Abstract
Background
Most tobacco smokers who wish to quit fail to reach their goal. One important, insufficiently emphasized aspect of addiction relates to the decision-making system, often characterized by dysfunctional cognitive control and a powerful drive for reward. Recent proof-of-principle studies indicate that transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex (DLPFC) can transiently modulate processes involved in decision-making, and reduce substance intake and craving for various addictions. We previously proposed that this beneficial effect of stimulation for reducing addictive behaviors is in part mediated by more reflective decision-making. The goal of this study was to test whether nicotine intake and decision-making behaviors are modulated by tDCS over the DLPFC in tobacco smokers who wished to quit smoking.Methods
Subjects received two five-day tDCS regimens (active or sham). Stimulation was delivered over the right DLPFC at a 2 mA during 30 min. Nicotine cravings, cigarette consumption and decision-making were assessed before and after each session.Results
Main findings include a significant decrease in the number of cigarettes smoked when participants received active as compared to sham stimulation. This effect lasted up to four days after the end of the stimulation regimen. In regards to decision-making, smokers rejected more often offers of cigarettes, but not offers of money, after they received active as compared to sham stimulation at the Ultimatum Game. No significant change was observed at the Risk Task with cigarettes or money as rewards.Conclusion
Overall, these findings suggest that tDCS over the DLPFC may be beneficial for smoking reduction and induce reward sensitive effects.Free full text

Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study
Abstract
Background
Most tobacco smokers who wish to quit fail to reach their goal. One important, insufficiently emphasized aspect of addiction relates to the decision-making system, often characterized by dysfunctional cognitive control and a powerful drive for reward. Recent proof-of-principle studies indicate that transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex (DLPFC) can transiently modulate processes involved in decision-making, and reduce substance intake and craving for various addictions. We previously proposed that this beneficial effect of stimulation for reducing addictive behaviors is in part mediated by more reflective decision-making. The goal of this study was to test whether nicotine intake and decision-making behaviors are modulated by tDCS over the DLPFC in tobacco smokers who wished to quit smoking.
Methods
Subjects received two five-day tDCS regimens (active or sham). Stimulation was delivered over the right DLPFC at a 2mA during 30 minutes. Nicotine cravings, cigarette consumption and decision-making were assessed before and after each session.
Results
Main findings include a significant decrease in the number of cigarettes smoked when participants received active as compared to sham stimulation. This effect lasted up to four days after the end of the stimulation regimen. In regards to decision-making, smokers rejected more often offers of cigarettes, but not offers of money, after they received active as compared to sham stimulation at the Ultimatum Game. No significant change was observed at the Risk Task with cigarettes or money as rewards.
Conclusion
Overall, these findings suggest that tDCS over the DLPFC may be beneficial for smoking reduction and induce reward sensitive effects.
1. INTRODUCTION
Most tobacco smokers who would like to quit smoking fail to achieve such goal (National Institute on Drug Abuse, 2012). Experimental studies have reported impaired decision-making processes in nicotine smokers. For instance, smokers as compared to non-smokers tend to take more risk (Lejuez et al., 2003a, 2005), and such behaviors seem to be reward sensitive: smokers display greater self-interest motives when dealing with cigarettes as compared to money at the Ultimatum Game (Takahashi, 2007). It has been suggested that such decision-making dysfunction is associated to addictive behaviors. More specifically, an unbalance between a weakened inhibitory control and an excessively powerful reward system would push the addict, when experiencing an urge to smoke, to balance his decision toward maladaptive options (Goldstein and Volkow, 2002; Hyman, 2007).
Decision-making processes, such as self-interest motives (Sanfey et al., 2003; Knoch et al., 2011) and risk taking (Rogers et al., 2004; Ernst et al., 2002), have been repeatedly associated with the dorsolateral prefrontal cortex (DLPFC, among a complex cortical and subcortical network; see also Krain et al., 2006) and can be influenced with noninvasive brain stimulation when applied over this region, independent of smoking habits. For instance, transcranial direct current stimulation (tDCS) over the DLPFC can induce a more conservative response style in the context of risk taking at the Risk Task (Fecteau et al., 2007a) and the balloon analog risk task (BART; Fecteau et al., 2007b), and low-frequency repetitive transcranial magnetic stimulation (rTMS) over the DLPFC can increase risk taking at the Risk Task (Knoch et al., 2006a) and self-centered motives at the Ultimatum Game (Knoch et al., 2006b, 2011). Conversely, high-frequency rTMS over the DLPFC during the delay-discounting task has been shown to modulate delayed discounting of gains and losses, enabling subjects to make less impulsive decisions (Sheffer et al. 2013). When applied to nicotine smokers, tDCS over the DLPFC can reduce cigarette intake (Boggio et al., 2010) and cue-induced nicotine craving (Fregni et al., 2008; Boggio et al., 2010). High-frequency rTMS over the DLPFC can also reduce nicotine smoking (Eichhammer et al., 2003; Amiaz et al., 2009) and craving (Johann et al., 2003; Amiaz et al., 2009; Li et al., 2013). Diminished craving has also been reported with tDCS or rTMS over the DLPFC for alcohol (Boggio et al., 2008), cocaine (Camprodon et al., 2007; Politi et al., 2008), and food (Uher et al., 2005; Fregni et al., 2007). A recent meta-analysis on the noninvasive brain stimulation (NIBS)-induced reduction of drug cravings intensity has determined that active stimulation (tDCS or rTMS) significantly decrease cravings compared to sham (Jansen et al., 2013). Of interest, the meta-analysis points out that there is no significant difference in the reduction of craving between stimulation of the left or right DLPFC, and the effect remains stable across substances. Although further analyses are needed to evaluate the mechanisms of action of tDCS and rTMS and their respective effect magnitude in this perspective, the results are promising.
