Abstract
Background
Despite widespread use of methamphetamine and other amphetamine-type stimulants (METH/AMPH), little is known about the long-term medical consequences of METH/AMPH abuse and dependence. Preclinical neurotoxicity findings raise public health concerns that these stimulants may damage dopamine neurons, resulting in dopamine-related disorders such as Parkinson's disease (PD).Methods
A retrospective design was used to examine statewide medical records (1996 through 2011) linked to the Utah Population Database. Individuals 30 years or older on December 31, 2011 were assigned to a METH/AMPH cohort (ICD-9-CM 304.4, 305.7, 969.7, E854.2; N=4935), a cocaine cohort (ICD-9-CM 304.2, 305.6, 968.5, E855.2; N=1867) or a population cohort unexposed to drugs or alcohol for control selection. A competing-risks, proportional hazards model was used to determine whether the METH/AMPH or cocaine cohorts were at increased risk of developing PD (ICD-9-CM 332.0) or PD/parkinsonism/essential tremor (PD/PT; ICD-9-CM 332.0, 332.1, 333.0, 333.1) compared to individually sex- and age-matched controls (5:1 control to case ratio; N=34,010).Results
In METH/AMPH users, we observed an increased risk of PD and PD/PT (HRPD=2.8, 95%CI 1.6-4.8, P<10(-3); HRPD/PT=3.1, 95%CI 1.9-4.9, P<10(-4)) compared to population-based controls. Conversely, cocaine users exhibited no elevated risk of PD compared to controls.Conclusions
We observed a near three-fold increased risk of PD in METH/AMPH users vs. controls which confirms prior observations and supports that PD risk in users may be higher than previous estimates. A suggestion that female and male users may differ in PD susceptibility warrants further study.Free full text

Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment
Abstract
Background
Despite widespread use of methamphetamine and other amphetamine-type stimulants (METH/AMPH), little is known about the long-term medical consequences of METH/AMPH abuse and dependence. Preclinical neurotoxicity findings raise public health concerns that these stimulants may damage dopamine neurons, resulting in dopamine-related disorders such as Parkinson’s disease (PD).
Methods
A retrospective design was used to examine statewide medical records (1996 through 2011) linked to the Utah Population Database. Individuals 30y or older on December 31, 2011 were assigned to a METH/AMPH cohort (ICD-9-CM 304.4, 305.7, 969.7, E854.2; N=4,935), a cocaine cohort (ICD-9-CM 304.2, 305.6, 968.5, E855.2; N=1,867) or a population cohort unexposed to drugs or alcohol for control selection. A competing-risks, proportional hazards model was used to determine whether the METH/AMPH or cocaine cohorts were at increased risk of developing PD (ICD-9-CM 332.0) or PD/parkinsonism/essential tremor (PD/PT; ICD-9-CM 332.0, 332.1, 333.0, 333.1) compared to individually sex- and age-matched controls (5:1 control to case ratio; N=34,010).
Results
In METH/AMPH users, we observed an increased risk of PD and PD/PT (HRPD=2.8, 95%CI 1.6–4.8, P<10−3; HRPD/PT=3.1, 95%CI 1.9–4.9, P<10−4) compared to population-based controls. Conversely, cocaine users exhibited no elevated risk of PD compared to controls.
Conclusions
We observed a near 3-fold increased risk of PD in METH/AMPH users vs. controls which confirms prior observations and supports that PD risk in users may be higher than previous estimates. A suggestion that female and male users may differ in PD susceptibility warrants further study.
1. INTRODUCTION
According to the 2006 National Survey on Drug Use and Health Utah ranks 17th among US states in reported use of methamphetamine (METH) within the previous year among those 12 years and older (0.94%; Substance Abuse and Mental Health Services Administration (SAMHSA), 2006) and nearly 4% of Utah high-school students reported using METH at least once in a recent survey (Centers for Disease Control and Prevention, 2011). Regionally, Utah is located within the western United States (US) where recent METH use rates are over twice as high as in the Midwest or the South, and 12 times as high as the Northeast; age at first use typically occurs in the late teens to early 20’s (SAMHSA, 2013). The budgets of community hospitals and treatment facilities are adversely affected as visits related to METH use account for 9% of emergency department patient visits nationally (SAMHSA, 2011). The individual and public health consequences of these reports are significant, considering potential adverse health outcomes of individuals who initially engage in METH use at a young age.
Despite widespread abuse of METH and amphetamine or other amphetamine-type stimulants (AMPH), little is known about the long-term consequences of METH/AMPH abuse and dependence. Methamphetamine and its metabolite amphetamine cause release of the neurotransmitter dopamine in the dopamine-rich striatal subdivisions of the mammalian brain (Kish, 2008). Both the plasmalemmal dopamine transporter and the vesicular monoamine transporter-2 seem to play critical roles in this neurotoxicity (Hanson et al., 2004). Pre-clinical data demonstrate that METH can damage dopamine nerve terminals in the striatum, but with largely an apparent sparing of cell bodies (Fleckenstein et al., 2007). In humans, imaging and postmortem studies indicate that METH abuse causes persistent dopaminergic deficits (Wilson et al., 1996; Volkow et al., 2001; Kitamura, 2009). These neurotoxicity findings raise public health concerns that METH/AMPH may damage dopamine neurons in humans, resulting in dopamine-related disorders such as PD (Caligiuri and Buitenhuys, 2005). This concern was raised in a small clinical study in which prolonged amphetamine exposure was more frequent in PD patients ages 40–64 than in spouse controls (Garwood et al., 2006), and later in a study of California inpatient hospital and death records (1990–2005) in which researchers reported patients had a 1.8-fold risk of developing PD in comparison to appendicitis controls (Callaghan et al., 2011). To evaluate the potential link between METH/AMPH dependence and PD expression in a statewide population, we conducted a retrospective cohort study of individuals in the Utah Population Database (UPDB) with linked electronic medical records from 1996–2011. Over 85% of those with medical records in Utah link to a person in the UPDB through a birth, death, driver license, or voter registration data to provide personal medical histories on over 3 million individuals.
In 2012, 19% of patients admitted for drug dependence in Utah claimed METH as their principal drug of choice, and women were almost twice as likely as men in Utah to have a meth-related hospital admission (27% versus 15%) second only to alcohol (Utah Department of Human Services, 2012). Widely recognized sex differences occur in all of phases of drug abuse; initiation, escalation, addiction, and relapse (Greenfield et al., 2010). Women begin regular use of illicit drugs at lower doses than do men; however, use escalates more rapidly into addiction and women are at greater risk for relapse after abstinence (Greenfield et al., 2010). In preclinical studies, female rats exhibit higher self-administration of METH and increased METH-seeking relapse relative to males (Roth and Carroll, 2004; Reichel et al., 2012). The effects of stimulants may be strongly influenced by endogenous hormones as estrogen increases and progesterone decreases the interaction of stimulants with reward-related systems in women (Anker and Carroll, 2011). Lifetime rates of mood and anxiety disorders which often co-occur with METH or AMPH abuse are significantly higher among women than men (Greenfield et al., 2010). Lower phosphocreatine levels, associated with depressive symptoms, were more pronounced in female METH users (Sung et al., 2013). Because of such gender differences, we investigated sex-specific risks of PD in the Utah population.
