Abstract
Free full text

Successful Protection against Tularemia in C57BL/6 Mice Is Correlated with Expansion of Francisella tularensis-Specific Effector T Cells
Abstract
Francisella tularensis is an intracellular, Gram-negative bacterium that causes the fatal disease tularemia. Currently, there are no licensed vaccines for tularemia and the requirements for protection against infection are poorly defined. To identify correlates of vaccine-induced immunity against tularemia, we compared different strains of the live vaccine strain (LVS) for their relative levels of virulence and ability to protect C57BL/6 mice against challenge with virulent F. tularensis strain SchuS4. Successful vaccination, as defined by survival of C57BL/6 mice, was correlated with significantly greater numbers of effector T cells in the spleen and lung. Further, lung cells and splenocytes from fully protected animals were more effective than lung cells and splenocytes from vaccinated but nonimmune animals in limiting intracellular replication of SchuS4 in vitro. Together, our data provide a unique model to compare efficacious vaccines to nonefficacious vaccines, which will enable comprehensive identification of host and bacterial components required for immunization against tularemia.
INTRODUCTION
Francisella tularensis is a Gram-negative, facultative intracellular pathogen with a wide host range that includes humans, rodents, and arthropods. It is the causative agent of a fatal illness termed tularemia. There are four major subspecies, but only two, F. tularensis subspecies tularensis and F. tularensis subspecies holarctica, are of clinical concern. F. tularensis subspecies tularensis is extremely infectious and highly virulent. Inhalation of fewer than 10 bacteria can result in lethal infection (1, 2). F. tularensis subspecies tularensis was also developed as a biological weapon by the former Soviet Union, the United States, and Japan (3). Thus, this bacterium remains a concern for both public health and its potential use as a biological weapon.
F. tularensis subspecies holarctica is equally infectious as F. tularensis subspecies tularensis in humans but is rarely lethal (4). Due to its reduced lethality in humans, a strain of F. tularensis subspecies holarctica was selected and developed as a live vaccine strain (LVS). The LVS was capable of protecting individuals against infection with modest numbers of virulent F. tularensis subspecies tularensis (2). However, LVS did not provide protection against exposure to more than 1,000 bacteria and the protection engendered by LVS appeared to wane over time. Furthermore, the basis of attenuation of LVS is not known, the mechanisms of protection have not been fully defined, and LVS can undergo spontaneous mutation resulting in enhanced attenuation and loss of protective efficacy (5, 6). For these reasons, LVS is not licensed for general use in the United States (7). Thus, there is a need to develop novel, effective vaccines directed against tularemia.
Although it is not licensed for use in humans, LVS remains an important tool for dissecting the requirements for vaccine-induced immunity against F. tularensis in experimental models. The majority of this work has been performed in mice since their use is convenient and cost-effective and since the outcomes seen with mice infected with F. tularensis exhibit many similarities to those observed in humans and/or human cells (2, 6, 8). LVS has been reported to protect BALB/c mice but not C57BL/6 mice from lethal intranasal challenge with virulent F. tularensis (9,–13). While studies with LVS in BALB/c mice have provided some information on basic requirements for vaccine-induced immunity against tularemia, determining the immune correlates of protection in BALB/c mice is challenging, as most tools for detailed dissection of the immune response in mice are not available for use in this strain. One solution to this problem is to establish a model of vaccine-induced immunity in C57BL/6 mice, as there are numerous immunological tools available for use in these mice. Moreover, C57BL/6 mice represent the favored strain for generating animals with targeted deletions in host genes known to contribute to defense against infectious disease, enabling a more comprehensive identification of host parameters required for protection. Ideally, development of a C57BL/6 model of vaccine-induced immunity against tularemia would also include comparison of minimally versus highly efficacious strains of LVS. This comparison should allow future identification of bacterial components associated with strains capable of triggering strong immunity against virulent F. tularensis.
In the study whose results are presented here, we utilized two different strains of LVS in C57BL/6 mice to develop such a model. We demonstrate that complete protection, as measured by survival, of C57BL/6 mice against infection with virulent F. tularensis strain SchuS4 is correlated with the relative virulence of the LVS strain used to vaccinate the host. Further, we provide ex vivo evidence for the host components that are associated with this strong vaccine-induced immunity.
MATERIALS AND METHODS
Bacteria.
Francisella tularensis subsp. tularensis strain SchuS4 was provided by Jeannine Peterson (Centers for Disease Control and Prevention, Fort Collins, CO). The F. tularensis subsp. holarctica live vaccine strain (LVS) ATCC 29684 (ATCC LVS) was provided by Karen Elkins (U.S. Food and Drug Administration, Rockville, MD). F. tularensis subsp. holarctica LVS (RML LVS) was provided by Jean Celli (Rocky Mountain Laboratories [RML], NIAID, NIH, Hamilton, MT). RML LVS was originally acquired by Fran Nano (University of Victoria, Victoria, British Columbia, Canada). The strain designations of both LVS isolates were confirmed by the absence of pdpD, absence of pilA, and deletion in the C terminus of FTT0918. Listeria monocytogenes was provided by Sing Sing Way (Cincinnati Children's Hospital, Cincinnati, OH). Francisella stocks were generated as previously described (14,–16). Briefly, bacteria were grown overnight in modified Mueller-Hinton (MMH) broth, and 1-ml aliquots were frozen at −80°C. Bacteria were thawed just prior to use. Inocula were confirmed by enumerating viable bacteria from serial dilutions plated on MMH agar. L. monocytogenes stock was generated as previously described (17). Briefly, bacteria were grown overnight in brain heart infusion (BHI) broth (Sigma) and 1-ml aliquots were frozen at −80°C. Bacteria were thawed just prior to use. Inocula were confirmed by enumerating viable bacteria from serial dilutions plated on BHI agar. The number of viable bacteria in frozen stock vials varied <1% over a 10-month period.
Mice.
Specific-pathogen-free, 6-to-8-week-old female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in sterile microisolator cages in the animal biosafety level 2 (ABSL-2) and ABSL-3 facilities at the RML. All research involving mice was conducted in accordance with Animal Care and Use guidelines, and animal protocols were approved by the Animal Care and Use Committee at RML. Infected mice were monitored regularly and euthanized at the first sign of illness.
Generation of BMM.
