the wall of the artery, immunohistochemical studies provide limited insight into cellular dynamics and molecular mechanisms involved. It has been suggested that some changes in the vessels are independent of trophoblast cells, occurring as part of the maternal response to pregnancy16; however, it is clear is that in the absence of trophoblastic invasion, the extent of this remodeling is significantly reduced, indicating that trophoblasts must play an active role in this process.
We developed a model to investigate the effect of these endovascular trophoblasts.7 Using this model, we show that when isolated unmodified nonplacental bed spiral arteries were cultured with trophoblasts in the lumen for 4 days, there was loss of endothelium from the vessel. We have shown induction of apoptosis in the endothelial layer, preceding loss of the endothelium from the vessel, and suggest that this is a mechanism through which trophoblasts influence vessel remodeling. Loss of spiral artery endothelium was also observed in a recent study using cocultures of decidual explants with invasive trophoblasts from villous explants; however, no mechanisms underlying this phenomenon were demonstrated in this study.17
A number of mechanisms trigger apoptosis in vascular cells, including stimulation of Fas (CD95).18 Binding of FasL to Fas in many cells leads to apoptosis of the Fas-bearing cell through mechanisms involving activation of caspases, particularly caspase-8.19 Spiral artery endothelial and smooth muscle cells were shown to express Fas. FasL can either be membrane-bound or cleaved by metalloproteinases to release the extracellular portion as soluble FasL (sFasL). The expression of FasL by trophoblast cells has been proposed as a mechanism for induction of apoptosis in maternal immune cells, thus providing a degree of immune privilege to the maternal-fetal interface,20,21 and it has been shown recently that first-trimester trophoblasts secrete FasL.22 The induction of apoptosis by trophoblasts is therefore not without precedent, but this is the first observation of this occurring in cells of the maternal vasculature. Whether the loss of the endothelium is a triggering event for subsequent changes in the smooth muscle layer remains to be determined, as does the fate of the apoptosed ECs; although it is interesting to note that trophoblasts can be phagocytic.23,24
To investigate the mechanisms by which trophoblasts may affect endothelial integrity, we used direct coculture techniques and time-lapse microscopy to monitor characteristic morphological changes of apoptosis. Trophoblast induction of endothelial apoptosis in coculture confirmed our observations from the vessel model. Apoptosis was observed using HUVEC-derived cells and primary decidual ECs, suggesting that the ability of trophoblast to induce this effect may not be limited to spiral artery ECs. A role for FasL/sFasL was determined by blocking antibodies, which completely abrogated the response. Our results suggest that a soluble factor is likely to be involved because the use of transwells prevented direct cell/cell interactions; however, apoptotic markers could still be detected in the ECs. Extension of this study to the vessel model confirmed a role for Fas/FasL because trophoblasts in the presence of the blocking antibody induced no apoptosis in the spiral artery ECs.
Vascular ECs are normally resistant to Fas-mediated apoptosis, although they express Fas constitutively.18 In human ECs, levels of FLIP have been shown to control apoptosis mediated by Fas.25 It is therefore interesting to postulate whether trophoblasts are not only inducing apoptosis through FasL/sFasL but are also affecting the downstream signaling pathway from the Fas receptor. Our coculture studies indicated that trophoblasts did not alter endothelial expression of FLIP, which either suggests that the Fas-mediated signaling is the initiating event, or, if it is the consequence of sensitization to Fas-mediated signals, that mechanisms other than FLIP expression are involved. It is also possible that factors produced by trophoblasts are affecting Fas or FasL expression or distribution on ECs,26 as has been suggested for trophoblast induction of immune cell apoptosis.27 It is unlikely that the response observed is specific to trophoblast/EC interactions because many cells will produce the same factors; however, in the context of the spiral artery in vivo, only extravillous trophoblasts are present and in contact with the maternal endothelium.
Studies of pregnancies in Fas and FasL-deficient mice (lpr and gld) have been controversial. Reduction in litter sizes and increased resorption rates have been reported in gld mice,28 whereas other studies have disputed the importance of Fas/FasL in murine pregnancy.29 However, we must exercise caution in extrapolating murine studies to human pregnancy, especially because it has been suggested that remodeling in murine arteries may be more associated with uterine natural killer cells than trophoblasts.30
It is unlikely that Fas/FasL interactions are the only factors of importance in human uterine artery remodeling in vivo. Because these adaptations are so crucial to establishing an adequate utero–placental blood flow and healthy pregnancy, it is highly likely that the regulation will prove more complex. Obviously, extrapolation from these in vitro observations to the complex in vivo situation must be done with caution; however, our results indicate that extravillous trophoblasts can induce endothelial apoptosis through Fas/FasL interactions and give the first substantial insight into a possible mechanism by which vascular remodeling may be occurring in the placental bed. Understanding the mechanisms by which this occurs in normal pregnancy will provide valuable information for determining why these vascular adaptations fail to occur in some pregnancies.
Acknowledgments
This study was supported by the British Heart Foundation (PG2001045). We thank Glenn Ferris for technical assistance, Rosemary Keogh for assistance with Western blot analysis, and John Aplin for helpful discussions.
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/01.atv.0000148547.70187.89
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/01.ATV.0000148547.70187.89
Citations & impact
Impact metrics
Article citations
Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells.
Cells, 13(16):1372, 17 Aug 2024
Cited by: 1 article | PMID: 39195262 | PMCID: PMC11352994
GPR65 inhibits human trophoblast cell adhesion through upregulation of MYLK and downregulation of fibronectin via cAMP-ERK signaling in a low pH environment.
Cell Commun Signal, 21(1):238, 18 Sep 2023
Cited by: 2 articles | PMID: 37723567 | PMCID: PMC10506227
A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia.
Cell Mol Life Sci, 80(2):44, 18 Jan 2023
Cited by: 11 articles | PMID: 36652019 | PMCID: PMC9849194
Estrogen Actions in Placental Vascular Morphogenesis and Spiral Artery Remodeling: A Comparative View between Humans and Mice.
Cells, 12(4):620, 14 Feb 2023
Cited by: 4 articles | PMID: 36831287 | PMCID: PMC9954071
Review Free full text in Europe PMC
Trans-differentiation of trophoblast stem cells: implications in placental biology.
Life Sci Alliance, 6(3):e202201583, 27 Dec 2022
Cited by: 3 articles | PMID: 36574992 | PMCID: PMC9797987
Go to all (94) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism.
Am J Pathol, 169(5):1863-1874, 01 Nov 2006
Cited by: 84 articles | PMID: 17071607 | PMCID: PMC1780207
Proapoptotic effect of metalloproteinase 9 secreted by trophoblasts on endothelial cells.
J Obstet Gynaecol Res, 37(3):187-194, 16 Dec 2010
Cited by: 3 articles | PMID: 21159039
An in vitro model of trophoblast invasion of spiral arteries.
Methods Mol Med, 122:59-74, 01 Jan 2006
Cited by: 8 articles | PMID: 16511975
Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling.
Placenta, 113:48-56, 03 May 2021
Cited by: 12 articles | PMID: 33985793 | PMCID: PMC8440336
Review Free full text in Europe PMC
Funding
Funders who supported this work.
British Heart Foundation (1)
Trophoblast invasion of spiral arteries: a novel in vitro model
Prof Judith Cartwright
Grant ID: PG/2001045/12838





