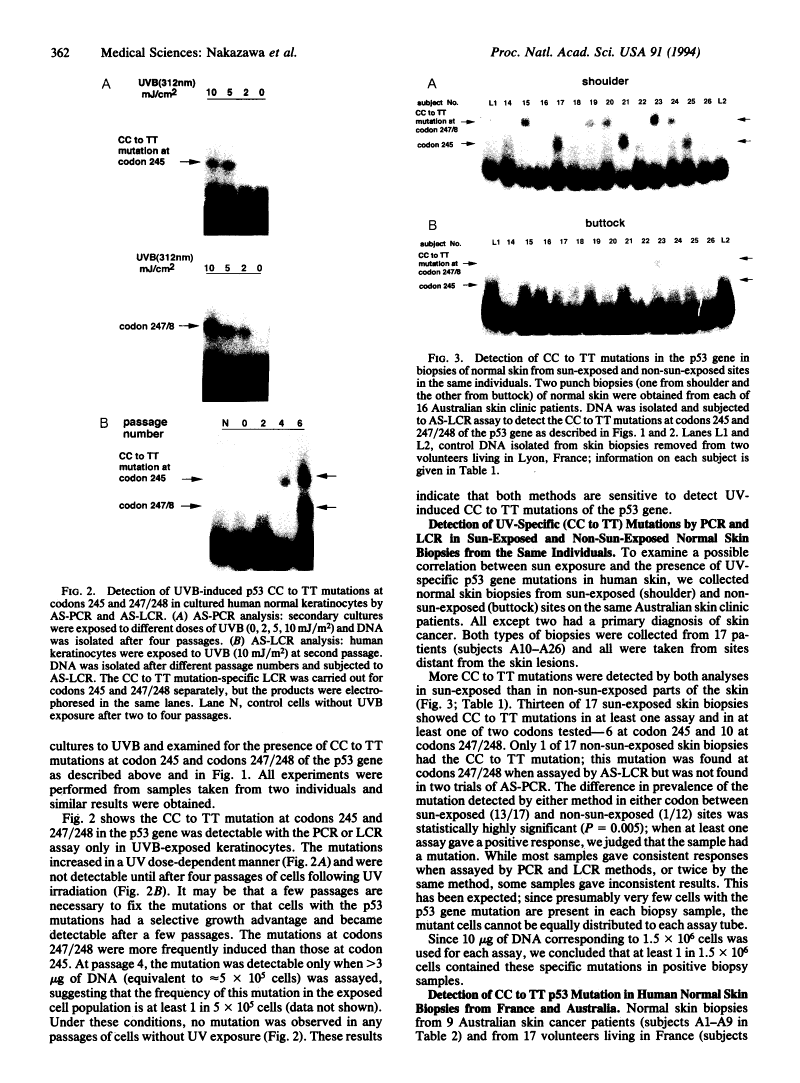
Abstract
Free full text

UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement.
Abstract
Many human skin tumors contain mutated p53 genes that probably result from UV exposure. To investigate the link between UV exposure and p53 gene mutation, we developed two methods to detect presumptive UV-specific p53 gene mutations in UV-exposed normal skin. The methods are based on mutant allele-specific PCRs and ligase chain reactions and designed to detect CC to TT mutations at codons 245 and 247/248, using 10 micrograms of DNA samples. These specific mutations in the p53 gene have been reported in skin tumors. CC to TT mutations in the p53 gene were detected in cultured human skin cells only after UV irradiation, and the mutation frequency increased with increasing UV dose. Seventeen of 23 samples of normal skin from sun-exposed sites (74%) on Australian skin cancer patients contained CC to TT mutations in one or both of codons 245 and 247/248 of the p53 gene, and only 1 of 20 samples from non-sun-exposed sites (5%) harbored the mutation. None of 15 biopsies of normal skin from non-sun-exposed or intermittently exposed sites on volunteers living in France carried such mutations. Our results suggest that specific p53 gene mutations associated with human skin cancer are induced in normal skin by solar UV radiation. Measurement of these mutations may be useful as a biologically relevant measure of UV exposure in humans and as a possible predictor of risk for skin cancer.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Wogan GN. Molecular epidemiology in cancer risk assessment and prevention: recent progress and avenues for future research. Environ Health Perspect. 1992 Nov;98:167–178. [Europe PMC free article] [Abstract] [Google Scholar]
- Albertini RJ, O'Neill JP, Nicklas JA, Heintz NH, Kelleher PC. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. [Abstract] [Google Scholar]
- Vogelstein B, Kinzler KW. Carcinogens leave fingerprints. Nature. 1992 Jan 16;355(6357):209–210. [Abstract] [Google Scholar]
- Balmain A, Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. [Abstract] [Google Scholar]
- Dipple A, Pigott M, Moschel RC, Costantino N. Evidence that binding of 7,12-dimethylbenz(a)anthracene to DNA in mouse embryo cell cultures results in extensive substitution of both adenine and guanine residues. Cancer Res. 1983 Sep;43(9):4132–4135. [Abstract] [Google Scholar]
- Abbott PJ, Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. [Abstract] [Google Scholar]
- Marion MJ, Froment O, Trépo C. Activation of Ki-ras gene by point mutation in human liver angiosarcoma associated with vinyl chloride exposure. Mol Carcinog. 1991;4(6):450–454. [Abstract] [Google Scholar]
- Barbin A, Tenenbaum L, Toman Z, Radman M, Bartsch H. recA-independent mutagenicity induced by chloroethylene oxide in E. coli. Mutat Res. 1985 Nov-Dec;152(2-3):157–159. [Abstract] [Google Scholar]
- Creech JL, Jr, Johnson MN. Angiosarcoma of liver in the manufacture of polyvinyl chloride. J Occup Med. 1974 Mar;16(3):150–151. [Abstract] [Google Scholar]
- Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. [Abstract] [Google Scholar]
- Ozturk M. p53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet. 1991 Nov 30;338(8779):1356–1359. [Abstract] [Google Scholar]
- Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. [Europe PMC free article] [Abstract] [Google Scholar]
- Seidman MM, Bredberg A, Seetharam S, Kraemer KH. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. [Europe PMC free article] [Abstract] [Google Scholar]
- Pierceall WE, Mukhopadhyay T, Goldberg LH, Ananthaswamy HN. Mutations in the p53 tumor suppressor gene in human cutaneous squamous cell carcinomas. Mol Carcinog. 1991;4(6):445–449. [Abstract] [Google Scholar]
- Somers KD, Merrick MA, Lopez ME, Incognito LS, Schechter GL, Casey G. Frequent p53 mutations in head and neck cancer. Cancer Res. 1992 Nov 1;52(21):5997–6000. [Abstract] [Google Scholar]
- Kress S, Sutter C, Strickland PT, Mukhtar H, Schweizer J, Schwarz M. Carcinogen-specific mutational pattern in the p53 gene in ultraviolet B radiation-induced squamous cell carcinomas of mouse skin. Cancer Res. 1992 Nov 15;52(22):6400–6403. [Abstract] [Google Scholar]
- Nakazawa H, Aguelon AM, Yamasaki H. Relationship between chemically induced Ha-ras mutation and transformation of BALB/c 3T3 cells: evidence for chemical-specific activation and cell type-specific recruitment of oncogene in transformation. Mol Carcinog. 1990;3(4):202–209. [Abstract] [Google Scholar]
- Kumar R, Sukumar S, Barbacid M. Activation of ras oncogenes preceding the onset of neoplasia. Science. 1990 Jun 1;248(4959):1101–1104. [Abstract] [Google Scholar]
- Nikitin AYu, Ballering LA, Lyons J, Rajewsky MF. Early mutation of the neu (erbB-2) gene during ethylnitrosourea-induced oncogenesis in the rat Schwann cell lineage. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9939–9943. [Europe PMC free article] [Abstract] [Google Scholar]
- Molès JP, Moyret C, Guillot B, Jeanteur P, Guilhou JJ, Theillet C, Basset-Sèguin N. p53 gene mutations in human epithelial skin cancers. Oncogene. 1993 Mar;8(3):583–588. [Abstract] [Google Scholar]
- Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, Halperin AJ, Baden HP, Shapiro PE, Bale AE, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4216–4220. [Europe PMC free article] [Abstract] [Google Scholar]
- Kanjilal S, Pierceall WE, Cummings KK, Kripke ML, Ananthaswamy HN. High frequency of p53 mutations in ultraviolet radiation-induced murine skin tumors: evidence for strand bias and tumor heterogeneity. Cancer Res. 1993 Jul 1;53(13):2961–2964. [Abstract] [Google Scholar]
- Reid TM, Loeb LA. Mutagenic specificity of oxygen radicals produced by human leukemia cells. Cancer Res. 1992 Mar 1;52(5):1082–1086. [Abstract] [Google Scholar]
- Hawley-Nelson P, Sullivan JE, Kung M, Hennings H, Yuspa SH. Optimized conditions for the growth of human epidermal cells in culture. J Invest Dermatol. 1980 Aug;75(2):176–182. [Abstract] [Google Scholar]
- Suzuki Y, Sekiya T, Hayashi K. Allele-specific polymerase chain reaction: a method for amplification and sequence determination of a single component among a mixture of sequence variants. Anal Biochem. 1991 Jan;192(1):82–84. [Abstract] [Google Scholar]
- D'Aquila RT, Bechtel LJ, Videler JA, Eron JJ, Gorczyca P, Kaplan JC. Maximizing sensitivity and specificity of PCR by pre-amplification heating. Nucleic Acids Res. 1991 Jul 11;19(13):3749–3749. [Europe PMC free article] [Abstract] [Google Scholar]