Bone marrow-derived macrophages (BMM) were generated as previously described (18). Briefly, femurs and tibias from 6-to-8-week-old C57BL/6J mice were flushed with Dulbecco's minimum essential medium (DMEM; Life Technologies). Cells were washed, resuspended in complete DMEM (cDMEM; DMEM supplemented with 10% heat-inactivated fetal calf serum [FCS], 0.2 mM l-glutamine, 1 mM HEPES, and 0.1 mM nonessential amino acids [NEAA], all from Life Technologies), and incubated in the presence of 20 ng/ml macrophage colony-stimulating factor (M-CSF; Peprotech) in a 75-mm flask for 2 days. Nonadherent cells were then collected, centrifuged, resuspended in cDMEM plus 20 ng/ml M-CSF, and incubated for 2 days in a 75-mm flask. Media and cytokine were replaced, and, following overnight incubation, cells were gently scraped off the flask, counted, and seeded at 8 × 104 cells/well in 48-well plates. Cells were utilized 2 days after plating. All cells had a phenotype consistent with immature macrophages: CD11b+/F480+/MHCIIlow/CD11c−/GR-1−.
Infection of BMM.
BMM were inoculated as previously described (19). Briefly, bacteria were diluted in cDMEM and BMM were infected at a multiplicity of infection (MOI) of 50 SchuS4 or 0.01 L. monocytogenes. MOIs were selected to result in similar levels of uptake of SchuS4 and L. monocytogenes. Bacteria were removed, and BMM were incubated with 50 μg/ml gentamicin–cDMEM to eliminate any remaining extracellular bacteria. BMM were washed extensively, the medium was replaced, and cells were incubated at 37°C and 7% CO2. Intracellular bacteria were enumerated by lysing cells at the indicated time points with sterile water. Cell lysates were serially diluted and plated on BHI or MMH agar plates and incubated at 37°C and 7% CO2 for 24 h (L. monocytogenes) or 48 h (SchuS4) before individual CFU were counted.
Cytokine quantification.
Concentrations of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-5 (IL-5), IL-6, IL-10, IL-13, IL-17A, CXCL1, CCL5, MIP1α, MIP-1β, and MCP-1 in tissues were determined using a cytometric bead array (CBA; BD Biosciences, San Jose, CA); concentrations of IL-12p40 were determined by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, San Jose, CA) using commercially available kits, following the manufacturer's instructions.
Inoculation of mice.
Mice were inoculated with the indicated strains of Francisella as previously described (15, 20). Briefly, bacteria were thawed and serially diluted in phosphate-buffered saline (PBS). Mice were anesthetized by intraperitoneal injection of 70 μl of 12.5 mg/ml ketamine–3.8 mg/ml xylazine. Then, mice were immediately intranasally inoculated with the indicated number of bacteria administered in a total volume of 25 μl in a single nasal passage. Mice were monitored closely and euthanized at the first sign of illness. As indicated, 30 days after inoculation of LVS, mice were intranasally infected with approximately 25 CFU/25 μl F. tularensis SchuS4.
Collection of tissue homogenate and enumeration of bacteria.
Bacteria were enumerated from the spleens and lungs as previously described (14, 15, 21). Briefly, organs were aseptically removed and placed in ice-cold tissue lysis buffer (150 mM Tris-HCl, 5 mM EDTA, 10 mM Trizma base) supplemented with phosphatase inhibitor mixture I, phosphatase inhibitor mixture II, and protease inhibitor mixture III (AG Scientific, San Diego, CA). Organs were homogenized by grinding tissues through a sterile S/S type 304 no. 60 wire mesh screen (Billeville Wire Cloth, Cedar Grove, NJ) using a 5-ml syringe plunger. An aliquot of tissue homogenate was then serially diluted in PBS and plated on MMH agar for enumeration of bacterial loads. The remaining tissue homogenate was centrifuged at 14,000 × g for 30 min at 4°C, and supernatants were stored at −80°C.
In vitro killing assay.
Lung cells and splenocytes were collected as previously described (9). Briefly, lungs and spleens were aseptically harvested from naive or LVS-inoculated mice 30 days after vaccination. Single-cell suspensions of spleens were generated, red blood cells were lysed using ACK lysis buffer (Life Technologies), and splenocytes were seeded in 6-well plates at 6.4 × 106 cells/well. Lungs were minced and digested with Liberase (Roche), and red blood cells were lysed with ACK lysis buffer and seeded into 6-well plates at 3.2 × 106 cells/well. Lung cells and splenocytes were stimulated with paraformaldehyde (PFA)-killed RML LVS, PFA-killed ATCC LVS (equivalent to 50 killed bacteria/cell), or phorbol myristate acetate (PMA) (10 ng/ml)–ionomycin (Ion) (1 μg/ml) (both from Sigma) for 24 h. Cells treated with cDMEM alone served as negative (mock) controls. Three hours after SchuS4 infection of BMM, lung cells or splenocytes were added to cultures at a ratio of 0.6:1 (4.8 × 104 lung cells/well) or 1.25:1 (1 × 105 splenocytes/well). These coculture conditions resulted in approximately 2.5 × 103, 3.9 × 103, and 3.6 × 103 T cells from the lungs and 2.6 × 104, 2.2 × 104, and 1.97 × 104 T cells from the spleens of naive, RML LVS-vaccinated, and ATCC LVS-vaccinated animals added to infected macrophages, respectively. One hour after infection of BMM with L. monocytogenes, lung cells or splenocytes were added at a ratio of 9:1 (7.2 × 105 lung cells/well) or 20:1 (1.6 × 106 splenocytes/well). Cocultures were incubated for 24 h before intracellular bacterial loads were determined as described above.
FACS.
Lung and spleen cell populations were assessed in LVS-vaccinated mice 30 days after inoculation. Lung cell and splenocyte populations of naive and vaccinated mice were assessed by flow cytometry as previously described, using the following antibodies in various combinations: allophycocyanin (APC)-CD4, eFluor450-CD8, fluorescein isothiocyanate (FITC)-CD44, and phycoerythrin (PE)-CD62L (eBioscience, San Diego, CA) (20, 21). Cells were stained in fluorescence-activated cell sorter (FACS) buffer (eBioscience) at room temperature, washed, and fixed in 2% paraformaldehyde. Cells were washed and resuspended in FACS buffer before data acquisition was performed using an LSR II instrument (BD Biosciences). Approximately 10,000 gated live-cell events were analyzed for each sample. Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis.
Statistically significant differences between two groups were determined using an unpaired two-tailed t test, with significance set at P < 0.05. Significance of differences in survival between groups was determined using a log-rank (Mantel-Cox) test with significance set at P = <0.05. The LD50 was determined using the Reed-Muench equation.
RESULTS
Determination of the LD50s of ATCC LVS and RML LVS in C57BL/6 mice.