It is projected that the number of people with PD in the US will double over the next 25 years; thus, the potential contribution of METH/AMPH abuse to PD incidence is a significant national health issue (Dorsey et al., 2007). The objectives of our study are to assess the findings of the California report (Callaghan et al., 2011), to identify population features relevant to the association between METH/AMPH use and PD, and to enhance generalizability of results by utilizing a statewide resource. Our study is novel in many respects. Using the UPDB, we accessed a statewide pool of individuals with long-term follow up and no history of illicit drug or alcohol abuse to provide an unexposed population from which we randomly selected controls for matching to exposed individuals with an indication of METH/AMPH or cocaine use. Our exposed drug cohorts are based not only on hospitalizations, but also on comprehensive outpatient records.
2. METHODS
2.1 Sources of data
2.1.1 Utah Population Database
The UPDB is a dynamic and rich resource located at the University of Utah that consists of computerized records for nearly eight million individuals spanning more than a century. The UPDB includes extensive genealogies, statewide vital records, driver license and voter registration records, and statewide inpatient records and links to clinic discharge records beginning in 1996. The discharge data hold up to eight fields per admission of International Classification of Diseases (ICD) codes mapped to version 9 (ICD-9-CM) reported to the Utah Department of Health from every healthcare system in Utah. These records are comprehensive, and are updated at least annually (Utah Population Database, 2014). Birth certificates data in UPDB (used to establish follow up in Utah) became comprehensively available statewide after 1939.
2.1.2 University of Utah and Intermountain Healthcare
In addition to statewide inpatient records, the UPDB contains a master index to link individuals who are patients in the University of Utah Healthcare (UUHC) statewide system of 4 hospitals and 30 community clinics to individuals in the UPDB. Likewise, the database contains an index that links records from the Intermountain Healthcare (IH) system of 22 hospitals and 185 physician clinics to UPDB persons. Using these record linkages, electronic medical records beginning in 1996 that include ICD-9-CM diagnoses (up to eight fields) and pharmacy orders were available for study. As the two largest healthcare providers in Utah, UUHC and IH account for more than 80% of all patient encounters in the state and they provide a valuable source of diagnoses in outpatient settings. We accessed UPDB and UUHC/IH records from 1996–2011 to obtain diagnoses of interest under an approved study protocol. These records comprise the ‘patient data’ referred to below. Approvals were received from the University of Utah’s Institutional Review Board, the Resource for Genetic Epidemiologic Research (governing body that reviews use of UPDB data), and Intermountain Healthcare’s privacy board to conduct this study.
2.2 Outcomes of interest and cohort definitions
2.2.1 Parkinson’s disease (PD) and PD/parkinsonism/essential tremor outcomes
The primary outcomes of interest were time from base line (January 1, 1996) to a subsequent index diagnosis of PD (defined as ICD-9 CM 332.0), or to a diagnosis of PD/parkinsonism/essential tremor (PD/PT) defined as: ICD-9 CM 332.0 (paralysis agitans); 332.1 (secondary parkinsonism); 333.0 (other degenerative diseases of the basal ganglia); and 333.1 (essential and other specified forms of tremor) in the patient data in any diagnostic position during January 1, 1996 to December 31, 2011. Patients with an indication of human immunodeficiency virus (HIV) based on ICD-9-CM 042 (HIV) or V08 (asymptomatic HIV infection status) were excluded, as HIV can result in parkinsonism-like symptoms (Tse et.al., 2004).
2.2.2 Exposed METH/AMPH cohort
Individuals (born in 1940 or later) who were 30 years or older on December 31, 2011 or date of last follow up (whichever occurred first), were assigned to a METH/AMPH cohort if the following criteria were met: (1) an ICD-9-CM code in any diagnostic position of 304.4 (amphetamine and other psychostimulant dependence), 305.7 (amphetamine or related acting sympathomimetic abuse), 969.7 (psychostimulant poisoning) or E854.2 (accidental psychostimulant poisoning) in the patient data; (2) no indication of PD/PT prior to the earliest METH/AMPH indication; (3) no indication of HIV as defined in 2.2.1; and (4) no indication of non-METH/AMPH illicit drug-use (ICD-9-CM codes 304.2, cocaine dependence; 305.6, cocaine abuse; 968.5, cocaine poisoning; E855.2, unintentional cocaine poisoning; 304.0, opioid dependence; 304.7, combination of opioid dependence with any other drug; 305.5, opioid abuse; 965.0, poisoning by opioids and related narcotics; E850.0–E850.2, accidental poisoning by heroin, methadone, or other opiates; 304.3, cannabis dependence; 305.2, cannabis abuse; 969.6, poisoning by cannabis or hallucinogens; 304.1, 304.5, 304.6, 304.8, 304.9, 305.3, 305.4, 305.9, other drug abuse or dependence conditions), or alcohol-use diagnoses (ICD-9-CM codes 303.0, acute alcoholic intoxication; 303.9, other and unspecified alcohol dependence; 305.0, alcohol abuse; 980.0, alcohol poisoning; E860.0, accidental poisoning by alcoholic beverages and other ethyl alcohol; E860.1, other and unspecified ethyl alcohol and its products) in the patient data. We identified 16,931 individuals in Utah age 30 or older at follow up with a METH/AMPH-use history and no indication of HIV. Of these, 11,985 individuals had an indication of other illicit drug or alcohol use (defined above) and 11 individuals had a prior PD/PT diagnosis and were therefore removed, resulting in a METH/AMPH cohort of 4,935 persons.
2.2.3 Exposed cocaine cohort
As a contrast to an exposed METH/AMPH cohort, we determined a ‘drug lifestyle’ cohort of individuals, born after 1939, with cocaine-use diagnoses who were age 30 years or older on December 31, 2011 or at date of last follow up (whichever occurred first). These individuals were assigned to a cocaine cohort if the following criteria were met: (1) an ICD-9-CM code in any diagnostic position of 304.2 (cocaine dependence), 305.6 (nondependent cocaine abuse), 968.5 (cocaine poisoning) or E855.2 (accidental cocaine poisoning) in the patient data; (2) no indication of PD/PT prior to the earliest indication of cocaine use; (3) no indication of HIV as defined in 2.2.1; and (4) no indication of METH/AMPH or other illicit drugs or alcohol use, defined above. We identified 7,952 individuals in Utah age 30 or older with one or more diagnoses of cocaine and no indication of HIV. Of these, 6,084 individuals with an indication of any other illicit drug or alcohol use and one individual with a prior PD/PT diagnosis were removed, resulting in a cocaine cohort of 1,867 persons.