We proposed that the beneficial effects of noninvasive brain stimulation over the DLPFC on decreasing addictive behaviors, including nicotine intake, reflect shifts in the role of the DLPFC related to decision-making behaviors (Fecteau et al., 2010). Specifically, we suggested that it might influence the individual’s response to smoking triggers, shifting decision-making processes to a more reflective mode, reducing reward seeking and resulting in significant reduction of cigarette intake. The goal of this study was thus to test whether tDCS applied over the DLPFC modulate nicotine intake and decision-making behaviors in tobacco smokers who wished to quit smoking. Based on our previous work (Fregni et al., 2008; Boggio et al., 2010), we first hypothesized that when smokers receive active as compared to sham stimulation, they will reduce smoking and report a decreased level of nicotine craving. Also, in keeping with our theoretical framework (Fecteau et al., 2010), we predicted that modulation of decision-making processes will be reward sensitive, that is modulation will be greater when the reward consists of cigarettes than money.
2. MATERIALS AND METHODS
2.1 Design
The present study was a crossover, blind at four levels (group allocator, subjects, tDCS provider, outcome assessor), randomized, sham-controlled design in which subjects received two five-day regimens (one active and one sham) of tDCS over the DLPFC. Three months separated the two tDCS regimens to avoid carryover effects. Subjects were asked to fill out a daily cigarette diary during the entire experiment and to perform decision-making tasks before and after each tDCS regimen.
2.2 Participants
Twelve adults (5 men; mean age of 36.3; range 21 to 64 years) took part of the study. One participant was categorized as “heavy smoker” (25> cigarettes per day), seven as “moderate smokers” (15–24 cigarettes per day) and four as “light smokers” (<15 cigarettes per day; Wilson et al., 1999). They were all in the contemplator stage, as assessed by the Prochaska and DiClemente questionnaire (Prochaska and DiClemente, 1983), indicating they wanted to quit smoking. Participants had no history of neurological or psychiatric disorders other than addiction for nicotine smoking and had normal physical and neurological exams. One participant (subject 2) was taking lisinopril for high blood pressure. Participants were also assessed on nicotine dependence (Fagerström Test for Nicotine Dependence; Fagerstrom et al., 1990) impulsivity (Barratt Impulsiveness Scale; Patton et al., 1995), mood (Beck Depression Inventory; Beck et al., 1996), sleep (Pittsburgh Sleep Quality Index; Buysse et al., 1988), food habits (General Craving Food Questionnaire-Trait; Cepeda-Benito et al., 2000), and handedness (Edinburgh Handedness Inventory). None of the participants dropped out of the study. Demographic and clinical measures are presented in Table 1.
Table 1
Demographic and clinical measures
| ID | Gender | Age | Handedness | N of cigarettes | Time | FTND | BIS | BDI | G-FCQ-T | PSQI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f | 62 | 84 | 25 | < 5 | 6 | 55 | 0 | 25 | 1 |
| 2 | m | 53 | 100 | 20 | 6–30 | 5 | 77 | 10 | 55 | 6 |
| 3 | f | 58 | 40 | 15 | 6–30 | 3 | 72 | 2 | 40 | 2 |
| 4 | m | 22 | 100 | 12 | 6–30 | 6 | 56 | 2 | 45 | 9 |
| 5 | f | 64 | 100 | 20 | < 5 | 7 | 68 | 1 | 35 | 2 |
| 6 | f | 21 | 30 | 15 | 6–30 | 4 | 54 | 9 | 38 | 9 |
| 7 | f | 20 | 100 | 15 | 31–60 | 2 | 73 | 2 | 27 | 12 |
| 8 | f | 21 | 63 | 10 | 31–60 | 2 | 73 | 4 | 42 | 5 |
| 9 | m | 25 | 100 | 20 | < 5 | 6 | 79 | 10 | 57 | 5 |
| 10 | m | 36 | 100 | 7 | 31–60 | 2 | 59 | 4 | 40 | 5 |
| 11 | f | 32 | 100 | 10 | 6–30 | 2 | 65 | 15 | 23 | 10 |
| 12 | m | 21 | 88 | 17 | 6–30 | 5 | 90 | 5 | 37 | 2 |
Number of cigarettes: average of cigarettes smoked per day before starting the experiment. Time: laps in minutes between awakening and first cigarette.
FTND: Fagerström Test for Nicotine Dependence BIS: Barratt Impulsiveness Scale 11. BDI: Beck Depression Inventory. G-FCQ-T: General Craving Food Questionnaire Trait. PSQI: Pittsburgh Sleep Quality Index.
Participants were screened for noninvasive brain stimulation contraindications (Wasserman, 1998; Rossi et al., 2009). All were naive to brain stimulation, the decision-making tasks, and were not informed about the experimental variables related to the cognitive tasks tested. Written informed consent was obtained from participants prior to inclusion in the study, which was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center. All stimulation sessions were conducted with strict adherence to current safety guidelines and recommendations at the Harvard-Thorndike Clinical Research Center and the Berenson-Allen Center for Noninvasive Brain Stimulation (Beth Israel Deaconess Medical Center, Harvard Medical School). A standardized questionnaire on side effects of stimulation (modified after Keel et al., 2001) and mood (as previously used in Fregni et al., 2008) were administrated following each stimulation session. All side effects reported by participants are listed in Table 2.
