Abstract
Free full text

5-Azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin.
Abstract
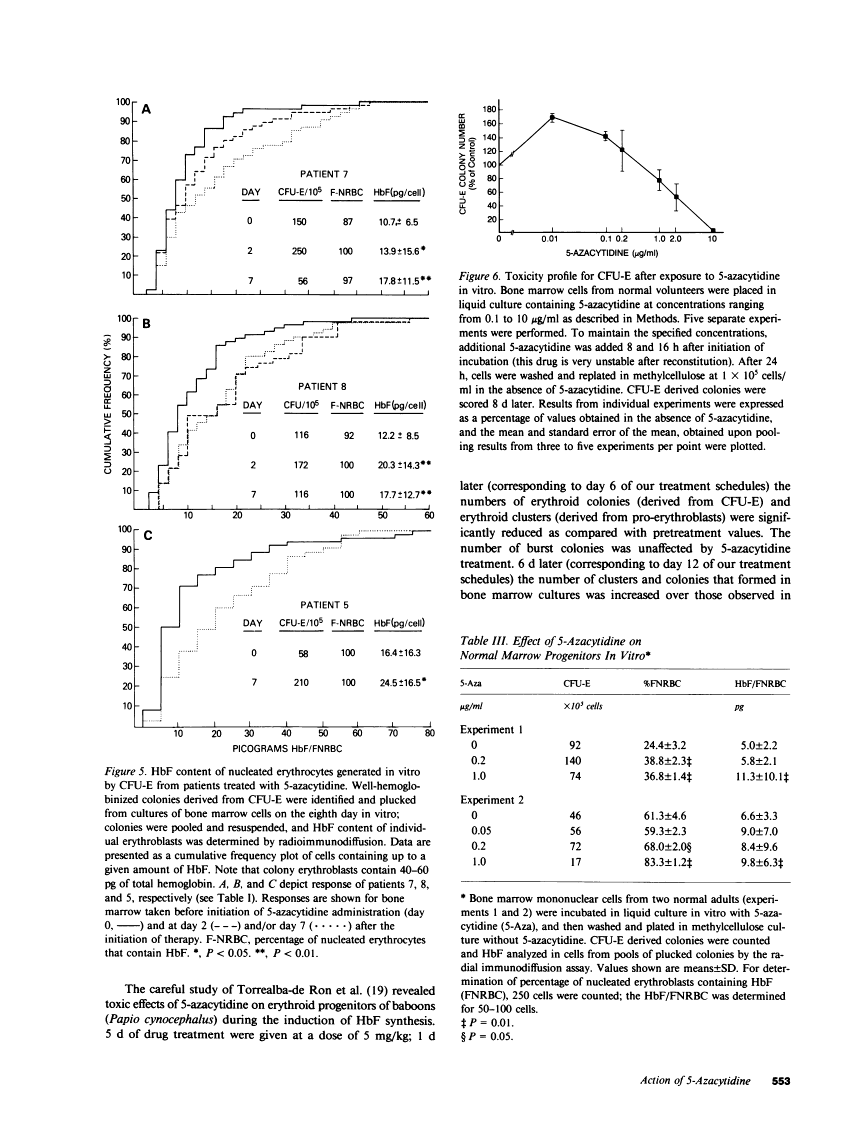
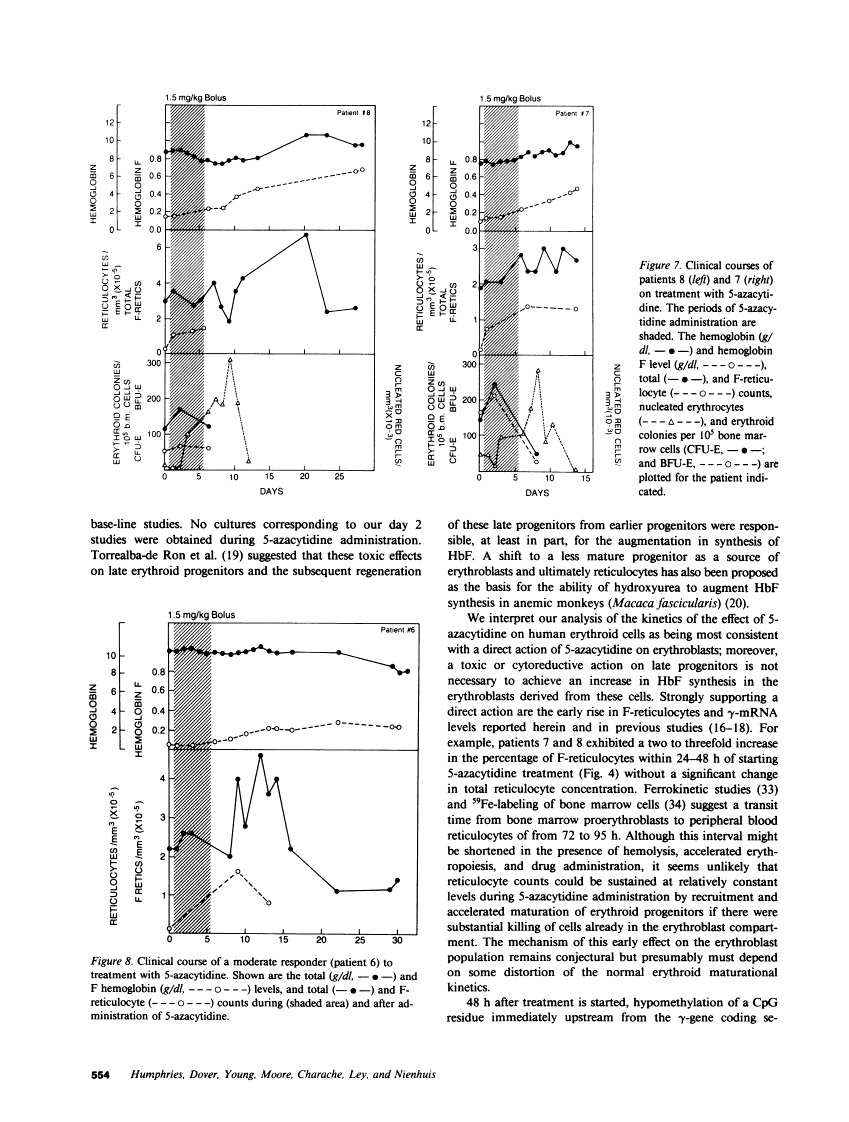
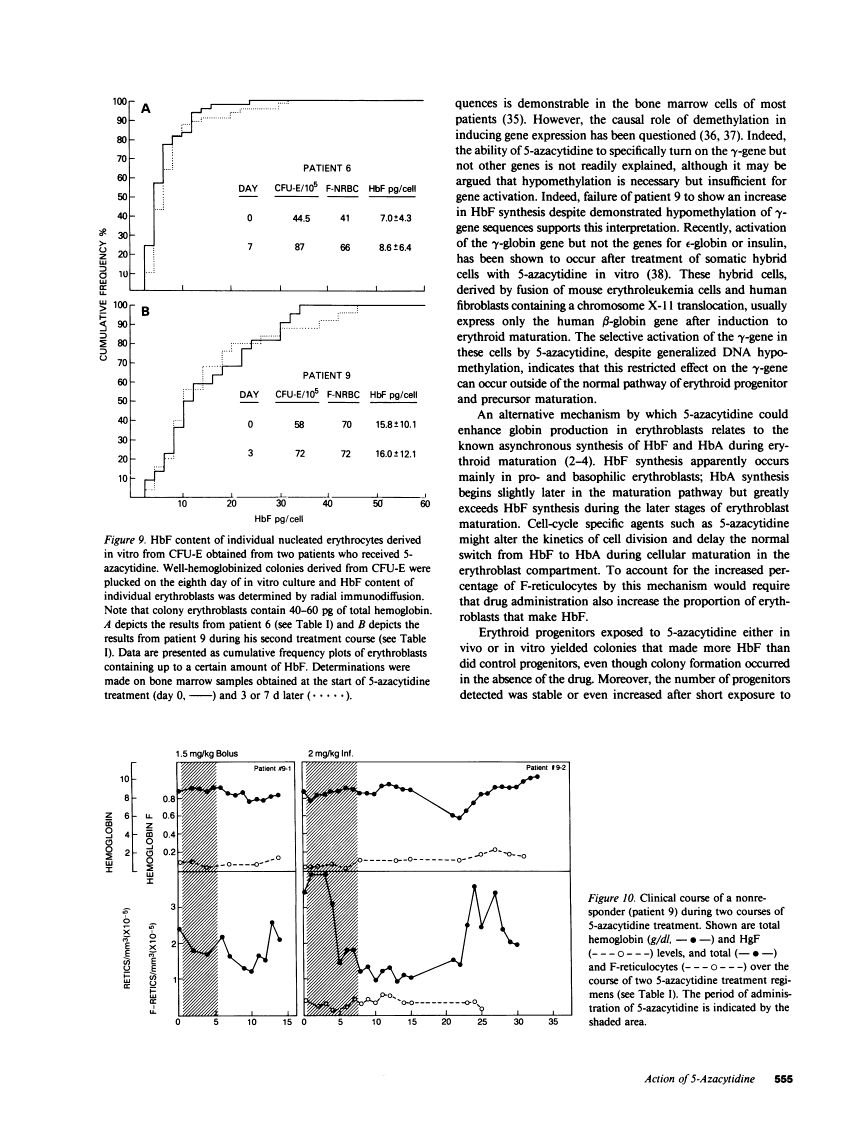
The effect of 5-azacytidine on erythroid precursors and progenitors was studied in nine patients with sickle cell anemia or severe thalassemia. Each patient received the drug intravenously for 5 or 7 d. 5-Azacytidine caused a four- to sixfold increase in gamma-messenger RNA concentration in bone marrow cells of eight of the nine patients and decreased the methylation frequency of a specific cytosine residue in the gamma-globin gene promoter in all nine patients. Within 2 d of the start of drug treatment there was a rise in the percentage of reticulocytes containing fetal hemoglobin (HbF; F-reticulocytes) without a significant change in the total number of reticulocytes, which suggested that there was a direct action of 5-azacytidine on erythroid precursors. Late erythroid progenitors (CFU-E), present in bone marrow after 2 d of drug administration, formed colonies containing an increased amount of HbF as compared with control colonies. Moreover, the number of CFU-E derived colonies was not decreased at these early times, which suggested that there was a direct action of 5-azacytidine on erythroid progenitors in the absence of cytotoxicity. Exposure of normal bone marrow cells in tissue culture to 5-azacytidine for 24 h reproduced both of these effects as judged during subsequent colony formation. The combined direct effects of 5-azacytidine on both the erythroid precursor and progenitor compartments resulted in an increase in HbF synthesis that was sustained for 2-3 wk. Toxicity to bone marrow as reflected by cytoreduction was evident after treatment in some patients but was not accompanied by an increase in HbF production. A correlation was found between the effects of 5-azacytidine on bone marrow, as assessed by in vitro measurements, and the hematological response of the individual patients to drug treatment.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer SH, Belding TK, Margolet L, Noyes AN. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975 Apr 25;188(4186):361–363. [Abstract] [Google Scholar]