2.2.4 Unexposed population cohort and control selection
As the exposed cohorts were retrospectively determined from statewide data, the unexposed cohort was designed to represent the Utah population, born 1940 or later, who were 30 years or older at year-end 2011 or date of last follow up, if earlier. Individuals were excluded from the population cohort who had a diagnosis of PD/PT prior to baseline, any indication of illicit drug- or alcohol-use diagnoses, or an HIV diagnosis in the patient data. From an unexposed population of 1.43 million individuals, controls were randomly selected and individually matched 5:1 to exposed case individuals on sex and birth year. In addition, controls had to have a follow-up period in Utah for at least as long as their respective case.
2.3 Statistical analyses
2.3.1 Proportional hazards model
A proportional hazards model was used to calculate the hazard ratio (HR), the ratio of hazard rates to measure how often an event occurs in one group compared to another group, over time. In our study, the HR was used to estimate the risk of a subsequent index diagnosis of PD or PD/PT in a competing-risks framework (Fine and Gray, 1999). As individuals with a history of illicit METH/AMPH or cocaine use are at increased risk of mortality compared to nonusers (Degenhardt and Hall, 2012), the competing risk of all-cause mortality was incorporated. We defined a subsequent index diagnosis to include outcomes with an index date coincident to the date of first exposure. This assumption is reasonable given age at first use for METH/AMPH averages 18 years (The National Survey on Drug Use and Health, 2006), while the average age at onset of PD is approximately 60 years (NIH National Institute of Neurological Disorders and Stroke). However, to minimize potential bias from PD/PT preceding an index indication of METH/AMPH, we removed individuals with zero event times in a secondary analysis. In case-only comparisons of drug-exposed cohorts, models were adjusted for birth year and sex, where appropriate.
2.3.2 Selection of matched controls
Exposed individuals in METH/AMPH or cocaine use cohorts were compared to randomly selected, unexposed population controls, individually matched 5:1 to exposed case subjects on sex and birth year. In addition, matched controls had to have a follow-up period in the UPDB at least as long as their respective exposed individual. To account for individual case-control matching, separate hazard functions were incorporated for each group (an exposed case and five associated controls).
2.3.3 Potential confounders
Cigarette smoking has been consistently associated with decreased PD risk (Wirdefeldt et al., 2011), while smoking rates and nicotine dependence among illicit drug users is high in comparison to non-drug users (Grant et al., 2004; Weinberger and Sofuoglu, 2009). A subset of models were adjusted for presence or absence of tobacco use in the patient data (ICD-9-CM codes 305.1, tobacco use disorders; 989.84, toxic effect of tobacco; and V15.82, history tobacco use history). In addition to gender, PD prevalence differs between racial and ethnic groups (Willis et al., 2013). Most individuals living in Utah are whites of non-Hispanic ethnicity (80%), with the largest minority population comprised of Hispanic or Latino individuals of any race (13%; Utah Governor’s Office of Planning and Budget, 2012). To avoid analysis where categorized subjects are potentially sparse, we did not adjust for race or ethnicity in our reported models. All analyses were performed using SAS® statistical software, version 9.4 (SAS/STAT 13.1).
2.4 Follow-up procedures
In population-based cohort studies, an accurate assessment of follow-up times in both exposed case and unexposed control cohorts merits careful consideration. Valid case-control comparisons using retrospective data depend on appropriate matching of exposure periods and longitudinal tracking of individuals. The UPDB records the date that each person in the database was last known to be residing in Utah based on recorded events and in their children, of an event indicating their mother or adoptive mother was known to be living in Utah, if this date is more recent (Curtin et al., 2013). However, assessing length of follow up for potential population-based controls can be problematic, if the first date an individual appears in Utah is unknown, particularly for those born outside of Utah. We developed an algorithm for determining a ‘first residence in Utah’ date to reduce potential bias from selecting controls new to Utah that may have been exposed elsewhere. By creating a statewide pool of individuals with adequate follow up based on time between an index event in Utah and the most recent event recorded in the UPDB, we help ensure appropriate matching of exposure periods between cases and controls in our study (Supplementary Material1).
3. RESULTS
3.1 Participant characteristics
Characteristics of exposed individuals in the METH/AMPH and cocaine cohorts in comparison to their respective matched population controls are shown in Table 1a. In the METH/AMPH cohort, 86% of individuals had ≥1 occurrence of ICD-9-CM code 305.7 (72%), 304.4 (23%), 969.7 (5%), or E854.2 (<0.5%) while 14% had diagnoses across multiple codes. A majority of users were diagnosed within the UUHC and IH healthcare systems (83%); the remainder were diagnosed from hospital discharge records of other providers. In the cocaine cohort, 90% of individuals had ≥1 occurrence of ICD-9-CM code 305.6 (76%), 304.2 (23%), 968.5 (1%), or E855.2 (<0.5%) and 77% were diagnosed within the UUHC and IH systems. Based on UPDB records, most subjects were white and non-Hispanic. Nearly half of METH/AMPH-exposed individuals in our study were women, with a younger median age at index exposure than their male counterparts. Male cocaine users outnumbered female users by nearly 2 to 1 (Table 1b). This is consistent with the profile of female users in Utah and elsewhere, who often report using METH/AMPH (the illicit drug of choice in women of childbearing age) to provide an energy boost or to lose weight; the so-called “Jenny Crank” program (Brecht et al., 2004).