- Törmänen VT, Pfeifer GP. Mapping of UV photoproducts within ras proto-oncogenes in UV-irradiated cells: correlation with mutations in human skin cancer. Oncogene. 1992 Sep;7(9):1729–1736. [Abstract] [Google Scholar]
- Wei Q, Matanoski GM, Farmer ER, Hedayati MA, Grossman L. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1614–1618. [Europe PMC free article] [Abstract] [Google Scholar]
- Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991 Jun 15;51(12):3075–3079. [Abstract] [Google Scholar]
- Meuth M. The structure of mutation in mammalian cells. Biochim Biophys Acta. 1990 Jun 1;1032(1):1–17. [Abstract] [Google Scholar]
- Vrieling H, Van Rooijen ML, Groen NA, Zdzienicka MZ, Simons JW, Lohman PH, van Zeeland AA. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. [Europe PMC free article] [Abstract] [Google Scholar]
- Kricker A, English DR, Randell PL, Heenan PJ, Clay CD, Delaney TA, Armstrong BK. Skin cancer in Geraldton, Western Australia: a survey of incidence and prevalence. Med J Aust. 1990 Apr 16;152(8):399–407. [Abstract] [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7516–7520. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.91.1.360
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/91/1/360.full.pdf
Citations & impact
Impact metrics
Article citations
Cutaneous Squamous Cell Carcinoma: An Updated Review.
Cancers (Basel), 16(10):1800, 08 May 2024
Cited by: 2 articles | PMID: 38791879 | PMCID: PMC11119634
Review Free full text in Europe PMC
Impact of coconut kernel extract on carcinogen-induced skin cancer model: Oxidative stress, C-MYC proto-oncogene and tumor formation.
Heliyon, 10(8):e29385, 16 Apr 2024
Cited by: 0 articles | PMID: 38665592 | PMCID: PMC11043960
A Comprehensive Assessment of Ultraviolet-Radiation-Induced Mutations in Flammulina filiformis Using Whole-Genome Resequencing.
J Fungi (Basel), 10(3):228, 20 Mar 2024
Cited by: 0 articles | PMID: 38535236 | PMCID: PMC10971301
Mutational spectrum of TP53 gene correlates with nivolumab treatment efficacy in advanced gastric cancer (TP53MUT study).
Br J Cancer, 129(6):1032-1039, 02 Aug 2023
Cited by: 2 articles | PMID: 37532830 | PMCID: PMC10491760
Focus on the Contribution of Oxidative Stress in Skin Aging.
Antioxidants (Basel), 11(6):1121, 06 Jun 2022
Cited by: 55 articles | PMID: 35740018 | PMCID: PMC9220264
Review Free full text in Europe PMC
Go to all (147) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Quantitative detection of ultraviolet-specific p53 mutations in normal skin from Japanese patients.
Cancer Epidemiol Biomarkers Prev, 6(6):433-438, 01 Jun 1997
Cited by: 15 articles | PMID: 9184777
UV-radiation-specific p53 mutation frequency in normal skin as a predictor of risk of basal cell carcinoma.
J Natl Cancer Inst, 90(7):523-531, 01 Apr 1998
Cited by: 48 articles | PMID: 9539248
Carcinogen-specific mutation pattern in the p53 tumour suppressor gene in UV radiation-induced basal cell carcinoma.
Int Arch Occup Environ Health, 75(4):272-276, 26 Jan 2002
Cited by: 14 articles | PMID: 11981662
The specificity of p53 mutation spectra in sunlight induced human cancers.
J Photochem Photobiol B, 28(2):115-124, 01 May 1995
Cited by: 68 articles | PMID: 7636632
Review