Table 2
Side effects reported by subjects at each stimulation sessions
| Subject ID | Sham stimulation | Active stimulation |
|---|---|---|
| 1 | Sleepiness (Day 1) | |
| 2 | Sleepiness (Day 1) | Headache (Day 1, Day 2) |
| Neck pain (Day 2) | ||
| Sleepiness (Day 1, Day 2) | ||
| Trouble concentrating (Day 1) | ||
| 3 | Headache (Day 1, Day 2) | |
| Neck pain (Day 2) | ||
| Sleepiness (Day 2) | ||
| 4 | Sleepiness (Day 2, Day 5) | |
| 10 | Sleepiness (Day 1) | Sleepiness (Day 3) |
| 11 | Sleepiness (Day 1, Day 5) Trouble concentrating (Day 1) | Tingling (Day 1) |
2.3 Smoking intake with the reported number of cigarettes smoked and carbon monoxide measurement
We measured the number of cigarettes smoked using a daily calendar (Boggio et al., 2010). Subjects were required to complete the calendar at the end of every day during each of the two arms (nine days for each arm). We measured levels of carbon monoxide before stimulation (Day 1) and immediately after stimulation (Day 5) using a carbon monoxide monitor (piCO+ Smokerlyzer; Bedfont, Scientific Ltd., Kent, UK).
2.4 Nicotine craving
Level of nicotine craving was characterized with a cue-provoked paradigm. We used videos displaying individuals smoking employed previously (Fregni et al. 2007; Boggio et al., 2010). In order to rate level of craving, we used the standardized Questionnaire of Smoking Urges from Tiffany and Drobes (1991). This questionnaire consists of four different subscales: Desire to smoke, Anticipation of positive outcome, Relief from negative affect, and Intention to smoke. This paradigm was administrated before stimulation (Day 1) and immediately after the last stimulation session (Day 5).
2.5 Ultimatum Game
Subjects performed two versions of the Ultimatum Game. The two versions were identical with the only difference of the type of reward. One version consisted of the original task in which subjects were rewarded with money (Guth et al., 1982), and in the modified version, subjects were rewarded with cigarettes. In this task, a proposer offers the subject to split a certain amount of money ($10). The subject can either accept or reject the offer. If he accepts the offer, the money is split as proposed, but if he rejects, none of them receive the money/cigarette. Most individuals reject low offers ($1 and $2) to punish the proposer as they consider the offer unfair and this punishment drive overwhelms self-centered motives (e.g., Sanfey et al., 2003; Knoch et al., 2006b). Although smokers show the same punishment behavior for money, they however accept unfair offers of cigarettes, showing greater self-centered motives in that context (Takahashi, 2007). The main outcome in this study was the acceptance rate. Data were analyzed for unfair offers ($1 and $2; 1 and 2 cigarettes), midfair offers ($3 and 3 cigarettes), and fair offers ($4 and $5; 4 and 5 cigarettes) for both the money and cigarettes version of the Ultimatum Game. Rewards were not real. There were 150 trials for each version. For each tDCS arm, subjects performed the two versions of the Ultimatum Game before stimulation (Day 1) and immediately after the last stimulation session (Day 5).
2.6 Risk Task
Subjects performed two versions of the Risk Task. The two versions were identical with the only difference being the type of reward. One version consisted of the original Risk Task in which subjects were rewarded with points (Rogers et al., 1999; Fecteau et al., 2007a). A modified version was also administrated, in which cigarettes constituted the reward. In both tasks, subjects were presented with six boxes and each box could either be pink or blue. The ratio of pink and blue boxes varies from trial to trial and can be 5:1, 4:2, or 3:3. Subjects have to pick the color of the box that hides the winning token. For each trial, the ratio of pink to blue boxes (level of risk) effectively determines the probability of findings the winning token and thus the level of risk of the choice. The winning color and levels of risk and reward changed from one trial to another, disrupting any possible winning stratagem or tactic. Subjects were thus not aware of any winning strategy to maximize their earnings and were not instructed in a way or another to adopt such a strategy. Subjects were rewarded with an amount of points or cigarettes for correctly guessing the color of the box hiding the winning token and punished by loosing points for picking the incorrect color. The amount of reward (or penalty) associated with any scenario varies (balance of reward) and is indicated on the screen. Importantly, the conflict inherent in risk taking is reflected by the fact that the largest reward is always associated with the least likely of the two outcomes (i.e., the most risky option). Also, it has been shown that addicts take more risk at the Risk Task as compared to nonaddicts (Rogers et al., 1999). The outcome measure in this study was the choice of low risk versus high risk options. The choice was calculated as the number of times subjects choose the low risk option on all trials excluding the neutral conditions (i.e., equal number of blue and pink boxes). Rewards were not real. There were 96 trials for each version. For each tDCS arm, subjects performed the two versions of the Risk Task at two time points: before tDCS (Day 1) and immediately after the last tDCS session (Day 5).