Table 1
| a. Characteristics of METH/AMPH and cocaine cohorts compared to unexposed controls, individually matched to exposed individuals on sex and birth year. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Exposed to METH/AMPH | Controls matched 5:1 | Exposed to Cocaine | Controls matched 5:1 | |||||
| N | % | N | % | N | % | N | % | |
| Total exposed or unexposed | 4,935 | 100 | 24,675 | 100 | 1,867 | 100 | 9,335 | 100 |
| Median(range) age at follow up | 40 | (30–71) | 42 | (29–71) | 43 | (30–70) | 47 | (30–71) |
| Mean(SD) yr of follow up | 6.8 | (4.3) | 7.1 | (4.3) | 8.0 | (4.9) | 8.7 | (4.7) |
| Median(range) age at exposure | 35 | (15–69) | – | – | 38 | (14–67) | – | – |
| Sex | ||||||||
| Female | 2,399 | 48.6 | 11,995 | 48.6 | 702 | 37.6 | 3,510 | 37.6 |
| Male | 2,536 | 51.4 | 12,680 | 51.4 | 1,165 | 62.4 | 5,825 | 62.4 |
| Reported race | ||||||||
| White | 4,292 | 87.0 | 23,236 | 94.2 | 1,308 | 70.1 | 8,678 | 93.0 |
| Not white | 308 | 6.2 | 890 | 3.6 | 290 | 15.5 | 349 | 3.7 |
| Unreported | 335 | 6.8 | 549 | 2.2 | 269 | 14.4 | 308 | 3.3 |
| Reported ethnicity | ||||||||
| Not Hispanic | 3,560 | 72.1 | 18,979 | 76.9 | 1,188 | 63.6 | 7,162 | 76.7 |
| Hispanic | 746 | 15.1 | 1,900 | 7.7 | 353 | 18.9 | 751 | 8.0 |
| Unreported | 629 | 12.7 | 3,796 | 15.4 | 326 | 17.5 | 1,422 | 15.2 |
| Vital status at end of study | ||||||||
| Dead | 287 | 5.8 | 106 | 0.4 | 204 | 10.9 | 77 | 0.8 |
| Alive | 4,648 | 94.2 | 24,569 | 99.6 | 1,663 | 89.1 | 9,258 | 99.2 |
| ICD–9 smoking indication | ||||||||
| No indication | 2,045 | 41.4 | 22,634 | 91.7 | 1,007 | 53.9 | 8,505 | 91.1 |
| Indicated | 2,890 | 58.6 | 2,041 | 8.3 | 860 | 46.1 | 830 | 8.9 |
| Age, index PD/PT diagnosis | ||||||||
| Median(range) | 49 | (25–69) | 44 | (20–68) | 58 | (45–63) | 45 | (21–64) |
| Years to index PD/PT diagnosis | ||||||||
| Mean(SD) | 2.0 | (1.8) | 2.9 | (3.5) | 5.8 | (3.9) | 5.1 | (3.9) |
| b. Characteristics of METH/AMPH sex-stratified cohorts compared to unexposed controls, individually matched to exposed individuals on sex and birth year. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | |||||||
| Exposed to METH/AMPH | Controls matched 5:1 | Exposed to METH/AMPH | Controls matched 5:1 | |||||
| N | % | N | % | N | % | N | % | |
| Total exposed or unexposed | 2,399 | 100 | 11,995 | 100 | 2,536 | 100 | 12,680 | 100 |
| Median(range) age at follow up | 41 | (30–71) | 40 | (30–71) | 41 | (30–71) | 42 | (30–71) |
| Mean(SD) yr of follow up | 6.9 | (4.3) | 7.2 | (4.3) | 6.4 | (4.3) | 6.7 | (4.3) |
| Median(range) age at exposure | 33 | (15–67) | – | – | 36 | (16–69) | – | – |
| Reported race | ||||||||
| White | 2,128 | 88.7 | 11,328 | 94.4 | 2,164 | 85.3 | 11,908 | 93.9 |
| Not white | 173 | 1.2 | 465 | 3.9 | 135 | 5.3 | 425 | 3.4 |
| Unreported | 98 | 4.1 | 202 | 1.7 | 237 | 9.4 | 347 | 2.7 |
| Reported ethnicity | ||||||||
| Not Hispanic | 1,845 | 76.9 | 9,391 | 78.3 | 1,715 | 67.6 | 9,588 | 75.6 |
| Hispanic | 373 | 15.6 | 932 | 7.8 | 373 | 14.7 | 968 | 7.6 |
| Unreported | 181 | 7.5 | 1,672 | 13.9 | 448 | 17.7 | 2,124 | 16.8 |
| Vital status at end of study | ||||||||
| Dead | 114 | 4.8 | 34 | 0.3 | 173 | 6.8 | 72 | 0.6 |
| Alive | 2,285 | 95.2 | 11,961 | 99.7 | 2,363 | 93.2 | 12,608 | 99.4 |
| ICD–9 smoking indication | ||||||||
| No indication | 906 | 37.8 | 10,892 | 90.8 | 1,139 | 44.9 | 11,742 | 92.6 |
| Indicated | 1,493 | 62.2 | 1,103 | 9.2 | 1,397 | 55.1 | 938 | 7.4 |
| Age, index PD/PT diagnosis | ||||||||
| Median(range) | 51 | (25–67) | 40 | (20–59) | 45 | (31–69) | 46 | (25–68) |
| Years to index PD/PT diagnosis | ||||||||
| Mean(SD) | 1.5 | (1.6) | 2.6 | (3.6) | 2.2 | (2.2) | 3.1 | (3.6) |
ABBREVIATIONS: METH/AMPH, methamphetamine, amphetamine and amphetamine-type stimulants; PD, Parkinson's disease; PD/PT, Parkinson’s disease/parkinsonism/essential tremor; SD, standard deviation.
The numbers of subjects with PD or PD/PT outcomes in the exposed cases and controls (overall and sex-stratified) are shown in Table 2. Of 30 individuals with an incident PD/PT diagnosis in the METH/AMPH exposed cohort, half had an ICD-9-CM 332.0 diagnosis of PD. Of 1,867 exposed individuals, only 4 subjects with a “pure” diagnosis of cocaine (no indication of other illicit drugs or alcohol) experienced a subsequent diagnosis of PD/PT, while 22 matched controls had a subsequent PD/PT diagnosis (15 with ICD-9-CM 332.0 in the medical record). Based on a limited subset of medications data, 3 METH/AMPH users and 5 matched controls with incident PD/PT had a pharmacy order of carbidopa-levodopa.
Table 2
Summary of models and number of outcomes in exposed case and control cohorts.
