Abstract
Free full text

Circulating tetrahydrobiopterin as a novel biomarker for abdominal aortic aneurysm
Abstract
Rupture of abdominal aortic aneurysm (AAA) is unpredictable and lethal. A clinically valid biomarker to monitor the disease has not been available. Based on our recent discoveries that uncoupled endothelial nitric oxide synthase (eNOS)/tetrahydrobiopterin deficiency plays a causal role in various models of AAA, the present study examined the relationship between circulating and tissue levels of tetrahydrobiopterin (H4B) in angiotensin II-infused hyperphenylalaninemia (hph-1) and apoE null mice. For apoE null mice, tissue and plasma H4B levels decreased time dependently, to 2.69 ± 0.15 and 1.99 ± 0.06 pmol/mg, respectively (from 4.86 ± 0.32 and 3.31 ± 0.13 pmol/mg at baseline) by week 3, when aneurysms developed. For hph-1 mice, tissue and plasma H4B levels decreased significantly to 1.02 ± 0.10 and 0.98 ± 0.09 pmol/mg, respectively (from 1.84 ± 0.18 and 1.48 ± 0.12 pmol/mg at baseline), by week 1, when aneurysms developed. Oral folic acid administration, which has been shown to improve aortic H4B levels to completely prevent or markedly decrease the incidence of AAA, significantly increased tissue and plasma H4B levels in both animal models starting at week 1. The two H4B measurements at all conditions showed significant linear correlation, suggesting that plasma H4B accurately predicts its tissue levels when H4B is either reduced or enhanced. Together, these data demonstrate that H4B levels decrease with AAA development and increase with folic acid treatment in two different murine models of AAA and that plasma H4B levels accurately reflect H4B levels in the tissue, suggesting that circulating H4B levels may be used clinically as a novel and powerful biomarker for the development and response to treatment of AAA.
despite advances in surgical correction of large abdominal aortic aneurysms (AAA) in recent years (5, 22), this mostly asymptomatic disease continues to claim more than 70,000 lives annually in the United States when unexpected rupture occurs (3). This is at least in part due to lack of sufficient diagnostic and monitoring tools for this severe human disease. Imaging technologies as diagnostic methods are not routinely utilized for populations at risk and not as convenient or sensitive as a potential blood-based biomarker. Some screening techniques also expose patients to radiation, which carries its own risks when repeated measurements are made (12). Furthermore, AAA size alone does not always accurately predict the risk of rupture (13), making early detection of the condition more important for early intervention to prevent silent growth and rupture. Therefore, it is essential to develop a biomarker that can accurately detect the development of AAA, and ideally, response to treatments.
Although there are currently no clinically accepted biomarkers for AAA, many candidates are under investigation. These include degradation products of elastin (18, 19) and collagen (28), proteases (20, 21), and inflammation markers such as cytokines (15, 31) and c-reactive protein (27). However, a clear biomarker has yet to emerge, as these markers are not AAA specific, and many of the studies have conflicting results (8, 23). Hence, there is urgent, unmet need for a sensitive and specific biomarker for AAA.
Recent studies from our laboratory have identified that uncoupling of endothelial nitric oxide synthase (eNOS), in which the normally nitric oxide (NO)-producing eNOS produces superoxide to induce oxidative stress, plays a causal role in the formation of AAA (10, 29). This uncoupling of eNOS is caused by a reduced bioavailability of tetrahydrobiopterin (H4B), which is the essential cofactor for proper eNOS coupling activity (4, 16, 17, 24). Hence, H4B holds potential as a biomarker for the development of AAA.