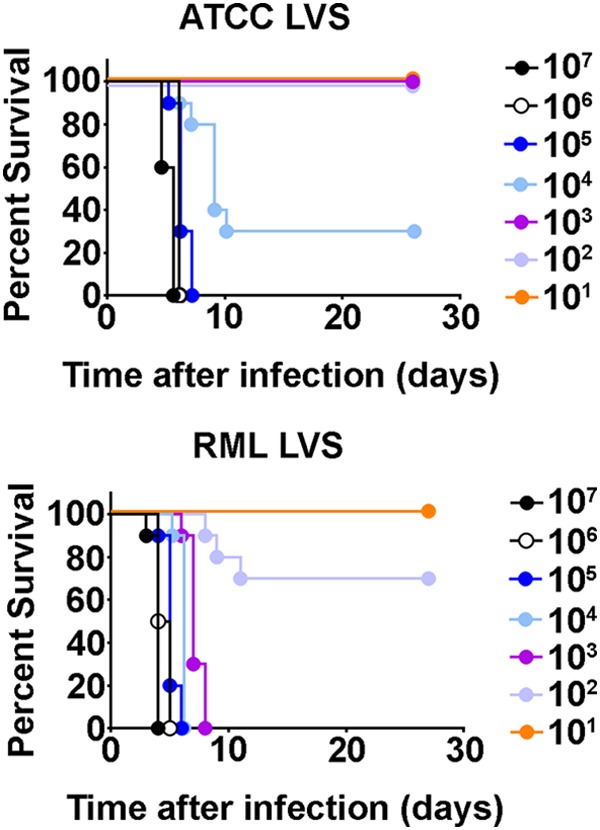
We previously reported that, unlike many strains of LVS, RML LVS induced sterilizing immunity in BALB/c mice challenged with SchuS4 as long as 90 days after vaccination with 100 CFU RML LVS (9, 12). Although not reported in our previous study, the 50% lethal dose (LD50) of RML LVS in BALB/c mice was approximately 1,000 CFU (C. M. Bosio, unpublished data). This was in contrast to ATCC LVS, which requires higher immunizing doses and does not protect against infection with virulent F. tularensis in challenges performed more than 75 days after immunization (8). Thus, we hypothesized that, similarly to the results seen with BALB/c mice, ATCC LVS and RML LVS may have different levels of virulence and protective efficacy in C57BL/6 mice. We first established whether ATCC LVS and RML LVS differed in their levels of virulence for C57BL/6 mice by determining the LD50 of each LVS strain in this mouse strain following intranasal infection. Significantly more mice inoculated with ≤103 CFU ATCC LVS survived than mice infected with >104 CFU ATCC LVS (P < 0.0001). Specifically, mice infected with 10,000 CFU or more of ATCC LVS exhibited 30% and 0% survival, respectively (Fig. 1). All mice that received ≤1,000 CFU ATCC LVS survived infection (Fig. 1). In contrast, significantly fewer mice inoculated with ≥1,000 CFU RML LVS survived infection than animals that received 102 or 101 CFU RML LVS (P < 0.0001) (Fig. 1). Mice that received 100 or 10 CFU RML LVS exhibited 70% or 100% survival, respectively (Fig. 1). Despite this difference in the levels of survival of animals inoculated with 100 or 10 CFU RML LVS, the survival rates of these two groups were not significantly different. The calculated LD50 of RML LVS was 173 CFU, whereas the LD50 of ATCC LVS was 9,320 CFU. These data indicate that, similarly to the data from BALB/c mice, RML LVS is more virulent than ATCC LVS in C57BL/6 mice.
Protective efficacy of LVS strains in C57BL/6 mice.
RML LVS provided superior protection in BALB/c mice, as measured by longevity of immunity against lethal SchuS4 challenge, compared to the results of previous studies using ATCC LVS (9). Since we observed an LD50 of RML LVS among C57BL/6 mice similar to that found in BALB/c mice, we reasoned that RML LVS might engender protective immunity against infection with SchuS4 among C57BL/6 mice whereas ATCC LVS would not. Thus, we next compared the abilities of the LVS strains to protect C57BL/6 mice against challenge with the SchuS4 virulent F. tularensis type A strain. All unvaccinated control mice succumbed to SchuS4 infection, with a mean time to death (MTD) of 4.6 days (Fig. 2). As previously reported, C57BL/6 mice immunized with ATCC LVS were very poorly protected against SchuS4, with only 10% of animals surviving infection, which was not significantly different from the survival rate of the naive controls (Fig. 2) (11, 22, 23). In contrast, significantly more mice vaccinated with 10 or 100 CFU RML LVS survived SchuS4 infection than animals given similar vaccinating doses of ATCC LVS (P = 0.0044 for both doses). Specifically, 50% and 100% of mice that were inoculated with 10 and 100 CFU RML LVS, respectively, survived SchuS4 infection (Fig. 2). Further, significantly more mice vaccinated with 100 CFU RML LVS survived SchuS4 infection than animals vaccinated with 10 CFU (P = 0.0328). Thus, RML LVS engendered superior protective immune responses directed against SchuS4 in C57BL/6 mice compared to ATCC LVS.
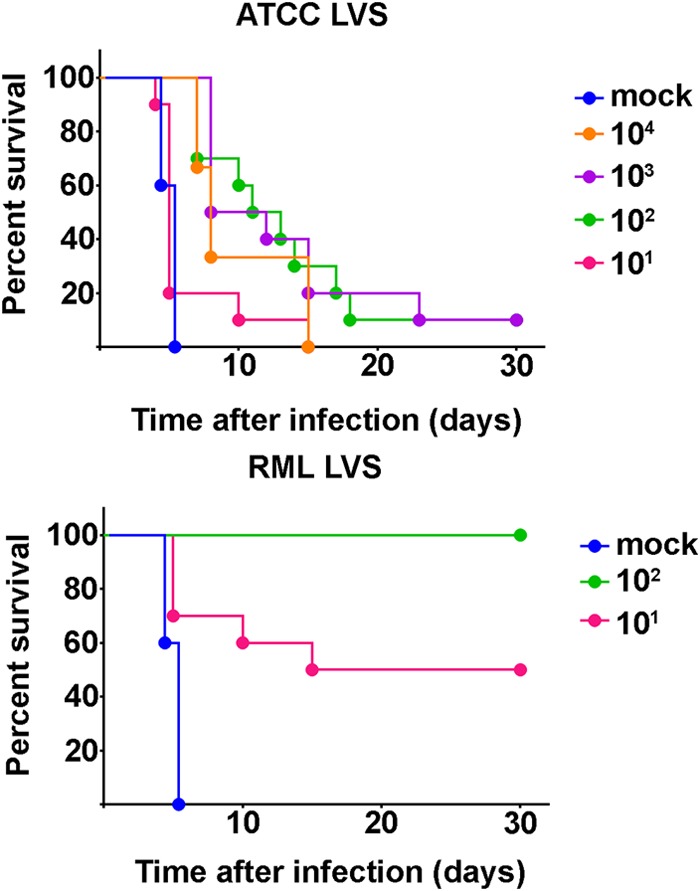
Protection of vaccinated mice against intranasal SchuS4 infection. Mice were immunized (n = 10/group) intranasally with the indicated doses of RML LVS or ATCC LVS. Unvaccinated mice (n = 5/group) served as negative controls. Thirty days after vaccination, mice were challenged intranasally with approximately 25 CFU F. tularensis strain SchuS4 and survival of infection was monitored. Data are representative of the results of two similarly designed experiments.