| Figures | Competing risks model (covariates) | Exposure | Outcomea | Number of outcomes | |
|---|---|---|---|---|---|
| Cases | Controls | ||||
| 1a. | Individual matching (sex, birth year) | METH/AMPH | PD | 15 | 27 |
| Females | 8 | 6 | |||
| Males | 7 | 21 | |||
| Case–only (birth year) | Females vs. Males | (see above) | — | ||
| 1b. | Individual matching (sex, birth year) | METH/AMPH | PD/PT | 30 | 49 |
| Females | 18 | 18 | |||
| Males | 12 | 31 | |||
| Case–only (birth year) | Females vs. Males | (see above) | — | ||
| 2. | Individual matching (sex, birth year) | Cocaineb,c | PD/PT | 4 | 22 |
| Case–only (sex, birth year) | Cocaine vs. METH/AMPH | PD/PT | 4 vs. 30 | — | |
| Case–only (sex, birth year) | METH/AMPH vs. cocaine | PD/PT | 30 vs. 4 | — | |
| 3a.d | Individual matching (sex, birth year) | METH/AMPH | PD>0 event time | 12 | 27 |
| Females | 8 | 6 | |||
| Males | 4 | 21 | |||
| Case–only (birth year) | Females vs. Males | (see above) | — | ||
| 3b.d | Individual matching (sex, birth year) | METH/AMPH | PD/PT>0 event time | 22 | 49 |
| Females | 15 | 18 | |||
| Males | 7 | 31 | |||
| Case–only (birth year) | Females vs. Males | (see above) | — | ||
3.2 Risk of Parkinson’s disease and Parkinson’s disease/parkinsonism/essential tremor
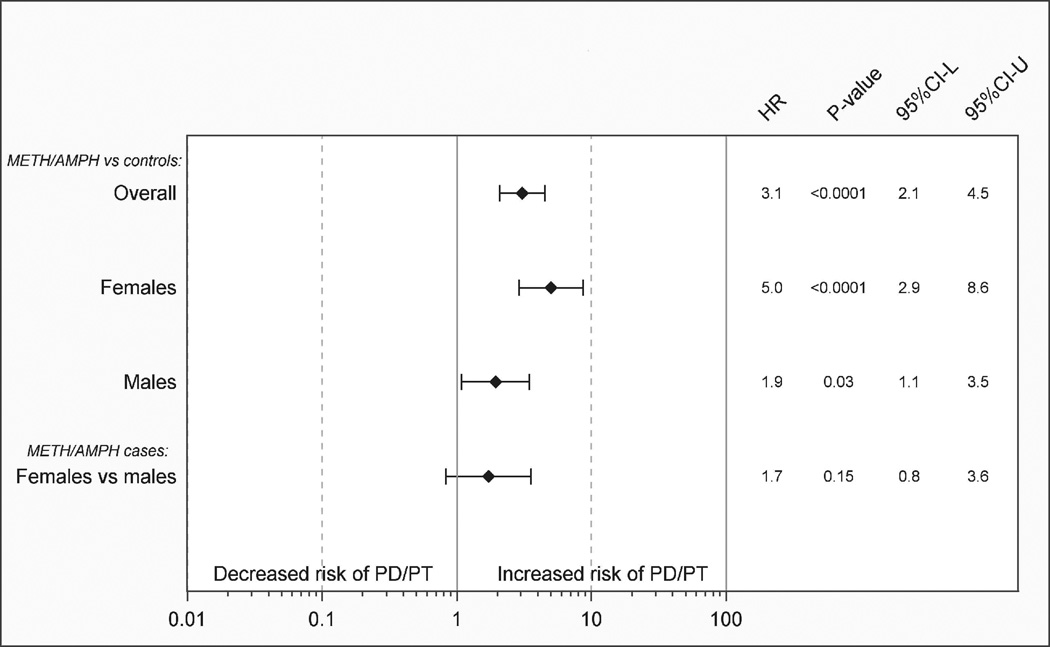
A summary of models upon which the findings presented in Figures 1a through 3b are based is shown in Table 2. Hazard ratios and 95% confidence intervals for PD or PD/PT in METH/AMPH exposed cases compared to individually matched controls are shown in Figures 1a and 1b, respectively. We observed an approximate 3-fold significant increased risk based on the HR in METH/AMPH users (men and women combined) compared to unexposed controls (HRPD=2.8, 95%CI=1.6–4.8; P<10−3 HRPD/PT=3.1, 95%CI=2.1–4.5; P<10−4). Although power was limited to examine risk in men and women separately where PD was defined as ICD-9-CM 332.0 (Figure 1a), the sex-stratified HRs were similar in magnitude to those observed for a more broadly-defined outcome of PD/PT (Figure 1b). Based on point-estimates of the HR, an increased risk of PD/PT in female exposed METH/AMPH cases compared to female controls (HRPD/PT=5.0, 95%CI=2.9–8.6; P<10−4) appeared to be larger than an increased risk observed in male METH/AMPH cases compared to male controls (HRPD/PT=1.9, 95%CI=1.1–3.5; P=0.03), although confidence intervals overlap. A case-only comparison of female vs. male METH/AMPH users was statistically non-significant (Figure 1b).
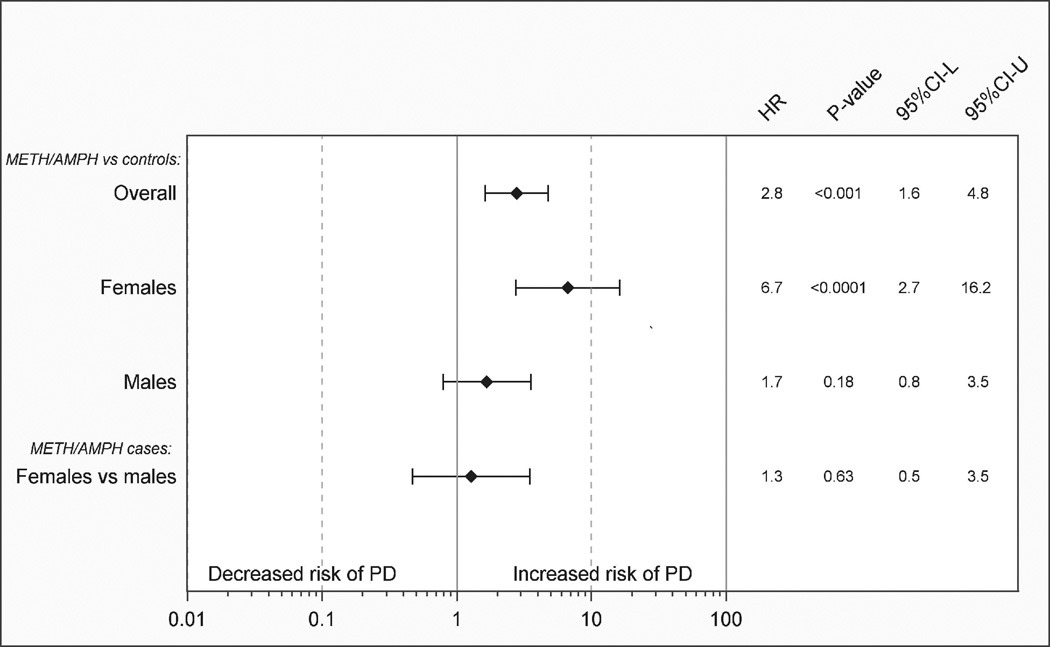
a. Methamphetamine/amphetamine use (METH/AMPH) and risk of Parkinson’s disease (PD). Hazard ratios (HR), P-values, and 95% confidence intervals (95%CI-L, 95%CI-U) for cases vs. population controls matched 5:1 on sex and birth year. Case-only female vs. male comparison (adjusted for birth year) is shown.
b. Methamphetamine/amphetamine and amphetamine-type stimulants (METH/AMPH) or cocaine use and risk of Parkinson’s disease/ parkinsonism/essential tremor (PD/PT). Hazard ratios (HR), P-values, and 95% confidence intervals (95%CI-L, 95%CI-U) for cases vs. population controls matched 5:1 on sex and birth year. Case-only female vs. male comparison (adjusted for birth year) is shown.
In contrast, the cocaine cohort exhibited no increased risk of PD/PT compared to unexposed controls, although the number of outcomes was extremely limited in the exposed subjects and estimates imprecise. In a case-only comparison of the cocaine and METH/AMPH cohorts (Figure 2), users of METH/AMPH appeared to be at an increased risk of PD/PT compared to cocaine users (HR=3.9, 95%CI=1.4–10.8; P=0.01). As tobacco exposure is a potential confounder, we further adjusted the caseonly model for presence or absence of a tobacco diagnosis. Adjustment for tobacco did not substantively impact the estimated risk of PD/PT in METH/AMPH vs cocaine cohorts (HRtobacco=1.9, 95:%CI 0.9.4.0; P=0.01; data not shown).