In this study, we examined the potential of circulating H4B levels as a biomarker for AAA. We used two animal models of AAA, the angiotensin II (ANG II)-infused apolipoprotein E (apoE) null mouse (6) and ANG II-infused hyperphenylalaninemia (hph-1) mouse (10). Aortic and plasma H4B levels significantly decreased to 2.69 ± 0.15 and 1.99 ± 0.06 pmol/mg, respectively, after 3 wk in apoE null animals (from 4.86 ± 0.32 and 3.31 ± 0.13 pmol/mg) and to 1.02 ± 0.10 and 0.98 ± 0.09 pmol/mg, respectively, after 1 wk in hph-1 mice (from 1.84 ± 0.18 and 1.48 ± 0.12 pmol/mg) when AAA started to develop. This response of depressed H4B levels remained until the end of the experimental protocol for both strains of animals. Oral administration of folic acid (FA), which has been shown to reduce AAA development in these two animal models (10, 29), increased both circulating and tissue levels of H4B starting at week 1, with H4B levels remaining enhanced until the end of the experiment. Linear correlation was found between tissue and plasma levels of H4B under both H4B reduced and enhanced conditions, suggesting that plasma levels of H4B accurately predicts its tissue levels. These data suggest that H4B may be used as a sensitive biomarker to track the development of AAA and its response to treatments.
MATERIALS AND METHODS
Chemical reagents.
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich in highest purity. Isoflurane was purchased from Piramal Healthcare.
Animals.
All animal and experimental procedures were approved by the Institutional Animal Care and Usage Committee at the University of California, Los Angeles (UCLA). All animals were bred in-house for experimental use. The apoE null founders were purchased from Jackson Laboratory (stock no. 002052). Animals were kept in ventilated cages, provided water and food ad libitum, and cared for by animal facility staff. Six- to 8-mo-old males were used for experiments. For the animal groups treated with FA, standard chow was replaced with in-house customized food tablets containing FA (15 mg·kg−1·day−1) for 2 days before ANG II infusion and then throughout the study period.
Infusion of ANG II by osmotic pump.
Animals were originally anesthetized in an isoflurane chamber with 5% isoflurane and then quickly moved to a nose cone supplying 1.5–2% isoflurane to maintain the anesthetic state. Hair was removed from a small area in the back between the shoulder blades, followed by disinfection with an iodine solution. A small incision was made at the site, and an osmotic pump (Alzet, model 2002 or 2004) containing ANG II (1 mg·kg−1·day−1 for apoE null, 0.7 mg·kg−1·day−1 for hph-1) in a sterile delivery solution (0.15 M NaCl, 1% acetic acid) was inserted under the skin to the left flank. The wound site was closed with surgical staples, and the animal was allowed to recover in a heated chamber.
Tissue collection.
After euthanasia by CO2, blood was removed from the animal through the ventricles using a syringe (28.5-gauge insulin syringe; Becton Dickinson). Plasma was separated from the blood by centrifugation for 10 min at 10,000 g at 4°C. For the aorta, the tissues were rapidly removed from the body after the collection of blood. The aortas were then rinsed in ice-cold PBS, followed by the removal of connective tissue and fat on ice.
Determination of H4B content by HPLC.
For the aorta, freshly isolated aortas were lysed in H4B lysis buffer (0.1 M phosphoric acid, 1 mM EDTA, 10 mM dl-dithiothreitol) and then centrifuged at 12,000 g for 3 min at 4°C in the dark. For plasma, equal volumes of plasma and H4B lysis buffer were mixed and incubated on ice for 20 min in the dark and then centrifuged at 12,000 g for 3 min at 4°C in the dark. The supernatant for both the aorta and plasma was subjected to oxidation in acidic (0.2 M trichloroacetic acid with 2.5% I2 and 10% KI) and alkalytic solutions (0.1 M NaOH with 0.9% I2 and 1.5% KI) as described previously (10, 24, 29, 35). After centrifugation, the supernatant was injected into a fluorescent detector-equipped HPLC system (Shimadzu) set at 350-nm excitation and 450-nm emission wavelengths. The resulting spectra contain peaks that represent total biopterin and oxidized biopterins. H4B was calculated from the difference between these peaks.
Statistical analysis.
All statistical analysis was carried out using the GraphPad Prism software package. Linear regression was calculated with a statistical threshold of P < 0.05. Comparison between multiple groups was done by ANOVA followed by the Newman-Keuls post hoc test with a significance level of P < 0.05.
RESULTS
Both plasma and tissue levels of H4B decrease with ANG II infusion in apoE null and hph-1 mice.
