Abstract
Free full text

Type-2 Innate Lymphoid Cells in Asthma and Allergy
Abstract
Type-2 innate lymphoid cells (ILC2) belong to an expanding family of innate lymphocytes that provide a potent source of immune effector cytokines at the initiation of immune responses. ILC2 arise, under the control of the transcription factors RORα and GATA3, from lymphoid progenitors in the bone marrow, to secrete type-2 cytokines including IL-5 and IL-13. Using experimental models, ILC2 have been implicated in allergic diseases, such as asthma and atopic dermatitis, but also in metabolic homeostasis. Furthermore, recent reports have indicated that ILC2 not only play roles at the initiation of type-2 immunity but can also contribute to chronic pathology, such as fibrosis, and can impact on the priming of the adaptive T-cell response. The identification of ILC2 in patients with allergic dermatitis and allergic rhinitis indicates that these cells may represent new therapeutic targets.
For some 25 years, after the characterization of different Th1 and Th2 cell subsets based on their distinct cytokine secretion profiles, a model prevailed based on the orchestration of immune responses by T cells through their release of a complex repertoire of cytokines (1). The model identified Th1 cells as driving type-1 immunity to intracellular pathogens and autoimmune diseases. By contrast, Th2 cells were proposed to control extracellular parasite infections but also underlie the immunopathology of allergy and asthma. We now recognize additional T helper cell subsets, including Th17 cells and T follicular helper cells, that via cytokine release have bespoke roles in regulating different aspects of immune responses.
However, a recent major paradigm shift has added another layer of complexity to immunoregulation by cytokines with the discovery of innate lymphoid cells (ILCs) (2). ILCs produce many Th cell–associated cytokines but do not express cell surface markers associated with other immune cell lineages (lineage-negative [Lin−]) and do not express a T-cell receptor. The ILC family now includes ILC1 (predominantly IFN-γ–expressing cells), ILC2 (predominantly IL-5– and IL-13–expressing cells), and ILC3 (predominantly IL-22– and IL-17–expressing cells) (3–6). Due to their recent discovery, our knowledge of these cells is still rather rudimentary, but major roles are emerging for ILCs in protective immunity against parasitic helminths (ILC2) (7–9) and bacteria (ILC1 and ILC3) (10–12), and in autoimmune disorders (ILC1 and ILC3) (13), allergic disease (ILC2) (14–16), and obesity (17–19).
Recent comprehensive reviews have described the identification and phenotypic characterization of ILC (3–6, 20). This review focuses on the most recent advances in our understanding of the factors that regulate ILC2 development and how these cells contribute to allergy and lung inflammation.
ILC2
ILC2 are found in the blood, spleen, intestine, liver, lung, fat-associated lymphoid clusters, and lymph nodes of mice (7–9). They are Lin−(CD3CD4CD8CD19CD11bCD11cFcεR1NK1.1Gr1−) CD45+CD127+Sca1+ICOS+KLRG1+CD25+CD117variableST2variableIL-17BRvariable. The human equivalents of ILC2 were reported in human lung parenchyma and bronchoalveolar lavage (BAL) fluid, as lineage-negative cells expressing IL-7Rα and the ST2 receptor for IL-33 (21). More comprehensively, Mjösberg and colleagues reported CD45hiCD127+CD117+CD161+(chemoattractant receptor-homologous molecule expressed on Th2 cells) CRTH2+ cells in peripheral blood, fetal gut, and the inflamed nasal polyps of patients with rhinosinusitis, that responded to IL-25 and IL-33 by producing IL-13 and IL-5 but not IL-17A or IL-22 (22).
Regulators of ILC2 Development
ILC Precursors
Progress in defining the transcriptional regulation of ILC differentiation has been rapid. In part this is because many of the factors that have been demonstrated to play important roles in ILC commitment have been discovered previously to regulate the development of other blood cell lineages. For example, GATA-binding protein 3 (GATA3) and T cell factor 1 (TCF1) have both been identified as important for regulating ILC2 development (23, 24). However, both of these factors have been demonstrated previously to be essential for normal T-cell development, and further investigation has revealed that GATA3 and TCF-1 are required for the generation of a progenitor downstream of the common lymphoid progenitor (CLPs) but upstream of the Id2+ ILC precursor and are required for multiple ILC subsets (25, 26) (Figure 1).