2.7 Transcranial Direct Current Stimulation
Each subject received the two types of stimulation conditions (active and sham) in a counterbalanced order. Studies on smokers have reported greater beneficial effects on smoking behaviors associated with anodal stimulation over the right DLPFCs coupled with cathodal stimulation over the left DLPFC. We therefore focused on this electrode arrangement (referred here as ‘right anodal/left cathodal’). This arrangement is presumed to enhance excitability in the right DLPFC and decrease it in the left DLPFC. A second stimulation condition was a sham stimulation control with the same electrodes arrangement as in the active stimulation (anodal over the left and cathodal over the right DLPFC). Direct current was induced by two saline-soaked surface sponge electrodes (35 cm2) and delivered by a batterydriven, constant current stimulator (neuroConn, Germany). For active stimulation, participants received a constant current of 2mA intensity during 30 minutes. The anode electrode was placed over F4 (international EEG 10/20 system) and the cathode electrode over F3. For sham stimulation, the electrodes were placed at the same position as for active stimulation (F4 and F3), but the stimulator was turned on to 2 mA only for the first 30 seconds and at the last 30 seconds of the 30-minute period so participants felt the itching sensation associated with ramping up and down the current, but received no active current otherwise. This method of sham stimulation has been shown to be reliable, at least for a 1 mA current (Gandiga et al., 2006), though may not totally blind participants when 2 mA is applied. Nonetheless, when explicitly asked at the end of the study, none of the fourteen participants believed to have undergone sham or active stimulation with a confidence level greater than 50%, suggesting successful blinding of the stimulation conditions.
2.8 Data analysis
All statistical analyses were performed using SPSS software (version 22.0, SPSS Inc., Chicago). Results with a P value ≤ 0.05 were considered significant for all statistical analyses. If appropriate, post hoc analyses were performed with correction for multiple comparisons.
3. RESULTS
3.1 Smoking intake
Smoking intake was first tested to investigate whether the number of cigarettes was different when subjects received active stimulation as compared to when they received sham stimulation. Data were analyzed with the Generalized Estimating Equations procedure (GEE) of SPSS. GEE is a type of ANOVA for repeated measures based on maximum likelihood estimation (MLE). It is more robust with missing data, heterogeneity of variance and non-normal distributions. Although our data are apparently normally distributed, we had three participants with missing data and a heterogeneous first-order autoregressive covariance matrix (heterogeneity of variance). The specification of this type of matrix [AR(1)] was included into the model declaration. Factors of stimulation (active, sham) and time (Day 1 [pre-stimulation] through Day 9) were analyzed. If the active stimulation changes the number of cigarettes across time, the interaction had to be statistically significant. The sequence (active stimulation as first arm and sham stimulation as second arm, sham stimulation as first arm and active as second arm) was used as a covariable.
The specified model had the best goodness-of-fit statistic (QICC = 5357). Results revealed significant main effects of stimulation (p=.0065) and time (p<.0001) with a significant interaction (p=,0005) and with no significant interference from the covariable (p=.1662). Please refer to Figure 1 for an illustration and Table 3 for the data.
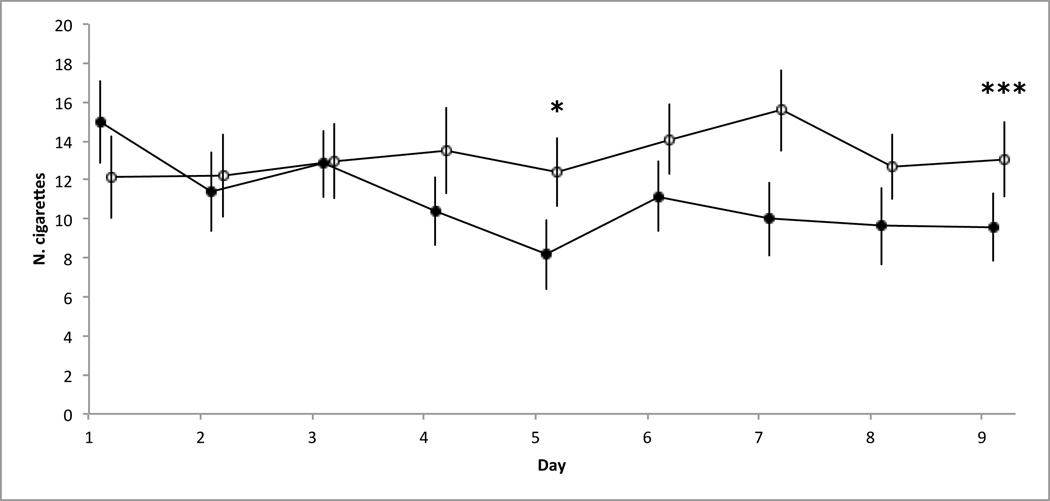
Group mean of the reported number cigarettes smoked for each day and stimulation conditions (active and sham). Bars represent SEMs. Means and SEMs are corrected to remove the covariable statistical impact (order of passation); * p<0.05, ** p<0.01, *** p<0.001. Significant results are shown after sequential Bonferonni correction. Active stimulation is represented with black circles and sham stimulation is represented with white circles. Values at Day 1 represent the baseline cigarette intake before initiation of stimulation; Day 2 to Day 5 during the stimulation; Day 6 to Day 9 during the followup assessment.