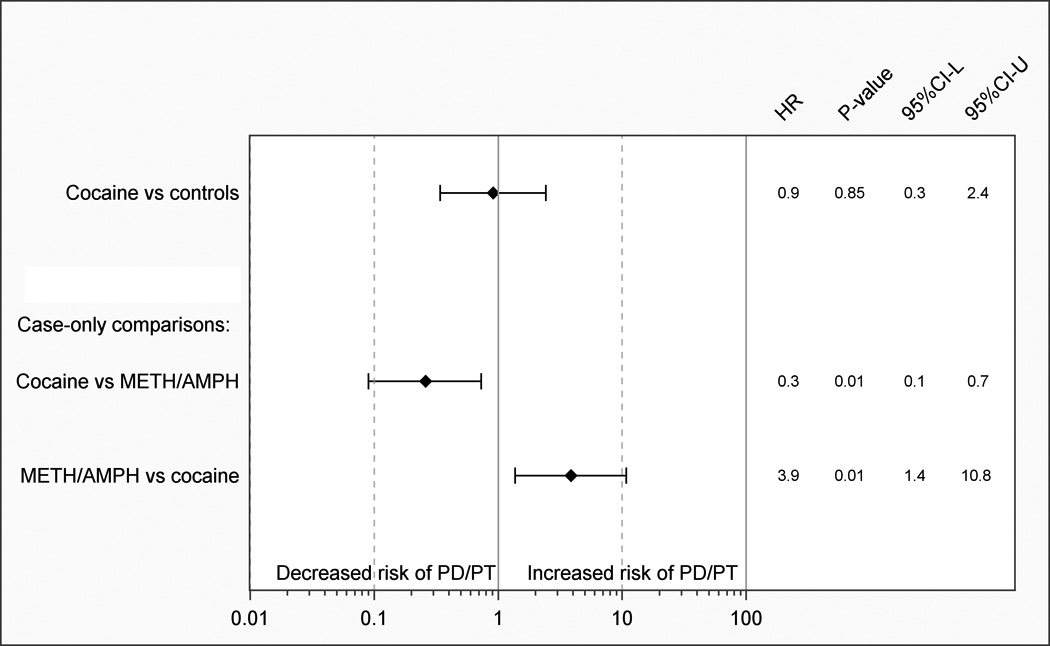
Cocaine use and risk of Parkinson’s disease/parkinsonism/essential tremor (PD/PT). Hazard ratios (HR), P-values, and 95% confidence intervals (95%CI-L, 95%CI-U) compared to population controls matched 5:1 on sex and birth year. Case-only cocaine and METH/AMPH cohort comparisons (adjusted for sex, birth year) are also shown.
We defined a subsequent outcome of PD or PD/PT to include subjects in which the index date of exposure and index diagnosis date of PD or PD/PT were coincident. To check the robustness of this assumption, we calculated estimates from models in which exposed subjects with a concurrent diagnosis of METH/AMPH and PD or PD/PT were excluded. We observed an increased risk of PD and PD/PT overall in men and women exposed to METH/AMPH in compared to controls (HRPD=2.2, 95%CI=1.2.–4.0; P<10−1; HRPD/PT=2.3, 95%CI=1.5–3.5 P<10−3); likewise, increased risk of PD and PD/PT was observed in the female subsample (Figures 3a and 3b, supplemental material available online). Compared to male METH/AMPH case subjects, an increased risk was observed for non-concurrent PD/PT only, just meeting statistical significance (see Figures 3a and 3b, available online2).
4. DISCUSSION
4.1 Study strengths
While confirming an earlier report of increased PD risk in a study of a northern California population, our analysis helps to further elucidate the nature of the association between METH/AMPH use and expression of PD. To our knowledge, it is the first retrospective cohort study of METH/AMPH and PD or PD/PT outcomes in the US conducted over an entire statewide population (Utah). Rather than relying on hospital-based controls, we were able to randomly select unexposed controls from the same population from which the exposed cohorts were identified. We are confident control subjects were living in Utah over the same exposure period as our case cohort members, and are less likely to have undetected drug use disorders diagnosed outside of Utah. Our study had access to both statewide inpatient discharge records (including admissions from an emergency department) and comprehensive outpatient clinic records from the largest healthcare providers of patient services in Utah. Thus we increase our chances of detecting exposures or PD at the clinic, prior to hospitalization for acute conditions. This population-based study would not be possible without the rich data resources in the UPDB. Although Utah ranks 34th in the nation in population, we were able to identify nearly 5,000 individuals with “pure” METH/AMPH use (no evidence of other illicit drugs or alcohol), and over 1,800 individuals with “pure” cocaine use in which diagnoses of PD/PT were almost nonexistent.
4.2 Study limitations
Our study is not immune to limitations. It is possible that METH/AMPH users have a history of multidrug or alcohol use without a corresponding diagnosis in the patient data. We believe that an analysis of a cocaine use cohort is appropriate; of almost 25,000 METH/AMPH or cocaine users (including “pure” and multidrug-use profiles) only 20% had an indication of both METH/AMPH and cocaine. Although we followed individuals over a 16-year period, subsequent diagnoses of PD were uncommon, and particularly rare in the cocaine cohort in which only a few outcomes were available to the analysis. Thus we were limited in our ability to provide precise estimates for the cocaine cohort and for sex-stratified analyses of METH/AMPH users, and results should be interpreted with caution.
Administrative claims data are limited in their ability to identify PD and treatment-seeking differences between illicit drug abusers and unexposed controls are a potential source of bias. There is no definitive ‘gold standard’ diagnostic test for PD in live patients, and studies evaluating the accuracy of ICD-9-CM codes are mixed (Swarztrauber et al., 2005; Noyes et al., 2007; White et al., 2007; Szumski and Cheng, 2009). White, et al. concluded a single occurrence of ICD-9-CM 332.0 for PD or 332.1 for parkinsonism to be insufficient, with a positive predictive values (PPV) of 57% and 39%, respectively. Szumski, et al. demonstrated that algorithms incorporating pharmacy data or clinic specialty can better identify PD compared to a single diagnostic code, 332.0. In a primarily male patient population, Swarztrauber, et al. reported administrative data did not adequately distinguish between PD and parkinsonism and that 333.0 (disorders of the basal ganglia) was highly predictive of parkinsonism (Swarztrauber et al., 2005). In a study of Medicare records, Noyes et al. concluded a claims-based determination of PD can be reliable. In addition to 332.0, they report using more PD-related diagnostic codes (332.1, 333.0, and 333.1) increased sensitivity while maintaining high specificity (99%), but PPV was reduced (60–67%) compared to 332.0 alone (71–79%). In a study of essential tremor in billing records of a neurological facility, Louis et al. reported the most common false-positive diagnosis of essential tremor was, in fact, PD (Louis et al., 2007). In order to maximize incident outcomes in our analysis we used an inclusive, multiple-code approach (hence, PD/PT) of Noyes, et al. to increase sensitivity although we acknowledge misclassification of subjects may have occurred. Although less precise, when patients with a PD diagnosis were defined as having 332.0, our findings corroborate those of the California report and are consistent with models of PD/PT as the outcome.