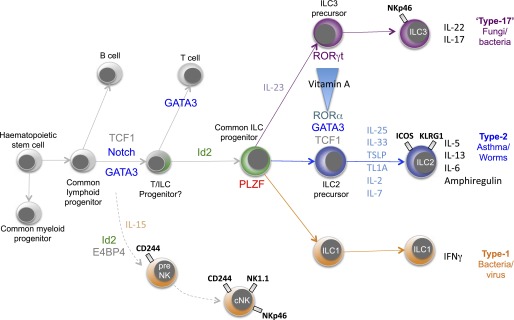
Transcriptional control of type-2 innate lymphoid cells (ILC2) development. Although the precise roles of specific transcription factors in ILC2 ontogeny are still to be elucidated, a general map is emerging in which a common lymphoid progenitor progressively commits to an ILC precursor. The data in this scheme are mainly derived from the mouse system. Subsequent activation of transcription factors such as RORγ (ILC3) and RORα (ILC2) restricts the lineages still further. GATA3 = GATA-binding protein 3; ICOS = inducible T cell costimulator; Id2 = inhibitor of DNA binding 2; KLRG1 = killer cell lectin-like receptor G1; NK = natural killer; PLZF = promyelocytic leukaemia zinc finger protein; TCF1 = T cell factor 1; TSLP = thymic stromal lymphopoietin.
ILC2 arise from CLPs in the bone marrow and, like all ILCs, require the transcriptional inhibitor Id2 for their development (7, 27, 28). This basic helix-loop-helix transcriptional regulator inhibits the activity of the E proteins (29), which have been implicated in the differentiation of B and T cells. Thus, suppression of these alternative cell fates represents a crucial step in the ILC developmental pathway.
Downstream of this Id2-dependent progenitor appears to lie an ILC precursor that is dependent on the transcription factor promyelocytic leukaemia zinc finger protein (PLZF) (encoded by Zbtb16), and this progenitor gives rise to ILC1, ILC2, and ILC3, but not classical natural killer (NK) cells or LTi (30) (Figure 1). Fate mapping demonstrated that CLPs, T cells, and B cells did not express PLZF during their development. Constantinides and colleagues went on to identify a rare PLZFhi cell subset in fetal liver and adult bone marrow that could give rise to all ILC lineages except LTi and classical NK cells (30). Unlike CLPs, these PLZFhi cells, which were Lin−CD127+CD117+α4β7hiGATA3+TOX+ (with a small proportion expressing Rorγt), did not generate T or B cells. Notably, deletion of PLZF in Zbtb16−/− mice only leads to the ablation of the ILC1 and ILC2 subsets, but not ILC3s, indicating that although PLZF is expressed in an ILC3 precursor, it is not essential for ILC3 development.
The timing and duration of Notch signals has also been shown to be critical for lymphoid differentiation and is required for the generation of mouse ILC2 from CLPs in vitro (27). Notch signaling is also important for the generation of human ILC2, and the use of a tuneable intracellular domain of Notch1 indicated that low levels of Notch signaling lead to T-cell development, whereas high concentrations lead to ILC2 differentiation (31). However, the requirement for the Notch pathway in vivo has yet to be ascertained.
Committed ILC2
In contrast to the more generic ILC commitment factors, the transcription factor RORγt (encoded by Rorc) has been identified as a key regulator of ILC3 cells (10, 32–34), and RORα has been identified as a key determinant of ILC2 differentiation (27, 35). However, even here there is overlap of transcription factor use, with Th17 cells requiring RORγt, and RORα also playing a subordinate role in Th17 cell development.
The transcription factor RORα has been shown to be critical for ILC2 development. RORα-deficient “Staggerer” mice, which carry a spontaneous deletion in the Rora gene (36), show severely impaired expansion of ILC2s in response to IL-25 injection, Nippostrongylus brasiliensis infection, or intranasal papain administration and fail to rapidly initiate characteristic type-2 immunity (27, 35). Although Rora mRNA can be detected in other ILC populations (albeit at considerably lower levels than in ILC2), the other ILC subsets are present at normal frequency in RORα-deficient mice (27, 35). RORα-deficient mice lack a population of ILC2-like cells in the bone marrow that appear to be immature ILC2 (Sca-1+, IL-7Rα+, CD25+ ST2+, IL-13−, IL-5−) and generate ILC2 robustly on adoptive transfer (35).