- Dover GJ, Boyer SH. Quantitation of hemoglobins within individual red cells: asynchronous biosynthesis of fetal and adult hemoglobin during erythroid maturation in normal subjects. Blood. 1980 Dec;56(6):1082–1091. [Abstract] [Google Scholar]
- Papayannopoulou T, Kalmantis T, Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. [Europe PMC free article] [Abstract] [Google Scholar]
- Papayannopoulou TH, Brice M, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2033–2037. [Europe PMC free article] [Abstract] [Google Scholar]
- Papayannopoulou T, Brice M, Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. [Europe PMC free article] [Abstract] [Google Scholar]
- Dover GJ, Ogawa M. Cellular mechanisms for increased fetal hemoglobin production in culture. Evidence for continuous commitment to fetal hemoglobin production during burst formation. J Clin Invest. 1980 Nov;66(5):1175–1178. [Europe PMC free article] [Abstract] [Google Scholar]
- Kidoguchi K, Ogawa M, Karam JD. Hemoglobin biosynthesis in individual erythropoietic bursts in culture. Studies of adult peripheral blood. J Clin Invest. 1979 Apr;63(4):804–806. [Europe PMC free article] [Abstract] [Google Scholar]
- Dover GJ, Boyer SH, Zinkham WH. Production of erythrocytes that contain fetal hemoglobin in anemia. Transient in vivo changes. J Clin Invest. 1979 Feb;63(2):173–176. [Europe PMC free article] [Abstract] [Google Scholar]
- Groudine M, Kohwi-Shigematsu T, Gelinas R, Stamatoyannopoulos G, Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. [Abstract] [Google Scholar]
- DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. [Europe PMC free article] [Abstract] [Google Scholar]
- Ley TJ, DeSimone J, Anagnou NP, Keller GH, Humphries RK, Turner PH, Young NS, Keller P, Nienhuis AW. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. [Abstract] [Google Scholar]
- Ley TJ, DeSimone J, Noguchi CT, Turner PH, Schechter AN, Heller P, Nienhuis AW. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983 Aug;62(2):370–380. [Abstract] [Google Scholar]
- Charache S, Dover G, Smith K, Talbot CC, Jr, Moyer M, Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. [Europe PMC free article] [Abstract] [Google Scholar]
- Torrealba-de Ron AT, Papayannopoulou T, Knapp MS, Fu MF, Knitter G, Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine. Blood. 1984 Jan;63(1):201–210. [Abstract] [Google Scholar]