To increase the number of outcomes available for study, we assumed index drug abuse likely precedes the first or index diagnosis of PD/PT in the electronic medical record and allowed identification of cohort membership coincident with any PD/PT outcome in the medical record. However, ignoring the possibility of an index diagnosis of disease preceding drug exposure is a limitation of this approach, and we estimated competing-risks models in which METH/AMPH cases with zero event times were excluded. The results in Figures 3a and 3b are consistent with our primary findings of an increased risk of PD or PD/PT in METH/AMPH exposed subjects.
We did not control for exposure to antipsychotic medications that with extended use may induce side effects that mimic PD, as access to detailed psychiatric diagnoses and related medications information were beyond the scope of our investigation and previously not-well studied (Srisurapanont et al., 2001). Bramness et al. (2012) suggest that psychotic complications subside quickly when amphetamine use is discontinued and thus unlikely to be mistaken for PD.
4.3 Contributions and future efforts
This research represents an important contribution in several respects. First, as statewide medical records are systematically added to the UPDB annually, we will be able to follow our exposed cohorts and unexposed population forward in time. We will focus our efforts on exploring multidrug use in relation to METH/AMPH and PD/PT or other adverse medical outcomes, particularly in relation to concurrent opioid or alcohol use as potential effect modifiers. As the UPDB is linked to extensive genealogy data and many study individuals have been identified within multi-generation families, we plan to examine a genetic basis for susceptibility to addiction and to develop measures of exposure intensity based on familial risk. We plan to improve measurement of the outcome of PD by developing algorithms using medical and other records available to the UPDB to accurately identify patients that are validated with chart review. Rates of cigarette smoking among METH/AMPH users in the US are estimated at 87–92% (Weinberger and Sofuoglu, 2009). In the Utah cohort, the proportion of ICD-9 tobacco diagnoses in METH/AMPH users (59%) likely understates nicotine exposure. We obtained self-reported smoking data from patient questionnaires administered at the time of service in a subset of 381 METH/AMPH individuals. Arguably more accurate than ICD-9 indicated tobacco (that may or may not be noted in the medical record), female METH/AMPH users reported they were current/ former smokers less often than male users (78% vs. 85%, respectively). Comprehensively assessing history of tobacco use at a statewide level will be critical to future investigations of the association of nicotine in both METH/AMPH addiction and development of PD.
We did not observe a statistically significant difference in risk of PD or PD/PT in female METH/AMPH users compared to male users. Point estimates suggest women who abuse METH/AMPH may be at an increased risk of PD or PD/PT than men who abuse stimulants compared to sex-matched controls. While intriguing, this observation should be interpreted cautiously as preclinical and clinical evidence of a sex difference between METH/AMPH exposure and dopaminergic deficits or dopamine disorders is sparse, and additional well-powered studies are necessary to provide support for a gender interaction. We plan to continue to follow our retrospective cohort forward in time and explore sex-specific differences in relation to comorbidities such as mood disorders, including any associated antipsychotic medications use. The Utah population is comprised primarily of whites, with a fast-growing community of Hispanic or Latinos. Our results may be representative of other areas of the US with a similar predominant Caucasian population, including northern California.
4.4 Conclusions
Our findings of an approximate 3-fold increased risk of PD or PD/PT in METH/AMPH exposed individuals, compared to both an unexposed control cohort and a cocaine use cohort, confirm those of the California study. Our study suggests the risk in those with a history of METH/AMPH dependence may be somewhat higher than previous estimates and although power was limited to detect an interaction, suggestive differences in risk estimates between women and men merit further study. Our investigation is the first, to our knowledge, to access an unexposed population cohort of statewide individuals with long-term follow up and no history of illicit drug use for comparison to exposed individuals with an indication of METH/AMPH or cocaine from outpatient clinic records as well as inpatient hospitalizations. Our findings provide additional evidence to support preclinical observations in animal models of METH use and development of Parkinson-like neurotoxicity may be mirrored in humans, which has profound implications for prevention and treatment of METH/AMPH disorders.
Supplementary Material
1
2
3
Acknowledgements
We gratefully acknowledge Alison Fraser (database management) and Yuan Wan (programming support) of the Huntsman Cancer Institute Pedigree & Population Resource, and Jef Huntington (Intermountain Healthcare) and Micky Daurelle (University of Utah Healthcare) for their efforts in the extraction of enterprise warehouse medical records.
Role of the funding source: This research was supported by the University of Utah Department of Pharmacology and Toxicology, and by the National Institutes of Health, National Institute on Drug Abuse (NIDA) R01 DA031883 to G. Hanson, PI. Partial support for all datasets within the Utah Population Database was provided by the University of Utah Huntsman Cancer Institute and the Huntsman Cancer Institute Cancer Center Support grant, P30 CA2014 from the National Cancer Institute (NCI). The National Institutes of Health (NIDA and NCI) did not have any role in the study design, analyses, interpretation of results, manuscript preparation, or approval to submit the final version of the manuscript for publication. The views expressed in this paper do not necessarily reflect those of NIDA or NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
11Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering ...
2Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering ...
AUTHOR DISCLOSURES
Conflict of interest: The authors declare that they have no conflict of interest.
Contributors: KC conceived the study design, prepared the manuscript, and supervised all statistical analyses and preparation of study datasets; AF helped to prepare and critically revise the manuscript; RJR and MJC participated in the design of the study and helped in the preparation of the manuscript; RJR supervised data extraction efforts at Intermountain Healthcare. KRS contributed to the research design and preparation of the manuscript. GHR conceived the study question and substantially participated in the study design and manuscript preparation. Dr. Karen Curtin (the lead author) had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analyses.
Ethical approval: The current study was approved by both the institutional review boards of the University of Utah and Intermountain Healthcare.
References
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci. 2011;8:73–96. [Abstract] [Google Scholar]
- Bramness JG, Gundersen OH, Guterstam J, Rognli EB, Konstenius M, Loberg EM, Medhus S, Tanum L, Franck J. Amphetamine-induced psychosis--a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatry. 2012;12:221. [Europe PMC free article] [Abstract] [Google Scholar]
- Caligiuri MP, Buitenhuys C. Do preclinical findings of methamphetamine-induced motor abnormalities translate to an observable clinical phenotype? Neuropsychopharmacology. 2005;30:2125–2134. [Abstract] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2011;120:35–40. [Abstract] [Google Scholar]
- Centers for Disease Control and Prevention. High School Youth Risk Behavior Survey, Utah. [Accessed on January 23, 2014];2011 Available at http://apps.nccd.cdc.gov/youthonline.