In addition to its role in early lymphocyte development, as discussed above, GATA3 is also required for downstream ILC2 maintenance and function. Using an Il13-Cre to delete conditional Gata3 alleles, GATA3 was shown to be essential for type-2 cytokine production (37). Furusawa and colleagues suggest that this results from IL-33 activation of the p38 mitogen-activated protein kinase, phosphorylation of GATA3, and the subsequent binding of GATA3 to the promoters of IL-5 and IL-13 (38). GATA3 expression has also been used to define an ILC2-committed Lin−Sca1+Id2+Gata3+ (or “LSIG”) precursor in the bone marrow (23), similar to immature natural helper cells (35). GATA3 appears important for the maintenance of these Id2-positive ILC2 precursors with deletion of Gata3 from Id2-ERt2Cre–expressing cells resulting in the selective ablation of ILC2 cells in vivo and their impaired survival in culture (23). Notably, partial silencing of Gata3 in human ILC2 resulted in their reduced responsiveness to IL-33 and thymic stromal lymphopoietin (TSLP), whereas ectopic expression of GATA3 in CRTH2– ILCs induced CRTH2 and ST2, resulting in an increased potential to produce type-2 cytokines (39).
Gfi1 is another transcription factor that has been demonstrated previously to play roles in hematopoiesis and Th2 cell development (40). Spooner and colleagues have now reported that Gfi1 also alters ILC2 function (41). Using Gfi1-reporter and knockout mice, the authors found that Gfi1 was necessary for IL-33 signaling, but not IL-25. In the absence of Gfi1, ILC2 uncharacteristically expressed IL-17A as well as IL-13, suggesting that Gfi1 is necessary for repressing the IL-17A pathway (41).
Spencer and colleagues have recently added to the factors that may influence ILC commitment by recognizing that vitamin A deficiency can lead to a reduction in the frequency of ILC3 but a reciprocal increase in ILC2 (42). They went on to show that administration of retinoic acid (a metabolite of vitamin A) to Rag2−/−Il2rg−/− mice induced ILC3, whereas treatment with a retinoic acid inhibitor resulted in ILC2 accumulation. Because purified mature ILC2 and ILC3 were resistant to external retinoic acid regulation, these results suggest that it is ILC precursors that are retinoic acid sensitive (Figure 1). Indeed, ILC2 precursors gave rise to increased numbers if IL-13–producing ILC2 when retinoic acid signaling was inhibited. Importantly, the authors went on to demonstrate that vitamin A deficiency resulted in increased susceptibility to gastrointestinal bacterial infection with Citrobacter rodentium but enhanced resistance to parasitic helminth infection.
ILC2 in Skin Allergy
Atopic dermatitis (AD) is a common pruritic inflammatory skin disease with a complex etiology that depends on genetic and environmental susceptibility factors. AD is associated with barrier dysfunction, and adaptive immune responses to common environmental allergens, characterized by the presence of Th2 cells and high levels of IL-13 and IL-4, have been detected in AD lesions (43, 44). The recent discovery of ILC2 has led to the investigation of the potential involvement of these cells in this disease, and ILC2 cells have been reported to have an increased frequency in mouse and human atopic lesional skin (43, 45). Both studies report the increased frequency of ILC2 in AD skin but offer different conclusions as to the critical cytokines responsible for ILC2 function in AD. Kim and colleagues reported that TSLP acted independently of IL-33 in mouse and human skin (43), and Salimi and colleagues found that IL-25, IL-33, and TSLP could all activate mouse and human skin ILC2 (45).
Salimi and colleagues concentrated on human ILC2 and defined lineage-negative, IL-7Rα+CRTH2+c-kit+ICOS+CD161+CD25+CCR4+CCR10+NKp46−CD56−, RORα+GATA3+ ILC2 that were resident in the skin of healthy control subjects, and which increased in frequency and expressed elevated ST2, IL-17BR, TSLPR, and KLRG1 in skin biopsies from the lesions of patients with AD (45). Increased transcripts encoding IL-33 and IL-25 were also detected in the cells in acute lesional atopic dermatitis skin as compared with control subjects, and in vitro cultured human ILC2 released type-2 cytokines and amphiregulin in response to IL-33 stimulation. These data are consistent with the elevated IL33 mRNA reported previously in the lesional skin of patients with AD and, similarly, the detection of higher levels of IL-25 in patients with AD (46–48). Notably, IL-33, and to a lesser extent TSLP (but not IL-25), induced migration of skin-derived human ILC2 (45, 49).