Kinetics of sublethal LVS infection in C57BL/6 mice.
We next determined the kinetics of sublethal infection of ATCC LVS and RML LVS in C57BL/6 mice. We hypothesized that the capability of RML LVS to induce protective immunity would correspond with differences in the course of infection compared to ATCC LVS. Mice were intranasally inoculated with sublethal doses of RML LVS (100 CFU) or ATCC LVS (1,000 CFU). These doses were chosen because on days 3 and 7 after vaccination, similar numbers of the two bacterial strains were recovered from the lungs (Fig. 3A). Furthermore, similar bacterial loads among mice vaccinated with either RML LVS or ATCC LVS were observed in the spleen on day 3 after vaccination. This suggested that, since mice were inoculated with 10-fold-fewer organisms, RML LVS was able to replicate more efficiently than ATCC LVS at the outset of infection and that the two strains of LVS colonized the spleen with similar proficiencies. Numbers of ATCC LVS bacteria recovered from the lung decreased on days 14 and 21 after inoculation compared to earlier time points, indicating that the mice were controlling replication of ATCC LVS. In contrast, although mice appeared to begin to control RML LVS infection by days 14 and 21 after inoculation, there were significantly more RML LVS bacteria in the lungs than were present in ATCC LVS-vaccinated mice at each of these time points (Fig. 3A). A similar scenario was observed in the spleens of RML LVS-vaccinated animals. Specifically, there were significantly more bacteria in the spleens of RML LVS-infected animals on days 7 and 14 than in those of ATCC LVS-infected mice (Fig. 3B). In addition, whereas mice appeared to be controlling replication of ATCC LVS by day 7 after infection, no reduction in the numbers of recoverable RML LVS in the spleen was detected until 14 days after infection (Fig. 3B). Despite the differences in the kinetics of sublethal infection with ATCC LVS and RML LVS, both strains were cleared from vaccinated mice within 28 days of inoculation (Fig. 3).
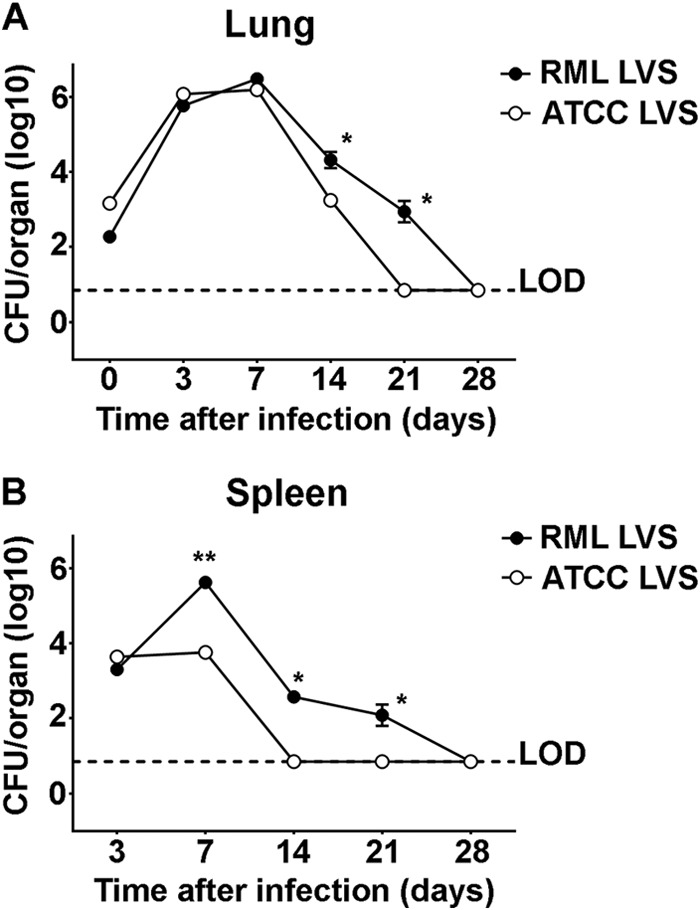
Replication and dissemination of infection in the lungs and spleens of vaccinated mice. Mice were inoculated (n = 10/group) intranasally with 100 CFU RML LVS or 1,000 CFU ATCC LVS. On days 3, 7, 14, 21, and 28 after inoculation, bacterial loads in lungs (A) and spleens (B) were assessed. * = P < 0.05. ** = P < 0.005. LOD = limit of detection. Data are representative of the results of two similarly designed experiments.
In addition to examining the kinetics of bacterial replication, dissemination, and clearance, we also compared changes in the production of cytokines and chemokines in the lungs and spleens of RML LVS-vaccinated and ATCC LVS-vaccinated mice throughout the course of infection. On day 3 after infection, there was significantly more IFN-γ, IL-6, TNF-α, IL-12p40, MIP-1β, KC, RANTES, and MCP-1 in the lungs of ATCC LVS-infected mice than in those of RML LVS-infected mice (Fig. 4). In contrast, on day 7 postinfection, lungs from RML LVS-infected mice contained significantly more MCP-1 than those from ATCC LVS-infected mice (Fig. 4). The lungs of these mice also exhibited significantly higher levels of RANTES and TNF-α on day 14 postinfection (Fig. 4). However, there were no significant increases of GM-CSF, IL-5, IL-10, IL-13, or IL-17A levels in the lungs of either group of LVS-vaccinated mice compared to lungs from naive animals (data not shown). In the spleen, both groups of vaccinated animals had an increased and prolonged presence of RANTES compared to naive controls. RML LVS-infected mice had significantly more IFN-γ in the spleen than ATCC LVS-infected mice 7 days after inoculation (Fig. 5). Similarly to the lung results, concentrations of GM-CSF, IL-5, IL-10, IL-13, or IL-17A in the spleens of LVS-vaccinated mice were not significantly different from those detected in spleens of naive animals (data not shown). Thus, protection afforded by RML LVS against SchuS4 challenge was associated with transient but significantly greater concentrations of MCP-1 in the lungs and of IFN-γ in the spleens and with the prolonged presence of TNF-α and a shift in the peak production of RANTES in the lungs of RML LVS-vaccinated mice.