- Letvin NL, Linch DC, Beardsley GP, McIntyre KW, Nathan DG. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984 Apr 5;310(14):869–873. [Abstract] [Google Scholar]
- Kantor JA, Turner PH, Nienhuis AW. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. [Abstract] [Google Scholar]
- Favaloro J, Treisman R, Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. [Abstract] [Google Scholar]
- Treisman R, Proudfoot NJ, Shander M, Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. [Abstract] [Google Scholar]
- Ley TJ, Anagnou NP, Pepe G, Nienhuis AW. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. [Europe PMC free article] [Abstract] [Google Scholar]
- Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Jeffreys AJ, Flavell RA. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. [Abstract] [Google Scholar]
- Dover GJ, Boyer SH, Bell WR. Microscopic method for assaying F cell production: illustrative changes during infancy and in aplastic anemia. Blood. 1978 Oct;52(4):664–672. [Abstract] [Google Scholar]
- Gregory CJ, Eaves AC. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977 Jun;49(6):855–864. [Abstract] [Google Scholar]
- Finch CA, Deubelbeiss K, Cook JD, Eschbach JW, Harker LA, Funk DD, Marsaglia G, Hillman RS, Slichter S, Adamson JW, et al. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. [Abstract] [Google Scholar]
- ALPEN EL, CRANMORE D. Cellular kinetics and iron utilization in bone marrow as observed by Fe59 radioautography. Ann N Y Acad Sci. 1959 Jun 25;77:753–765. [Abstract] [Google Scholar]
- Tanaka K, Appella E, Jay G. Developmental activation of the H-2K gene is correlated with an increase in DNA methylation. Cell. 1983 Dec;35(2 Pt 1):457–465. [Abstract] [Google Scholar]
- Ley TJ, Chiang YL, Haidaris D, Anagnou NP, Wilson VL, Anderson WF. DNA methylation and regulation of the human beta-globin-like genes in mouse erythroleukemia cells containing human chromosome 11. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6618–6622. [Europe PMC free article] [Abstract] [Google Scholar]
- Papayannopoulou T, Torrealba de Ron A, Veith R, Knitter G, Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984 May 11;224(4649):617–619. [Abstract] [Google Scholar]