Interestingly, Salimi and colleagues proposed a novel mechanism by which ILC2 may contribute to the sensing of barrier dysfunction (45). Skin resident human ILC2 express high levels of the inhibitory receptor KLRG1 (23), and this raised the possibility that this was required to suppress ILC2 activation. Thus, skin-derived ILC2 were cultured with IL-33 in the presence or absence of E-cadherin, an adhesion protein pivotal for maintaining the integrity of epithelia and a known ligand of KLRG1. Strikingly, the presence of E-cadherin down-regulated the expression of GATA3, IL-13, IL-5, and amphiregulin by ILC2. Because atopic dermatitis lesions are associated with E-cadherin down-regulation, this may lead to the release of a brake that would normally control the expression of type-2 cytokines, leading to their hyperproduction by ILC2. Overproduction of wound-healing regulators, such as amphiregulin, has been implicated previously in barrier repair and the pathogenesis of atopic dermatitis (50).
To investigate experimentally the role of ILC2 in vivo, both Kim and colleagues (43) and Salimi and colleagues (45) used an MC903-induced mouse model of atopic dermatitis–like inflammation. This was achieved using three different mouse models of ILC2 deficiency, antibody depletion of CD25 cells (43) or CD90+ cells (45) in Rag1−/− mice and RORα-deficient chimeric mice. All three approaches supported a role for ILC2, independently of T cells, in the initiation of skin inflammation. Using knockout mice deficient in IL-25, IL-33, or TSLP, the relative contribution of these cytokines to skin inflammation was also determined (45). IL-25 and IL-33 were the predominant ILC2-inducing cytokines in response to skin challenge, with TSLP having a less marked role. This contrasts with the report that TSLP is the primary cytokine inducing skin ILC2 and that this response was independent of IL-33 (43). One difference between these studies was the mouse strain background used, and this may underlie some of these differences. However, in combination with the human study data it appears likely that IL-25, IL-33, and TSLP are all involved in the regulation of ILC2 and allergic skin inflammation, and this overlap may have significant therapeutic implications.
In addition to being activated via IL-33, IL-25, and TSLP, the eicosanoid prostaglandin D2 (PGD2), which binds to CRTH2, has been shown recently to be a potent inducer of human ILC2 (49, 51, 52). PGD2 enhanced ILC2 expression of IL-4, IL-5, IL-9, IL-13, and, among others, granulocyte macrophage colony-stimulating factor and IL-3, and also acted as a potent chemoattractant for ILC2. Notably, degranulating human mast cells are a major source of PGD2 during allergy, and mast cell–derived PGD2 efficiently up-regulated type-2 cytokine expression and migration by human ILC2. Mast cell degranulation is caused predominantly by IgE/FcεRI crosslinking, and these data suggest that ILC2 may be attracted and activated during the adaptive type-2 response. It is noteworthy that intravital imaging of mouse ILC2 in the skin indicated that they interacted with mast cells and produced IL-13 (53). Another eicosanoid signaling pathway has also been reported for mouse lung ILC2. These cells express the cysteinyl leukotriene receptor 1 (CysLT1R) and respond to leukotriene D4 by expressing type-2 cytokines in vitro and in vivo (54). Notably, the leukotriene inhibitor montelukast can inhibit cytokine production by both mouse and human ILC2 (52, 54), suggesting that part of the therapeutic effect of this drug in asthma may be due to blocking ILC2 activation. Notably, human ILC2 also express the pro-resolvin receptor ALX/FRP2, which binds the resolving lipoxin A4 (51). Like leukotrienes and prostaglandins, lipoxin A4 is derived enzymatically from arachidonic acid. However, unlike these proinflammatory mediators, lipoxin A4 has antiinflammatory properties and has been used to inhibit airway hyperreactivity and inflammation (55). Indeed, lipoxin A4 inhibited IL-13 production by ILC2 from human peripheral blood that was cocultured with IL-2, IL-25, IL-33, and PGD2 (51). These changes were rather minor but indicate that this pathway may represent a therapeutic target in the suppression of ILC2.