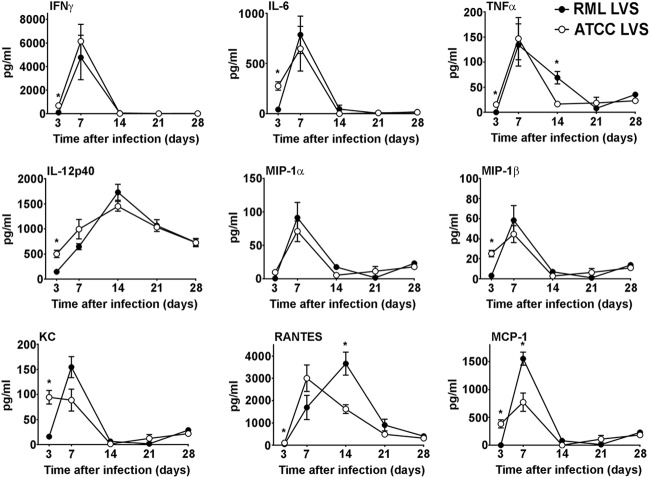
Changes in cytokine and chemokine levels in the lungs after immunization with RML LVS or ATCC LVS. Mice were infected (n = 10/group) intranasally with 100 CFU RML LVS or 1,000 CFU ATCC LVS. Mice were euthanized on days 3, 7, 14, 21, and 28 postinfection, and concentrations of the indicated cytokines and chemokines were determined in the lungs using cytometric bead array analysis or ELISA. * = P < 0.05. Error bars represent standard deviations (SD). Data are representative of the results of two similarly designed experiments.
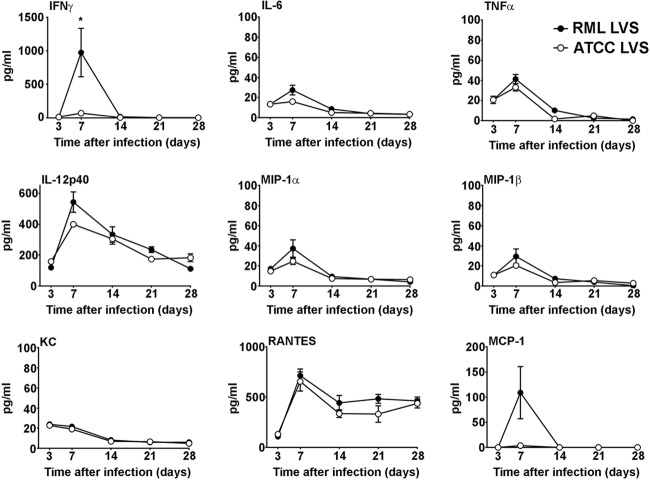
Changes in cytokine and chemokine levels in the spleens after immunization with RML LVS or ATCC LVS. Mice were infected (n = 10/group) intranasally with 100 CFU RML LVS or 1,000 CFU ATCC LVS. Mice were euthanized on days 3, 7, 14, 21, and 28 postinfection, and concentrations of the indicated cytokines and chemokines were determined in the spleens using cytometric bead array analysis or ELISA. * = P < 0.05. Error bars represent SD. Data are representative of the results of two similarly designed experiments.
Enumeration of effector T cells in the lung and spleen.
Control of intracellular replication of virulent F. tularensis is dependent on the presence of CD4+ and CD8+ T cells (23). We have previously established that vaccine-induced immunity in BALB/c mice vaccinated with RML LVS was correlated with increased numbers of activated, IFN-γ-producing T cells in the spleen (9). Given the ability of RML LVS to protect C57BL/6 mice against a lethal SchuS4 infection, we hypothesized that, similarly to BALB/c mice, C57BL/6 mice vaccinated with RML LVS would have higher numbers of effector memory T (TEM) cells in target organs than mice inoculated with ATCC LVS. Further, lung cells and splenocytes from RML LVS-vaccinated mice would exhibit a superior ability to control intracellular replication of SchuS4 in an ex vivo assay. Thirty days after vaccination, RML LVS-inoculated and ATCC LVS-inoculated animals had numbers of total lung cells and splenocytes similar to those seen with naive mice (Fig. 6A and and7A).7A). However, mice that received either strain of LVS had significantly more CD4+ and CD8+ TEM cells (CD44hi/CD62L−) in the lung and spleen than naive animals (Fig. 6A and and7A;7A; see also Fig. S1 in the supplemental material). While RML LVS-vaccinated animals had significantly higher percentages of CD8+ TEM in the lung and CD4+ and CD8+ TEM cells in the spleen than ATCC LVS-inoculated mice, the overall numbers of CD4+ and CD8+ TEM cells in the lungs and spleens of vaccinated animals were not significantly different from each other (Fig. 6A and and7A;7A; see also Fig. S1 in the supplemental material). This suggests that RML LVS-vaccinated animals had higher ratios of CD8+ TEM in the lung and CD4+ and CD8+ TEM cells in the spleen than ATCC LVS-inoculated mice.