Table 3
Mean number of cigarettes during the course of the study
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Active Stimulation | |||||||||
| Mean | 14.99 | 11.41 | 12.82 | 10.41 | 8.16 | 11.16 | 10.24 | 9.69 | 9.6 |
| SEM | 2.02 | 1.96 | 1.62 | 1.64 | 1.69 | 1.69 | 1.82 | 1.89 | 1.65 |
| Sham Stimulation | |||||||||
| Mean | 12.16 | 12.24 | 12.99 | 13.49 | 11.51 | 14.07 | 15.69 | 12.69 | 13.06 |
| SEM | 2.04 | 2.05 | 1.84 | 2.09 | 2.13 | 1.70 | 2.01 | 1.55 | 1.83 |
GENLIN post hoc analyses, and effect size calculation with Cohen’s d, were conducted to test for differences between active and sham stimulation conditions at each day. A Cohen's d value of -.5 indicates that the active stimulation caused a decrease of the reported number of cigarette equivalent to one half standard deviation. Before the sequential Bonferroni correction, active and sham stimulation conditions have different impact on all days except days 2 and 3: Day 1 (pre-stimulation; p=.04; d=0.39), post-stimulation Day 2 (p=.67; d=−0.12), Day 3 (p=.95; d=−0.03), Day 4 (p=.02; d =−0.45), Day 5 (p=.007; d =−0.71), Day 6 (p=.012; d =−0.47), Day 7 (p=.008; d =−0.85), Day 8 (p=.002; d =−0.51), Day 9 (p=.00006; d =−0.58). After the sequential Bonferroni correction, conditions are different only on day 5 (p=.05) and day 9 (p=.0009). On day 5, active stimulation decreased the number of cigarette by 4,24. On day 9, this decrease is of 3,46 cigarettes.
Furthermore, regression slopes revealed a significant slope for the active stimulation condition (p=.03), but not for the sham stimulation condition (p=.28). Finally, we compared regression coefficient between the two stimulation conditions, which indicate a significant difference between the two slopes (p=.02).
Level of carbon monoxide was analyzed using Wilcoxon test between active and sham stimulation conditions (change from baseline at Day 1 to post-stimulation at Day 5). There was no significant effect of stimulation, though a trend was noted (p=.06).
3.2 Nicotine craving
We tested whether stimulation had an effect cue-induced smoking craving using Wilcoxon test between active and sham stimulation conditions (change from baseline at Day 1 to post-stimulation at Day 5) for each of the four subscales of the standardized Questionnaire of Smoking Urges (Tiffany and Drobes, 1991). There was a significant difference for the Desire to smoke scale (p=.02), but not for the remaining scales: Anticipation of positive outcome (p=.37), Intention to smoke (p=.33), and Relief from negative affect (p=.64).
3.3 Ultimatum Game
In order to test whether stimulation had an effect on performance at the Ultimatum Game, the percent of change of acceptance rate of offers between baseline at Day 1 and post-stimulation at Day 5 was entered as dependent variable with stimulation (active, sham) and type of offer (unfair, mid-fair, fair) as independent fixed variables for each version of the Ultimatum Game. We analyzed the graphic representation of performance data from the two experiences (monetary reward and cigarettes) to ensure data fitted the normal distribution, and proceeded with two-way ANOVAs. For the Ultimatum Game with cigarettes as reward, there was an effect of stimulation (F(1,11)=6.932, p=.02), but no effect of type of offer (F(2,22)=.812; p=.46). Please refer to Figure 2 for an illustration and Table 4 for raw data. For the Ultimatum Game with money as reward, there were no effects of stimulation (p=.10) or type of offer (p=.20).
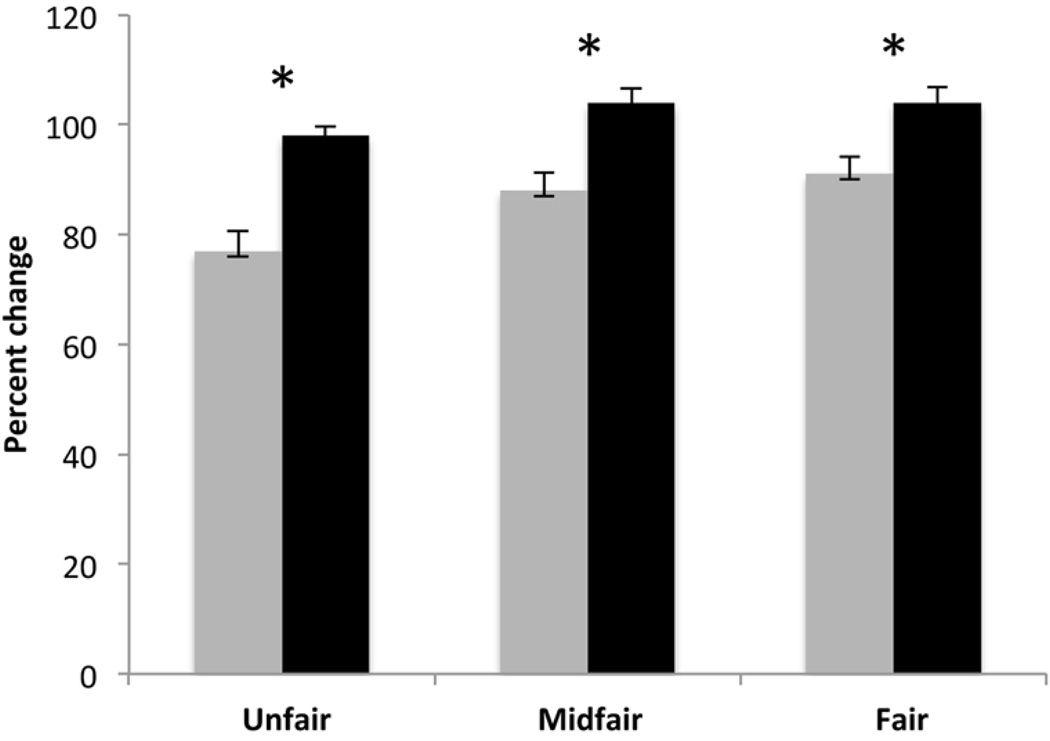
Group mean percent changes in the choice of accepting offers of cigarettes at the Ultimatum Game for each type of offer and stimulation condition (active and sham). Bars represent Standard Error of Mean; * p<0.05. Active stimulation is represented with grey bars; sham stimulation is represented with black bars.