- Curtin K, Smith KR, Fraser A, Pimentel R, Kohlmann W, Schiffman JD. Familial risk of childhood cancer and tumors in the Li-Fraumeni spectrum in the Utah Population Database: implications for genetic evaluation in pediatric practice. Int. J. Cancer. 2013;133:2444–2453. [Europe PMC free article] [Abstract] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. [Abstract] [Google Scholar]
- Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999;94:496–509. [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. [Abstract] [Google Scholar]
- Garwood ER, Bekele W, McCulloch CE, Christine CW. Amphetamine exposure is elevated in Parkinson's disease. Neurotoxicology. 2006;27:1003–1006. [Abstract] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry. 2004;61:1107–1115. [Abstract] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr. Clin. North Am. 2010;33:339–355. [Europe PMC free article] [Abstract] [Google Scholar]
- Hanson GR, Rau KS, Fleckenstein AE. The methamphetamine experience: a NIDA partnership. Neuropharmacology. 2004;47(Suppl. 1):92–100. [Abstract] [Google Scholar]
- Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178:1679–1682. [Europe PMC free article] [Abstract] [Google Scholar]
- Kitamura O. Detection of methamphetamine neurotoxicity in forensic autopsy cases. Leg. Med. (Tokyo) 2009;11(Suppl. 1):S63–S65. [Abstract] [Google Scholar]
- Louis ED, Applegate LM, Rios E. ICD-9 CM code 333.1 as an identifier of patients with essential tremor: a study of the positive predictive value of this code. Neuroepidemiology. 2007;28:181–185. [Abstract] [Google Scholar]
- National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) Parkinson's Disease Backgrounder [Google Scholar]
- Noyes K, Liu H, Holloway R, Dick AW. Accuracy of Medicare claims data in identifying Parkinsonism cases: comparison with the Medicare current beneficiary survey. Mov. Disord. 2007;22:509–514. [Abstract] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl.) 2012;223:371–380. [Europe PMC free article] [Abstract] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl.) 2004;172:443–449. [Abstract] [Google Scholar]
- Srisurapanont M, Kittiratanapaiboon P, Jarusuraisin N. Treatment for amphetamine psychosis. Cochrane Database Syst. Rev. 2001 CD003026. [Abstract] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. [accessed on January 23, 2014];The National Survey on Drug Use and Health (NSDUH) Report, State Estimates of Past Year Methamphetamine Use. 2006 http://www.samhsa.gov/data/2k6/stateMeth/StateMeth.pdf.
- Substance Abuse and Mental Health Services Administration. National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: 2011. Office of Applied Studies. Drug Abuse Warning Network, 2008. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. Results from the 2012 National Survey on Drug Use and Health (NSDUH): Summary of National Findings. HHS Publication No. (SMA) 2013:13–4795. [Google Scholar]
- Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, Hellem TL, Huber RS, Fiedler KK, Harrell RE, Nickerson BR, Kim SE, Jeong EK, Renshaw PF. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend. 2013;129:102–109. [Europe PMC free article] [Abstract] [Google Scholar]
- Swarztrauber K, Anau J, Peters D. Identifying and distinguishing cases of parkinsonism and Parkinson's disease using ICD-9 CM codes and pharmacy data. Mov. Disord. 2005;20:964–970. [Abstract] [Google Scholar]
- Szumski NR, Cheng EM. Optimizing algorithms to identify Parkinson's disease cases within an administrative database. Mov. Disord. 2009;24:51–56. [Europe PMC free article] [Abstract] [Google Scholar]
- Tse W, Cersosimo MG, Gracies JM, Morgello S, Olanow CW, Koller W. Movement disorders and AIDS: a review. Parkinsonism Relat. Disord. 2004;10:323–334. [Abstract] [Google Scholar]
- Utah Department of Human Services. Division of Substance Abuse and Mental Health. Annual Report, 2012. [accessed on January 23, 2014];2012 http://www.dsamh.utah.gov/docs/Annual%20report%202012.pdf.
- Utah Governor's Office of Planning and Budget. Census Brief: Race and Ethnicity in Utah (2010) Salt Lake City: Utah; 2012. [Google Scholar]
- Utah Population Database. Huntsman Cancer Institute at the University of Utah. [accessed on January 25, 2014];Table of Records Available. 2014 http://healthcare.utah.edu/huntsmancancerinstitute/research/updb/data/ [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–382. [Abstract] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am. J. Drug Alcohol Abuse. 2009;35:12–17. [Europe PMC free article] [Abstract] [Google Scholar]
- White D, Moore S, Waring S, Cook K, Lai E. Identifying incident cases of parkinsonism among veterans using a tertiary medical center. Mov. Disord. 2007;22:915–923. [Abstract] [Google Scholar]
- Willis AW, Schootman M, Kung N, Racette BA. Epidemiology and neuropsychiatric manifestations of Young Onset Parkinson's Disease in the United States. Parkinsonism Relat. Disord. 2013;19:202–206. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996;2:699–703. [Abstract] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur. J. Epidemiol. 2011;26(Suppl. 1):S1–S58. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.drugalcdep.2014.10.027
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4295903?pdf=render
Citations & impact
Impact metrics
Article citations
Microglia contribute to methamphetamine reinforcement and reflect persistent transcriptional and morphological adaptations to the drug.
Brain Behav Immun, 120:339-351, 03 Jun 2024
Cited by: 1 article | PMID: 38838836 | PMCID: PMC11269013
No evidence of structural abnormality of the substantia nigra in adult attention-deficit/hyperactivity disorder: a pilot cross-sectional cohort study.
Front Psychiatry, 15:1395836, 30 May 2024
Cited by: 0 articles | PMID: 38873538
The Common Denominators of Parkinson's Disease Pathogenesis and Methamphetamine Abuse.
Curr Neuropharmacol, 22(13):2113-2156, 01 Jan 2024
Cited by: 1 article | PMID: 37691228 | PMCID: PMC11337683
Review Free full text in Europe PMC
Prevention of Parkinson's Disease: From Risk Factors to Early Interventions.
CNS Neurol Disord Drug Targets, 23(6):746-760, 01 Jan 2024
Cited by: 1 article | PMID: 37326115
Review
Modeling methamphetamine use disorder and relapse in animals: short- and long-term epigenetic, transcriptional., and biochemical consequences in the rat brain.
Neurosci Biobehav Rev, 155:105440, 29 Oct 2023
Cited by: 1 article | PMID: 38707245
Review
Go to all (93) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs.
Drug Alcohol Depend, 120(1-3):35-40, 26 Jul 2011
Cited by: 154 articles | PMID: 21794992
Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders.
Mov Disord, 25(14):2333-2339, 01 Oct 2010
Cited by: 86 articles | PMID: 20737543
Comparative hazards of acute myocardial infarction among hospitalized patients with methamphetamine- or cocaine-use disorders: A retrospective cohort study.
Drug Alcohol Depend, 188:259-265, 26 Apr 2018
Cited by: 4 articles | PMID: 29793190
Methamphetamine use and future risk for Parkinson's disease: Evidence and clinical implications.
Drug Alcohol Depend, 187:134-140, 10 Apr 2018
Cited by: 31 articles | PMID: 29665491
Review
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P30 CA2014
NIDA NIH HHS (1)
Grant ID: R01 DA031883