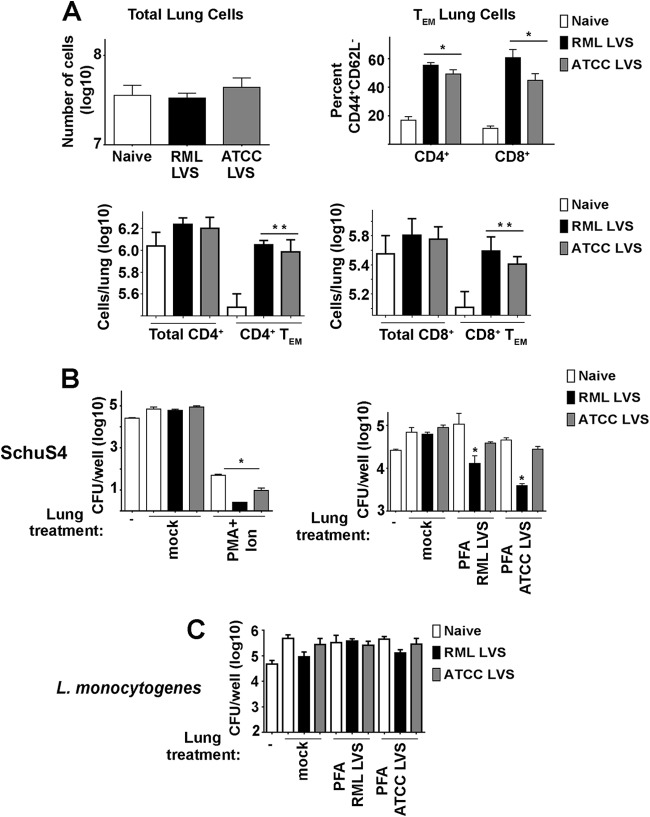
Comparison of lung cell populations of RML LVS- and ATCC LVS-vaccinated mice. Single-cell suspensions of lung cells from naive mice or animals vaccinated with RML LVS or ATCC LVS were generated. Cells were counted and then stained for surface expression of CD4, CD8, CD62L, and CD44 and analyzed by flow cytometry. (A) Effector memory T cells (TEM) were characterized as CD8+ CD44+ CD62L− or CD4+ CD44+ CD62L−. Data represent CD44+ CD62L− percentages of total CD4+ or CD8+ cells. * = P < 0.05 compared to naive and ATCC LVS-vaccinated mice. ** = P < 0.05 compared to naive mice. (B) Lung cells were stimulated as indicated for 24 h and added at a ratio of 0.6:1 to cultures of SchuS4 infected BMM 3 h after infection. At 21 h later (24 h after infection of BMM), numbers of intracellular SchuS4 bacteria were quantitated. * = P < 0.05 compared to mock-treated cells. (C) Lung cells were stimulated as indicated for 24 h and added at a ratio of 9:1 to L. monocytogenes-infected BMM 1 h after infection. At 23 h later (24 h after infection of BMM), numbers of intracellular L. monocytogenes bacteria were quantitated. Error bars represent SD. Data are representative of the results of three similarly designed experiments.
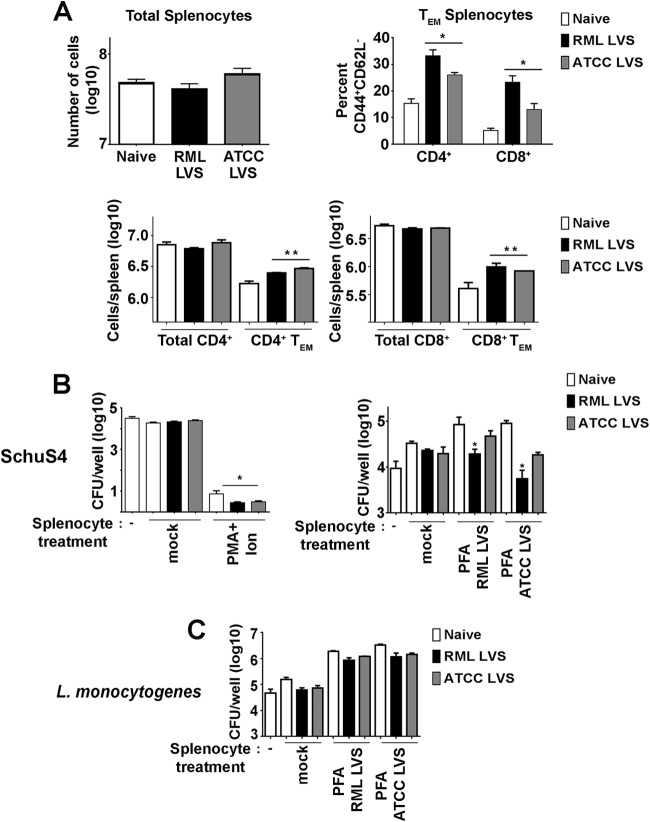
Comparison of splenocyte populations of RML LVS- and ATCC LVS-vaccinated mice. Single-cell suspensions of splenocytes from naive mice or animals vaccinated with RML LVS or ATCC LVS were generated. Cells were counted and then stained for surface expression of CD4, CD8, CD62L, and CD44 and analyzed by flow cytometry. (A) Effector memory T cells (TEM) were characterized as CD8+ CD44+ CD62L− or CD4+ CD44+ CD62L−. Data represent CD44+ CD62L− percentages of total CD4+ or CD8+ cells. * = P < 0.05 compared to naive and ATCC LVS-vaccinated mice. ** = P < 0.05 compared to naive mice. (B) Splenocytes were stimulated as indicated for 24 h and added at a ratio of 1.25:1 to cultures of SchuS4-infected BMM 3 h after infection. At 21 h later (24 h after infection of BMM), numbers of intracellular SchuS4 bacteria were quantitated. * = P < 0.05 compared to mock splenocytes. (C) Splenocytes were stimulated as indicated for 24 h and added at a ratio of 20:1 to L. monocytogenes-infected BMM 1 h after infection. At 23 h later (24 h after infection of BMM), numbers of intracellular L. monocytogenes bacteria were quantitated. Error bars represent SD. Data are representative of the results of three similarly designed experiments.
To determine if differences in T cell effector populations among LVS-vaccinated mice correlated with differences in the abilities of lung cells or splenocytes to control SchuS4, we tested the capability of cells harvested 30 days after vaccination to limit intracellular replication of SchuS4 among BMM infected in vitro. Neither unstimulated lung cells nor splenocytes from naive and vaccinated mice controlled intracellular growth of SchuS4 (Fig. 6B and and7B).7B). Both lung cells and splenocytes nonspecifically stimulated with PMA and ionomycin significantly reduced the number of intracellular SchuS4 bacteria 24 h after infection, regardless of the source of the cells (Fig. 6B and and7B).7B). This indicated that the inability of unstimulated cells to control SchuS4 did not represent an inherent defect in the ability of cells from immune animals to participate in control of the intracellular replication of SchuS4. We next determined if stimulation with reagents more specific to Francisella would reveal differences in the abilities of lung cells and splenocytes from LVS-vaccinated animals to control SchuS4 replication more efficiently than naive cells. Thus, we incubated lung cells and splenocytes with killed RML LVS or ATCC LVS prior to addition to cultures of infected BMM. Similarly to unstimulated cells, neither the presence of naive lung cells nor that of splenocytes incubated with either strain of killed LVS altered intracellular replication of SchuS4 (Fig. 6B and and7B).7B). In contrast, the addition of either lung cells or splenocytes from vaccinated mice stimulated with either killed ATCC LVS or killed RML LVS resulted in a significant decrease in the levels of recoverable intracellular SchuS4 compared to the results seen with cocultures containing naive cells (Fig. 6B and and7B).7B). Consistent with improved protection against SchuS4 challenge in vivo, the presence of lung cells or splenocytes from RML LVS-vaccinated mice stimulated with either strain of killed LVS resulted in significantly lower numbers of SchuS4 bacteria recovered from macrophages than were seen with cocultures containing cells obtained from ATCC LVS-immunized mice (Fig. 6B and and7B).7B). Importantly, neither lung cells nor splenocytes from mice vaccinated with either strain of LVS and activated with killed LVS contributed to control of intracellular replication of L. monocytogenes (Fig. 6C and and7C).7C). This demonstrated that the ability of LVS immune cells to control SchuS4 replication was specific for Francisella-infected macrophages. Together, these data suggest that superior control of SchuS4 infection among RML LVS-vaccinated C57BL/6 mice in vivo was correlated with an increased ratio of pulmonary and splenic TEM cells and a superior ability of activated lung cells and splenocytes to specifically control intracellular replication of SchuS4 in vitro.