To examine whether tissue and plasma levels of H4B track AAA development, we measured H4B from a well-established animal model of AAA, the ANG II-infused apoE null mouse. Previous studies have shown that in these mice, infusion of ANG II for 4 wk results in ~90% incidence of AAA (6, 29). Our group has also shown that aortic H4B levels were reduced in these animals at 4 wk of ANG II infusion (29). In the present study, we examined whether tissue and plasma levels of H4B correlatively decrease with ANG II infusion. Indeed, aortic and plasma H4B levels decreased time dependently by week 3 to 2.69 ± 0.15 and 1.99 ± 0.06 pmol/mg, respectively (from 4.86 ± 0.32 and 3.31 ± 0.13 pmol/mg at baseline), when aneurysms developed (Fig. 1A, n = 4–5 per group, P < 0.01). There was no additional decrease by week 4 from week 3.
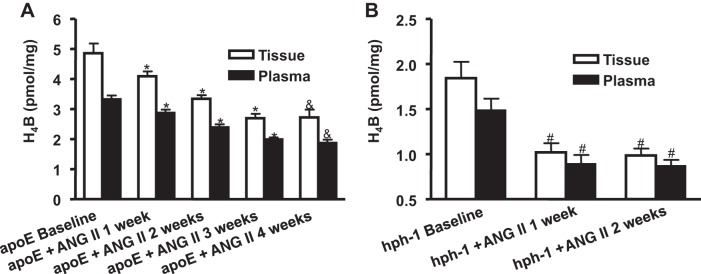
Both plasma and tissue levels of tetrahydrobiopterin (H4B) decrease with ANG II infusion in apolipoprotein (apoE) null and hyperphenylalaninemia (hph-1) mice. H4B levels were determined from plasma and aortas in apoE null mice (A; n = 4–5 each) and hph-1 mice (B; n = 4 each) at baseline and then weekly for 4 or 2 wk, respectively, after ANG II infusion. Results show that in apoE null animals, H4B levels significantly decreased after ANG II infusion in a time-dependent fashion up to week 3, when aneurysm developed, and remained unchanged until week 4. For hph-1 animals, H4B levels significantly decreased by week 1, when aneurysm developed, and then remained unchanged to week 2. *P < 0.05 vs. preceding weeks. &P < 0.05 vs. baseline and weeks 1 and 2. #P < 0.01 vs. baseline.
Furthermore, we performed similar experiments on the recently established ANG II-infused hph-1 model, which develops severe AAA in ~80% of the animals after 2 wk of ANG II infusion (10). Tissue and plasma H4B levels decreased significantly to 1.02 ± 0.10 and 0.98 ± 0.09 pmol/mg, respectively (from 1.84 ± 0.18 and 1.48 ± 0.12 pmol/mg at baseline), by week 1, when aneurysms developed (Fig. 1B, n = 4 each group, P < 0.01). The levels stayed low through week 2. These results show that both tissue and plasma H4B levels decrease with AAA development in two different, well-established murine models of AAA.
Plasma and tissue levels of H4B correlate linearly in ANG II-infused apoE null and hph-1 mice.
We next examined whether plasma H4B levels accurately reflect the abundance of H4B in the tissue. Linear correlation between tissue and plasma H4B was calculated for the above data for both animal models. Figure 2, A and B, shows that plasma H4B levels significantly correlated with tissue H4B levels in both animal models (n = 23, P < 0.0001 for apoE null mice; n = 12, P = 0.0017 for hph-1 mice).