Table 4
Mean percent changes in acceptance during the Ultimatum Game
| Type of offer | Unfair | Midfair | Fair |
|---|---|---|---|
| Active Stimulation | |||
| Mean percent change | 77 | 88 | 91 |
| SEM | 3.56 | 3.23 | 3.19 |
| Sham Stimulation | |||
| Mean percent change | 98 | 104 | 104 |
| SEM | 1.65 | 2.54 | 2.83 |
3.4 Risk Task
We sought to determine whether stimulation had an effect on performance at the Risk Task between active and sham stimulation entering the percent of change in the number of low-risk choice between baseline at Day 1 and post-stimulation at Day 5. We analyzed the data with a two-way ANOVA with type of stimulation (active and sham) and risk level (low and high) as two independent multi-levels within-subjects variables, and percent of change in performance as our dependent variable. Each risk level (low and high) was also segregated according to four levels of reward balance, as described in the Risk Task. Effects of stimulation or risk level alone were not significant for money (risk level, p= .59; stimulation, p =.62) or cigarettes (risk level, p=.14; stimulation, p =.54) as reward. The interaction between type of stimulation and risk level was not significant for both types of reward. In a secondary analysis, we sought to determine whether there were significant differences between active and sham stimulation, between baseline at Day 1 and post-stimulation at Day 5, when comparing the percent of change in performance at the two risk levels, without differentiating each risk levels according to the reward balance (thus, using a less specific performance criterion). Using the same statistical model, we found no significant effect of the type of stimulation and risk level (high and low) and this, with both money (risk level, p=.63; stimulation, p =.09) and cigarettes (risk level, p=.60; stimulation, p =.84) as reward. Hence, the risk task analyses seem to demonstrate that active stimulation did not significantly influence performance at the Risk Task, independently from the risk level associated to the task.
4. DISCUSSION
The present study aimed at promoting smoking reduction by testing the role of a fundamental concept of decision-making proposed by Goldstein and Volkow (2002) with a noninvasive brain stimulation approach. Specifically, this study was designed to test whether addictive behaviors and decision-making processes were modulated when tDCS was applied over the DLPFC in tobacco smokers. Main findings include significant reduction of the reported number of cigarettes smoked after subjects received active stimulation, but not when they received sham stimulation. This supports previous work (Boggio et al., 2010). Moreover, extends prior findings revealing that the decreased number of cigarettes was maintained even four days after the end of the active stimulation regimen.
Results from this work also suggest that some processes of decision-making behaviors are modulated when comparing performance before and after active tDCS in smokers, and that this effect is reward sensitive. Specifically, smokers rejected more often offers at the Ultimatum Game when the reward was cigarettes, but not money, after they received active as compared to sham stimulation. Processes involved in making a decision in the Ultimatum Game include self-interest motives and punishment to a peer (i.e., when you reject an offer you refrain your peer from receiving the reward). In this context, it seems that smokers act as protecting themselves from getting cigarettes, but also preventing the peers from receiving cigarettes. This was observed regardless of the fairness of the offers: they were were rejecting more often fair, midfair and unfair offers. Thus, smokers were not specifically punishing the peers of being unfair; they were presumably more driven by self-interest motives (e.g., reduce or quit smoking) than by desires of altruistic punishment of others. These effects might not be solely due to tDCS but the tDCS-induced bevavioral changes as well (e.g., smoking fewer cigarettes, diminished craving levels). Performance at the Risk Task with rewards of points and cigarettes remained similar when smokers received active and sham stimulation. These findings indicate that risk taking in this context was not modulated by stimulation for either salient (i.e., cigarettes) or non salient (i.e., points) rewards.
In Fecteau et al. (2007), there was supression of risk taking at the Risk Task with points as rewards after a single session of tDCS in healthy subejcts. In Knoch et al. (2006), there an an increase of acceptance rate at the Ultimatum Game after a single session of low-frequency repetitive transcranial magnetic stimulation applied over the right DLPFC in healthy subjects. Findings from the present work differ from that of these previous studies. Several factors likely contribute to this. First, baseline performance may differ between smokers and healthy subjects. Some studies reported difference in performance between individuals with addictions and healthy individuals at decision-making tasks (Lejuez et al., 2003; Bickel et al., 1999; but see Takahashi et al., 2007). Second, the effects of repeated sessions of tDCS are likely different from that of a single sesison of tDCS or rTMS. Third, behavioral-induced changes might have played a role in this study as well. We cannot rule out whether observed changes in smoking behaviors (e.g., reduced number of cigarettes smoked and/or suppressed desire to smoke) partially explained decision-making behaviors tested here, or vice-versa.