DISCUSSION
F. tularensis subspecies tularensis is a highly infectious, lethal bacterium. Its potential to be used as a biological weapon highlights the necessity to develop efficient vaccines to combat this pathogen. Although a live vaccine strain was developed during the mid-20th century, several concerns (such as its unknown mechanisms of attenuation and protection) have precluded its being made available for public use (5, 6). To partially address these concerns, development of novel vaccines targeting tularemia would ideally be acellular or comprised of specific antigens. However, a major obstacle to developing new, effective vaccines for tularemia is that the immune correlates of protection against F. tularensis infection remain largely undefined. In the present study, we sought to develop a model by which we could directly compare and contrast effective versus noneffective vaccines targeting F. tularensis with the aim of identifying new correlates of immunity. To accomplish this goal, we utilized C57BL/6 mice vaccinated with two different strains of LVS, i.e., ATCC LVS and RML LVS, which have differing levels of efficacy against SchuS4 infection in mice.
We first determined the LD50 of these two LVS strains and characterized the kinetics of infection following administration of a sublethal vaccinating dose. The LD50 for ATCC LVS was more than 50 times greater than the LD50 for RML LVS (Fig. 1). Furthermore, despite the presence of similar numbers of bacteria in the lungs on days 7 and 14 and equivalent levels of colonization of the spleen at day 3, RML LVS was cleared more slowly than ATCC LVS in both organs. It is unclear why these two strains of LVS have such marked differences in their LD50 and kinetics of clearance. Previous work has shown that a spontaneous phase shift resulting in modifications of LVS LPS which also causes a shift from gray to blue colonies correlates with further attenuation of the bacterium, rendering it far less protective than its parent strain (6, 24). However, we did not observe any qualitative differences in the lipopolysaccharides (LPS) of RML LVS or ATCC LVS as measured by changes in colony color or Western blot analysis of LPS and capsule (data not shown). A second explanation for the differences in the levels of attenuation of these strains of LVS is that they differ in their abilities to replicate in the intracellular compartment. We compared the abilities of ATCC LVS and RML LVS to replicate in macrophages in vitro and observed that RML LVS replicated to modest but significantly greater numbers in primary cells (data not shown). Successful replication of all subspecies and strains of Francisella is dependent on their ability to escape the endosome and gain access to the cytosol within the first 2 h of infection (25). Therefore, the minimal difference in the levels of intracellular replication of RML LVS and ATCC LVS in vitro as well as in vivo may be due to differential abilities to escape the endosome at the outset of infection. However, we did not observe any differences in escape kinetics of RML LVS or ATCC LVS among macrophages in vitro (data not shown). While this does not rule out the possibility that there are alterations in intracellular trafficking in vivo, these observations strongly suggest that the enhanced attenuation of ATCC LVS compared to RML LVS is more likely associated with the bacterium's ability to directly replicate in the cytosol.
Replication of bacteria in the cytosol of host cells is often paired with upregulation of bacterial genes associated with metabolism. For example, upon entry into the cytosol, L. monocytogenes increases expression of genes required for purine synthesis, and mutants that lack one or more of these genes have restricted intracellular replication and are attenuated in vivo (26). Similarly, Francisella strains with deletions in purine synthesis genes are defective for intracellular replication and are attenuated in vivo (27, 28). In addition to dependence on genes that participate in the purine biosynthesis pathway, Francisella strains with mutations in genes required for efficient iron acquisition, pyrimidine biosynthesis, and glycolysis have exhibited various levels of attenuation (29,–31). Thus, it is possible that the marked difference in the attenuation levels observed in RML LVS and ATCC LVS is due to spontaneous mutations in one or more genes required for efficient metabolism. Our laboratory is currently conducting a genetic screen to identify mutations in the genomes of RML LVS and ATCC LVS that may contribute to their differences in virulence and vaccine efficacy.
In addition to the difference in the levels of attenuation of RML LVS and ATCC LVS in mice, we also observed that these strains differed in their abilities to induce protective immunity against SchuS4 challenge. Specifically, the animals vaccinated with RML LVS all survived infection with SchuS4 whereas the mice vaccinated with ATCC LVS did not. This suggested that measurable differences in the development of innate and adaptive immune responses among animals vaccinated with RML LVS versus ATCC LVS could represent correlates of vaccine-induced immunity. We addressed this possibility by first examining production of cytokines and chemokines in target organs following vaccination with either strain of LVS. Others have used cytokine/chemokine profiling to create a map of soluble mediators associated with vaccine-induced immunity against homologous rechallenge with LVS or exposure to SchuS4. Unsurprisingly, given that Francisella bacteria are intracellular pathogens, early induction of IFN-γ has been routinely associated with development of protective immune responses (9, 10, 23, 32,–34). In addition to IFN-γ, control of LVS infection was also shown to require TNF-α, IL-6, T-bet, and IL-12Rβ2 (35). In a study comparing levels of production of cytokines and chemokines among LVS-immunized BALB/c mice (which were protected from SchuS4 infection) versus C57BL/6 mice (which were not immune to SchuS4 infection), protection was associated with the presence of IL-1β, IL-6, KC, MCP-1, and MIP-1β (32). Furthermore, the concentration and timing of production of IFN-γ, MCP-1, and TNF-α partially predicted the degree of protection against SchuS4 infection in BALB/c mice (32). In agreement with that study, we observed significant increases in levels of IFN-γ in the spleens and MCP-1 in the lungs and a prolonged presence of TNF-α in the lungs of RML LVS-vaccinated animals compared to the results seen with mice receiving ATCC LVS. Thus, transient increased expression of IFN-γ, MCP-1, and TNF-α was associated with strong protective immunity against SchuS4 in vaccinated C57BL/6 mice. These data suggest that this combination of soluble mediators may directly influence the development of protective immune responses. Indeed, while it is well appreciated that IFN-γ produced by T cells directly aids in the control of replication of intracellular pathogens, IFN-γ also contributes to the expansion of antigen-specific T cells (36, 37). Similarly, MCP-1 has been associated with optimal development of IFN-γ-secreting effector T cells following fungal infections (38), although the ability of MCP-1 to positively contribute to expansion of these cells may be dependent on both the source and site of infection (39). Finally, TNF-α produced by antigen-specific T cells has been shown to limit replication of Mycobacterium tuberculosis, and membrane-bound TNF-α present on lymphocytes of LVS-immune mice directly contributed to control of intracellular replication of LVS (40, 41). Together, these observations suggest that the specific cytokine/chemokine profile induced following vaccination with RML LVS should result in greater expansion of effector T cells, which, in turn, contributes to superior control of SchuS4 infection compared to the results seen with ATCC LVS-vaccinated mice.