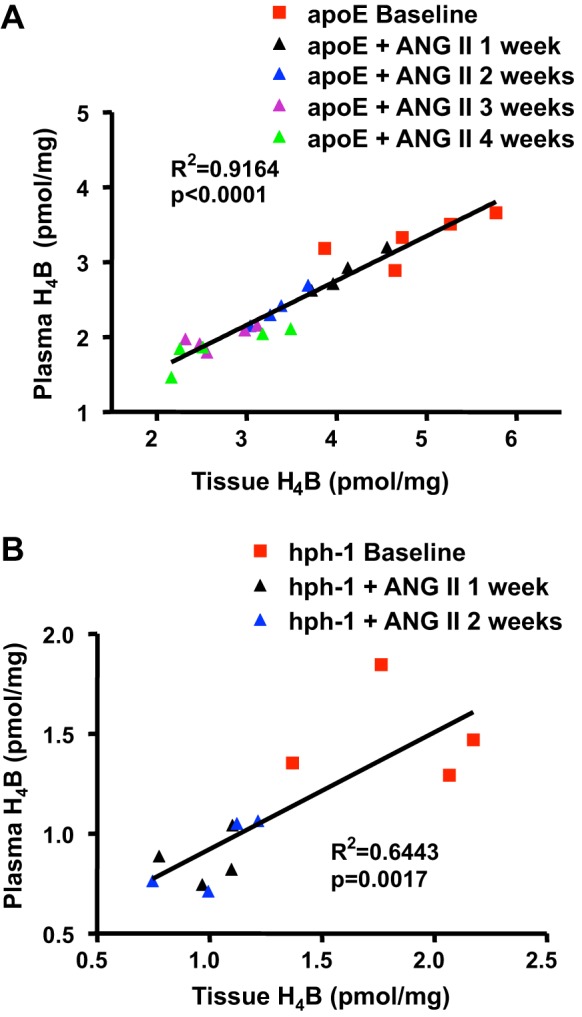
Plasma H4B levels correlate linearly with aortic H4B levels in ANG II-infused apoE null and hph-1 mice. H4B levels were determined in plasma and aortas at baseline and then weekly after ANG II infusion in apoE null mice (A; n = 23) and hph-1 mice (B; n = 12). Results show that plasma H4B levels correlated linearly with aortic H4B levels and decreased with ANG II infusion in both mouse models of abdominal aortic aneurysm (AAA).
Plasma and tissue levels of H4B increased with FA administration in ANG II-infused apoE null and hph-1 mice.
Oral administration of FA in ANG-II infused mice has been shown in the past to reduce the incidence of AAA through the improvement of tissue H4B bioavailability and eNOS coupling activity (10, 29). In the present study, we examined whether tissue and plasma levels of H4B also reflect this change, and how H4B levels change over time. As is obvious in Fig. 3, A and B, tissue and plasma levels of H4B significantly increased in response to FA treatment in ANG II-infused apoE null and hph-1 mice. The results show that H4B significantly increased at 1 wk and stayed elevated through the study period for both models (n = 4–5 each group, P < 0.05).
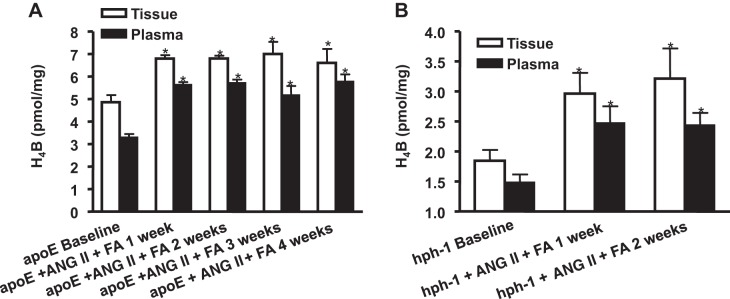
Both plasma and tissue levels of H4B increase with folic acid (FA) treatment in ANG II-infused apoE null and hph-1 mice. H4B levels were determined in plasma and aortas from apoE null mice (A; n = 4–5 each) and hph-1 mice (B; n = 4 each) at baseline and then weekly for 4 or 2 wk, respectively, after ANG II and FA treatment. Results show that FA treatment was able to significantly increase plasma and tissue H4B levels in both mouse models of AAA, up to the end of the experimental protocol. *P < 0.05 vs. baseline.
Plasma H4B levels accurately predict enhanced tissue H4B levels in response to FA.