4.1 Limitations
There are some limitations in this work. First, the present results comes a small sample size. There is a need to replicate these findings with a larger sample of subjects. Second, we failed to find a significant change in level of carbon monoxide. Subjects were thus smoking a significant lesser number of cigarettes but did not equally reduce the level of carbon monoxide (although one might consider there was a trend: p =.06), suggesting that they might have inhaled more each cigarette when they received active as compared to sham stimulation. Another limitation of this work is that we measured the level of certainty of the administered stimulation condition (active, sham) with a visual analog scale, but we did not measure accuracy per se, which might have resulted in different judgment on blindness of the stimulation condition. Finally, it should be noted that the rewarding cigarettes were not actually given to the subjects after the completion of the tasks. We made this decision because first we believe that providing smokers who wished to quit with real cigarettes was not ethical. Second, real and virtual rewards appear to be processed in a similar way. For instance, it has been shown that real and virtual reward induced similar behavioral responses (Bowman and Turnbull, 2003) and brain activation patterns (Miyapuram et al., 2012). Future work should further characterize smokers as slow and fast metabolizers using H3/cotinine ratios as this might influence the findings. Future work should also include neuroimaging measures to identify the neural substrates that are modulated by tDCS and associated with decreased in the number of cigarettes smoked. Here, we cannot state whether both electrodes or either the anodal or cathode electrode caused the observed effects. We can only say that these effects were found when comparing active to sham stimulation by applying the anode over F4 and cathode over F3.
4.2 Conclusion
In sum, results from this work suggest that stimulation with tDCS may help to reduce nicotine smoking in adults by acting onto the reward system as performance on the Ultimatum Game was reward sensitive. Future work should include longer follow-up to test whether tDCS might help maintaining this reduction and even promote smoking cessation.
Acknowledgements
We would like to thank our participants and Dr. Jean Leblond from the CIRRIS for statistical advice.
Funding
This work was supported by grants from the Canada Research Chair and Canadian Institutes of Health Research to SF and by the Harvard-Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center integrated in the Harvard Clinical and Translational Science Center, supported by grants M01-RR- 01066 and UL1 RR025758 from the National Center for Research Resources; by grants from the National Institutes of Health (K24RR018875) to APL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
There are no competing financial interests in relation to the work described.
Author Contributions
All authors have contributed equally to this manuscript, and approve its contents and submission.Contributor Information
Shirley Fecteau, Laboratory of Canada Research Chair in Cognitive Neuroscience, Centre Interdisciplinaire de recherche en réadaptation et intégration sociale, Centre de Recherche l’Institut Universitaire en Santé Mentale de Québec, Medical School, Laval University, Canada; Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, USA.
Sara Agosta, Center for Neuroscience and Cognitive Systems@UniTn, Italian Institute of Technology, Italy; Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, USA.
Antoine Hone-Blanchet, Laboratory of Canada Research Chair in Cognitive Neuroscience, Centre Interdisciplinaire de recherche en réadaptation et intégration sociale, Centre de Recherche l’Institut Universitaire en Santé Mentale de Québec, Medical School, Laval University, Canada.
Felipe Fregni, Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, USA; Laboratory of Neuromodulation, Spaulding Rehabilitation Hospital, Harvard Medical School, USA.
Paulo Boggio, Núcleo de Neurociências, Centro de Ciências Biológicas e da Saúde, Universidade Presbiteriana Mackenzie, Brazil.
Domenic Ciraulo, Boston University School of Medicine, Boston Medical Center, USA.
Alvaro Pascual-Leone, Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, USA; Institut Guttmann de Neurorehabilitació, Universitat Autónoma, Spain.
REFERENCES
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–660. [Abstract] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. 2nd edn. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Boggio P, Liguori P, Sultani N, Rezende L. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neuroscience. 2009;463:82–86. [Abstract] [Google Scholar]
- Bowman CH, Turnbull OH. Real versus facsimile reinforce on the Iowa Gambling Task. Brain Cogn. 2003;53:207–210. [Abstract] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. [Abstract] [Google Scholar]
- Boggio P, Sultani N, Fecteau S, Merabet L. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. [Abstract] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih M-C, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. [Abstract] [Google Scholar]
- Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the state and trait food-cravings questionnaires. Behav. Ther. 2000;31:151–173. [Abstract] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J. Clin. Psychiatry. 2003;64:951–953. [Abstract] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. [Abstract] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69:763–765. [Abstract] [Google Scholar]
- Fecteau S, Fregni F, Boggio PS, Camprodon JA, Pascual-Leone A. Neuromodulation of decision-making in the addictive brain. Subst. Use Misuse. 2010;45:1766–1786. [Europe PMC free article] [Abstract] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J. Neurosci. 2007a;27:12500–12505. [Europe PMC free article] [Abstract] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, Fregni F. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J. Neurosci. 2007b;27:6212–6218. [Europe PMC free article] [Abstract] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche M. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J. Clin. Psychiatry. 2008a;69:32–40. [Abstract] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome F, Nitsche M, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008b;51:34–41. [Europe PMC free article] [Abstract] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006;117:845–850. [Abstract] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. [Europe PMC free article] [Abstract] [Google Scholar]