- Dover GJ, Boyer SH, Charache S, Heintzelman K. Individual variation in the production and survival of F cells in sickle-cell disease. N Engl J Med. 1978 Dec 28;299(26):1428–1435. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci111731
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/111731/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci111731
Article citations
5-Aza-4'-thio-2'-deoxycytidine, a New Orally Bioavailable Nontoxic "Best-in-Class": DNA Methyltransferase 1-Depleting Agent in Clinical Development.
J Pharmacol Exp Ther, 379(3):211-222, 09 Sep 2021
Cited by: 11 articles | PMID: 34503994 | PMCID: PMC9164309
Review Free full text in Europe PMC
Challenges in Diagnosing Myelodysplastic Syndromes in the Era of Genetic Testing: Proceedings of the 13th Workshop of the European Bone Marrow Working Group.
Pathobiology, 86(1):62-75, 06 Jul 2018
Cited by: 1 article | PMID: 29982244 | PMCID: PMC6482987
Pharmacological Induction of Human Fetal Globin Gene in Hydroxyurea-Resistant Primary Adult Erythroid Cells.
Mol Cell Biol, 35(14):2541-2553, 18 May 2015
Cited by: 15 articles | PMID: 25986606 | PMCID: PMC4475914
Characterization of Hydroxymethylation Patterns in the Promoter of β-globin Clusters in Murine Fetal Livers.
DNA Cell Biol, 27 Feb 2015
Cited by: 0 articles | PMID: 25723376
Strongly suspicious hemolysis caused by azacitidine in a myelodysplastic syndrome patient.
Geriatr Gerontol Int, 14(4):1006-1007, 01 Oct 2014
Cited by: 0 articles | PMID: 25327907
Go to all (40) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine.
Blood, 63(1):201-210, 01 Jan 1984
Cited by: 47 articles | PMID: 6197114
Enhanced fetal hemoglobin production by phenylacetate and 4-phenylbutyrate in erythroid precursors derived from normal donors and patients with sickle cell anemia and beta-thalassemia.
Blood, 82(7):2203-2209, 01 Oct 1993
Cited by: 70 articles | PMID: 7691251
5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia.
Blood, 62(2):370-380, 01 Aug 1983
Cited by: 133 articles | PMID: 6191799
Induction of hemoglobin F synthesis in patients with beta thalassemia.
Annu Rev Med, 36:485-498, 01 Jan 1985
Cited by: 8 articles | PMID: 2581500
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: HL-02799
Grant ID: HLAM-28022
NIDA NIH HHS (1)
Grant ID: RCDA-1K0400689