To further examine whether plasma levels of H4B accurately predict tissue levels of H4B in response to FA treatment, linear correlation between the two measurements was performed with the addition of the FA data. Figure 4, A and B, demonstrates linear correlation between plasma and tissue levels of H4B from all of the apoE (n = 41) and hph-1 (n = 20) animals used in this study, respectively. The results show significant (P < 0.001) linear correlation between tissue and plasma levels of H4B in both animal models with the FA-enhanced measurements, suggesting that plasma level of H4B accurately predicts H4B level in the tissue when H4B levels are either reduced or elevated.

Plasma H4B levels correlate linearly with aortic H4B levels in response to FA treatment. H4B levels were determined in plasma and aortas at baseline and then weekly after ANG II infusion in both apoE null mice (A; n = 41) and hph-1 mice (B; n = 20) with and without oral administration of FA. Results show that plasma H4B levels significantly correlate linearly with aortic H4B levels, and both were elevated in response to FA treatment.
DISCUSSION
The most significant results from this study are as follows: 1) H4B levels in both tissue and plasma decrease time dependently along with the development of AAA in both ANG II-infused apoE null and hph-1 mice; and 2) tissue and plasma levels of H4B linearly correlate with each other in both apoE null and hph-1 mice in both H4B reduced and enhanced conditions. These data suggest a novel biomarker role of circulating H4B for the development and therapeutic responses of AAA.
Although surgical treatment of AAA has been mostly successful despite some risks (5, 22), the detection of the smaller aneurysms still remains problematic. Since AAA is largely asymptomatic, most patients with this condition are not aware of its presence, which can result in silent growth and deadly ruptures. AAA detection is still achieved through imaging techniques, such as ultrasound. However, targeted examination for AAA for the at-risk population is not commonly performed, and hence its detection has mostly been incidental. Furthermore, imaging techniques are generally inadequate in predicting the progression of the aortic expansion, leading to many patients receiving unnecessary surgical procedures (2, 9).
In this study, we examined the potential of H4B as a biomarker for AAA. H4B is a critical cofactor for the proper “coupling” function of the enzyme eNOS. At normal physiological ranges, H4B levels determine the “coupling” state of eNOS, resulting in production of the vasoprotective NO (32, 33). However, under pathological conditions when H4B levels are deficient, eNOS can become “uncoupled” to produce superoxide anion (4, 16, 17, 24, 35). Oxidative stress has been observed in previous studies to be a major contributing factor in the development of AAA (7, 10, 11, 29, 30, 34). An initial production of oxidants is expected to induce eNOS uncoupling to prolong oxidative stress, and in our laboratory, we have shown that eNOS uncoupling plays a causal role in AAA development in two different animal models of AAA, which is accompanied by decreased tissue levels of H4B (10, 29). These observations promoted us to examine the possibility of using decreasing H4B as a convenient, circulating biomarker for the development of AAA. Indeed, we found remarkable and consistent linear correlation between tissue and circulating levels of H4B that predicts development of AAA in both H4B reduced and enhanced conditions.
Whereas the results of this study importantly establish that H4B deficiency accurately tracks the development of AAA in animal models, H4B deficiency itself is not an AAA specific condition. Different degrees of H4B deficiency and eNOS uncoupling have been shown in cardiovascular disorders such as atherosclerosis (1, 17, 25), hypertension (16, 26), and diabetes (24, 35). Nonetheless, it does not rule out whatsoever that AAA is predictable and monitorable with the most severe H4B deficiency, which at its specific range becomes fairly specific as a diagnostic and treatment biomarker for AAA. Our additional unpublished data from animal and human studies support this notion strongly. In addition, the biomarker detects early molecular changes that impossible for imaging techniques to detect. The advantages of the biomarker therefore include specificity, sensitivity (for early detection), and applicability to both disease development and responses to treatment.
In this study, we used different groups of animals for each time point. Ideally, the sampling of the same animals over the course of the experiment would have allowed for the use of paired statistical tests and eliminated some of the problems of individual variability. However, this was technically not possible because our preliminary experiments showed that AAA rate was affected by repeated plasma draws from the animals (data not shown). Therefore, it was necessary to use different groups of animals for each time point. On the other hand, this allowed for the measurement of tissue levels of H4B at every time point, which afforded a better picture of how H4B changes within the aorta as AAA develops.