- Güth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. J. Econ. Behav. Organ. 1982;3:367–388. [Google Scholar]
- Hyman SE. The neurobiology of addiction: implications for voluntary control of behavior. Am. J. Bioeth. 2007b;7:8–11. [Abstract] [Google Scholar]
- Jansen JM, Daam JG, Maarten WJK, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci. Biobehav. Rev. 2013 In Press. [Abstract] [Google Scholar]
- Johann MN, Wiegand R, Kharraz A, Bobbe G, Sommer G, Hajak G, Wodarz N, Eichhammer P. Repetitive transcranial magnetic stimulation in nicotine dependence. Psychiatrische Praxis. 2003;30(Suppl. 2):129–131. [Abstract] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. 2001;112:720. [Abstract] [Google Scholar]
- Knoch D, Gianotti LRR, Baumgartner T, Fehr E. A neural marker of costly punishment behavior. Psychol. Sci. 2010;21:337–342. [Abstract] [Google Scholar]
- Knoch D, Gianotti LRR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J. Neurosci. 2006a;26:6469–6472. [Europe PMC free article] [Abstract] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006b;314:829–832. [Abstract] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. [Abstract] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Exp. Clin. Psychopharm. 2003;11:26–33. [Abstract] [Google Scholar]
- Lejuez CW, Aklin W, Bornovalova M, Moolchan E. Differences in risk-taking propensity across inner-city adolescent ever- and never-smokers. Nicotine Tob. Res. 2005;7:71–79. [Abstract] [Google Scholar]
- Li X, Hartwell KJ, Owens M, LeMatty T, Borckardt JJ, Hanlon CA, Brady KT, George MS. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol. Psychiatry. 2013;73:714–720. [Europe PMC free article] [Abstract] [Google Scholar]
- Miyapuram KP, Tobler PN, Gregorios-Pippas L, Schultz W. BOLD responsein reward regions to hypothetical imaginary monetary rewards. Neuroimage. 2012;59:1692–1699. [Europe PMC free article] [Abstract] [Google Scholar]
- National Institute on Drug Abuse. Topics in Brief, Tobacco Addiction Research Update. 2012 http://www.drugabuse.gov/sites/default/files/tobacco_1.pdf.
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995;51:768–774. [Abstract] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict. 2008;17:345–346. [Abstract] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J. Consult. Clin. Psychol. 1983;51:390–395. [Abstract] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J. Neurosci. 1999;20:9029–9038. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry. 2004;55:594–602. [Abstract] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanfey AG. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–1758. [Abstract] [Google Scholar]
- Sheffer CE, Mennemeier M, Landes RD, Bickel WB, Brackman S, Dornhoffer J, Kimbrell T, Brown G. Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J. Subst. Abuse Treat. 2013;45:206–214. [Europe PMC free article] [Abstract] [Google Scholar]
- Takahashi T. Economic decision-making in the ultimatum game by smokers. Neuroendocrinol. Lett. 2007;28:659–661. [Abstract] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Addiction. 1991;86:1467–1476. [Abstract] [Google Scholar]
- Uher R, Yoganathan D, Mogg A, Eranti S. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol. Psychiatry. 2005;58:840–842. [Abstract] [Google Scholar]
- Wassermann E. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroen. Clin. Neuro. 1998;108:1–16. [Abstract] [Google Scholar]
- Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev. Med. 1999;29:139–144. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.drugalcdep.2014.03.036
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4242508?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.drugalcdep.2014.03.036
Article citations
Unveiling the grip of mobile phone addiction: an in-depth review.
Front Psychiatry, 15:1429941, 02 Oct 2024
Cited by: 0 articles | PMID: 39415886 | PMCID: PMC11479953
Review Free full text in Europe PMC
Is Transcranial Direct Current Stimulation Effective for Cognitive Dysfunction in Substance Use Disorders? A Systematic Review.
Brain Sci, 14(8):754, 27 Jul 2024
Cited by: 0 articles | PMID: 39199449 | PMCID: PMC11352984
Review Free full text in Europe PMC
Transcranial random noise stimulation (tRNS) improves hot and cold executive functions in children with attention deficit-hyperactivity disorder (ADHD).
Sci Rep, 14(1):7600, 31 Mar 2024
Cited by: 1 article | PMID: 38556535 | PMCID: PMC10982302
The effect of bipolar bihemispheric tDCS on executive function and working memory abilities.
Front Psychol, 14:1275878, 03 Jan 2024
Cited by: 0 articles | PMID: 38235279 | PMCID: PMC10791995
A systematic review and meta-analysis of neuromodulation therapies for substance use disorders.
Neuropsychopharmacology, 49(4):649-680, 12 Dec 2023
Cited by: 9 articles | PMID: 38086901 | PMCID: PMC10876556
Review Free full text in Europe PMC
Go to all (81) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of transcranial direct current stimulation on the number of smoked cigarettes in tobacco smokers.
PLoS One, 14(2):e0212312, 14 Feb 2019
Cited by: 5 articles | PMID: 30763404 | PMCID: PMC6375608
Transcranial direct current stimulation of the prefrontal cortex reduces cigarette craving in not motivated to quit smokers: A randomized, sham-controlled study.
Addict Behav, 120:106956, 23 Apr 2021
Cited by: 6 articles | PMID: 33940337
Effects of psychoeducation combined with transcranial direct current stimulation on reducing cigarette craving and consumption in male smokers.
Addict Behav, 141:107643, 06 Feb 2023
Cited by: 1 article | PMID: 36791642
Transcranial Direct Current Brain Stimulation Increases Ability to Resist Smoking.
Brain Stimul, 9(2):191-196, 23 Oct 2015
Cited by: 37 articles | PMID: 26572280 | PMCID: PMC4789149
Funding
Funders who supported this work.
CIHR
NCRR NIH HHS (5)
Grant ID: UL1 RR025758
Grant ID: K24RR018875
Grant ID: M01-RR-01066
Grant ID: UL1RR025758
Grant ID: K24 RR018875