In support of this hypothesis, we observed that lungs and spleens from mice vaccinated with RML LVS contained significantly greater percentages of TEM cells than those from ATCC LVS-vaccinated mice (Fig. 6A and and7A;7A; see also Fig. S1 in the supplemental material). Moreover, both lung cells and splenocytes from RML LVS-vaccinated mice were more efficient at controlling intracellular SchuS4 replication in macrophages than cells from ATCC LVS-vaccinated mice (Fig. 6B and and7B).7B). Since similar numbers of CD4+ and CD8+ T cells and TEM cells were present in cultures containing cells harvested from LVS-vaccinated mice, this suggests that cells derived from RML LVS-vaccinated animals are functionally different than those present in ATCC LVS-vaccinated mice. Interestingly, cells from lungs from vaccinated animals were able to reduce numbers of intracellular SchuS4 bacteria to levels similar to those observed in cocultures containing splenocytes from the same animals despite adding fewer lung cells and lung T cells to infected macrophages. This suggests that lung cells may be more effective in controlling replication of intracellular SchuS4 than splenocytes. Future studies in our laboratory are aimed at determining the mechanism by which immune cells from RML LVS-vaccinated mice control SchuS4 replication in addition to determining the specific cells responsible for this control. Taken together, these findings will aid in determining the correlates of successful vaccine-induced immunity that are triggered following vaccination with a protective strain of LVS.
This report is the first to show that C57BL/6 mice can be fully protected against virulent F. tularensis infection, as measured by survival (Fig. 2), and that protection is dependent on the LVS strain used for vaccination. Interestingly, ATCC LVS has been shown to protect BALB/c mice but not C57BL/6 mice (11, 42, 43). Wu et al. showed that BALB/c mice were able to mount a T cell-mediated immune response to ATCC LVS, but C57BL/6 mice were not, and that this correlated with the difference in protection between these mouse strains. In agreement with observations by Wu et al., we demonstrated that C57BL/6 mice expanded populations of effector T cells following vaccination and that the number of these effectors was correlated with protective immune responses against SchuS4. In addition to this observation, we provide evidence that LVS-vaccinated C57BL/6 mice generated lung cell and splenocyte populations that specifically controlled SchuS4 replication in vitro. Thus, our data suggest that the C57BL/6 mice were fully capable of developing T cell-mediated immunity against virulent F. tularensis and that generation of solid protection against tularemia is not necessarily dependent on the strain of mouse but is rather dependent on the vaccinating agent used to provoke immunity. There are a wide variety of immunological tools and mouse lines with specific deletions in immune-related genes available on a C57BL/6 background. Thus, performing continued comparative studies utilizing this mouse strain and vaccines with various protective capabilities will advance our understanding of the requirements and correlates of vaccine-induced immunity against tularemia.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
We thank Lydia Roberts for her comments and suggestions pertaining to the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00648-14.
REFERENCES
Articles from Clinical and Vaccine Immunology : CVI are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/cvi.00648-14
Read article for free, from open access legal sources, via Unpaywall:
https://cvi.asm.org/content/cdli/22/1/119.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/cvi.00648-14
Article citations
Current vaccine strategies and novel approaches to combatting Francisella infection.
Vaccine, 42(9):2171-2180, 08 Mar 2024
Cited by: 0 articles | PMID: 38461051
Review
Contribution of Lipid Mediators in Divergent Outcomes following Acute Bacterial and Viral Lung Infections in the Obese Host.
J Immunol, 209(7):1323-1334, 24 Aug 2022
Cited by: 3 articles | PMID: 36002235 | PMCID: PMC9529825
The O-Ag Antibody Response to Francisella Is Distinct in Rodents and Higher Animals and Can Serve as a Correlate of Protection.
Pathogens, 10(12):1646, 20 Dec 2021
Cited by: 3 articles | PMID: 34959601 | PMCID: PMC8704338
Circulating T Cells Are Not Sufficient for Protective Immunity against Virulent Francisella tularensis.
J Immunol, 208(5):1180-1188, 11 Feb 2022
Cited by: 2 articles | PMID: 35149529 | PMCID: PMC8881340
Differential Immune Response Following Intranasal and Intradermal Infection with Francisella tularensis: Implications for Vaccine Development.
Microorganisms, 9(5):973, 30 Apr 2021
Cited by: 4 articles | PMID: 33946283 | PMCID: PMC8145380
Review Free full text in Europe PMC
Go to all (27) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Models derived from in vitro analyses of spleen, liver, and lung leukocyte functions predict vaccine efficacy against the Francisella tularensis Live Vaccine Strain (LVS).
mBio, 5(2):e00936, 08 Apr 2014
Cited by: 24 articles | PMID: 24713322 | PMCID: PMC3993856
Protection of vaccinated mice against pneumonic tularemia is associated with an early memory sentinel-response in the lung.
Vaccine, 35(50):7001-7009, 06 Nov 2017
Cited by: 4 articles | PMID: 29102170
An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain.
Vaccine, 26(41):5276-5288, 08 Aug 2008
Cited by: 61 articles | PMID: 18692537 | PMCID: PMC2652725
Progress, challenges, and opportunities in Francisella vaccine development.
Expert Rev Vaccines, 15(9):1183-1196, 03 May 2016
Cited by: 11 articles | PMID: 27010448
Review
Funding
Funders who supported this work.