In conclusion, this study demonstrates a novel biomarker role of circulating H4B for AAA. Both plasma and tissue levels of H4B decreased with ANG II infusion until the development of AAA, suggesting that it can accurately predict the development of the disease. Furthermore, plasma H4B level accurately predicted tissue H4B levels under both H4B enhanced and reduced conditions, showing that plasma H4B alone can be used as a biomarker. Together, these results suggest that circulating H4B levels may be used clinically as a powerful biomarker for the development of AAA and its response to treatments.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL077440; (to H. Cai), HL088975; (to H. Cai), HL108791; (to H. Cai, D. G. Harrison), and HL119968 (to H. Cai) and by American Heart Association Established Investigator Award 12EIA8990025 (to H. Cai).
AUTHOR CONTRIBUTIONS
K.L.S. performed experiments; K.L.S. and H.C. analyzed data; K.L.S. and H.C. interpreted results of experiments; K.L.S. and H.C. prepared figures; K.L.S. and H.C. drafted manuscript; K.L.S. and H.C. edited and revised manuscript; K.L.S. and H.C. approved final version of manuscript; H.C. conception and design of research.
REFERENCES
Articles from American Journal of Physiology - Heart and Circulatory Physiology are provided here courtesy of American Physiological Society
Full text links
Read article at publisher's site: https://doi.org/10.1152/ajpheart.00444.2014
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4255016
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Combination of folic acid with nifedipine is completely effective in attenuating aortic aneurysm formation as a novel oral medication.
Redox Biol, 58:102521, 16 Nov 2022
Cited by: 5 articles | PMID: 36459715 | PMCID: PMC9713368
Diagnostic and predictive values of circulating tetrahydrobiopterin levels as a novel biomarker in patients with thoracic and abdominal aortic aneurysms.
Redox Biol, 56:102444, 17 Aug 2022
Cited by: 2 articles | PMID: 36116158 | PMCID: PMC9486112
Oxidative stress in genetically triggered thoracic aortic aneurysm: role in pathogenesis and therapeutic opportunities.
Redox Rep, 26(1):45-52, 01 Dec 2021
Cited by: 18 articles | PMID: 33715602 | PMCID: PMC7971305
Review Free full text in Europe PMC
Targeting feed-forward signaling of TGFβ/NOX4/DHFR/eNOS uncoupling/TGFβ axis with anti-TGFβ and folic acid attenuates formation of aortic aneurysms: Novel mechanisms and therapeutics.
Redox Biol, 38:101757, 13 Oct 2020
Cited by: 24 articles | PMID: 33126053 | PMCID: PMC7585948
NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets.
Nat Rev Cardiol, 17(3):170-194, 07 Oct 2019
Cited by: 223 articles | PMID: 31591535 | PMCID: PMC7880919
Review Free full text in Europe PMC
Go to all (8) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid.
Hypertension, 59(1):158-166, 14 Nov 2011
Cited by: 76 articles | PMID: 22083158 | PMCID: PMC3668799
Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice.
PLoS One, 9(2):e88899, 18 Feb 2014
Cited by: 45 articles | PMID: 24558445 | PMCID: PMC3928303
NOX isoforms in the development of abdominal aortic aneurysm.
Redox Biol, 11:118-125, 19 Nov 2016
Cited by: 36 articles | PMID: 27912196 | PMCID: PMC5133668
Diagnostic and predictive values of circulating tetrahydrobiopterin levels as a novel biomarker in patients with thoracic and abdominal aortic aneurysms.
Redox Biol, 56:102444, 17 Aug 2022
Cited by: 2 articles | PMID: 36116158 | PMCID: PMC9486112
Funding
Funders who supported this work.
NHLBI NIH HHS (8)
Grant ID: HL077440
Grant ID: R01 HL119968
Grant ID: R01 HL088975
Grant ID: R01 HL108701
Grant ID: HL088975
Grant ID: HL119968
Grant ID: HL108791
Grant ID: R01 HL077440