ILC2 in Lung Allergy and Inflammation
In mice, lung-resident ILC2 responding to IL-25, IL-33, and TSLP have been shown to contribute to airway inflammation and hyperreactivity, induced by viral or allergen challenge (14–16, 21, 22, 56) (Figure 2). These studies focused on the initiation of type-2 responses often in short-term models where the interaction of ILC2 with the adaptive immune response was not measured. A recent report demonstrates that in addition to mediating acute type-2 inflammation, ILC2 can also influence adaptive immunity (57). ILC2-deficient mice (RORα-deficient) develop an impaired Th2 cell response to inhaled allergen (papain), and this can be reversed by the reconstitution of ILC2 by adoptive transfer. This defect was due to a deficiency in ILC2-derived IL-13 that was shown to be critical for efficient induction of Th2 cells. Although IL-13 did not act directly on naive CD4 T cells, it was required for the efficient migration of activated dendritic cells to the draining lymph node of the lung. The deficit in primed dendritic cells in the secondary lymphoid organs resulted in impaired Th2 cell priming. Thus, ILC2 play roles both during the early induction of effector functions and by enhancing adaptive Th2-driven immunity (Figure 2).

Type-2 innate lymphoid cells (ILC2) contribute to diverse type-2 immune responses. Stimuli such as allergens or parasitic worms lead to the release of ILC2-inducing factors such as IL-25, IL-33, and TSLP from the epithelium. These cytokines cause ILC2 to proliferate and produce type-2 cytokines and type-2 effector pathways.
The potential importance of ILC2 in tissue repair has also led to investigation of their roles in fibrosis. After infection with the H1N1 influenza virus, ILC2 were found to express amphiregulin that has been demonstrated to play roles as a regulator of wound healing and was required for restoring epithelial integrity and lung function (21). IL-33 is a potent activator of lung ILC2, and treatment of lung with IL-33 rapidly induces genes important for maintaining epithelial barrier function, including various keratin molecules (e.g., Krt14) and a number of late cornified envelope molecules (e.g., Lce3a), but also collagens (58). Indeed, a role for IL-33 and ILC2 in hepatic fibrosis has been reported recently (59). Notably, IL-33 has also been implicated as a factor in regulating airway remodelling in children with severe steroid-resistant asthma (60). Levels of IL-33 correlated with increased reticular basement membrane thickness in endobronchial biopsies, and mouse studies in which neonatal mice were dosed with house dust mite allergens indicated that, in contrast to IL-13 levels, IL-33 concentrations were steroid resistant. A similar link between IL-33 stimulation in the context of ovalbumin (OVA) challenge in the lung and steroid resistance was recently reported to be due to TSLP (61). When mice were dosed with only IL-33, the inflammatory response could be inhibited by treatment with dexamethasone. By contrast, low doses of IL-33 in the presence of OVA administration led to steroid-resistant inflammation in which the ILC2 population was preferentially more resistant to steroid treatment than T cells. Further investigation indicated that TSLP, induced in the IL-33/OVA model, caused the ILC2 to become resistant to steroid-mediated cell death via a STAT5 pathway. Strikingly, a STAT5 inhibitor, pimozide (an antipsychotic drug), reversed the effects of TSLP in vivo, resulting in ILC2 now becoming susceptible to dexamethasone treatment.
However, IL-33 is not the only epithelium-derived ILC2 growth and differentiation factor that can induce fibrosis. Using a house dust mite allergen stimulation model in adult mice, and with the coadministration of an adenoviral vector expressing Smad2 to enhance a remodelling phenotype, Gregory and colleagues reported that anti–IL-25 abrogated peribronchial collagen deposition and airway hyperreactivity (62). This correlated with a moderate reduction of IL-5 and IL-13 and a more profound decrease in IL-33 and TSLP. A further report has shown that IL-25 leads to the generation of IL-13–producing ILC2 that are sufficient to drive collagen deposition in the lungs of mice challenged with the type-2 immune response–inducing schistosome eggs (63). The authors also reported that IL-25 and ILC2 were increased in the BAL of patients with idiopathic pulmonary fibrosis.
Thus, IL-33, IL-25, and TSLP can all activate ILC2, and there is a paucity of information defining whether there is any specificity in the cytokine elicited in response to a particular allergen. For example, although IL-33 is induced most rapidly in response to intranasal challenge with Alternaria fungal allergens, in its absence IL-25 is up-regulated, although with slower kinetics, and induces ILC2 expansion (64). Similarly, IL-33 plays a more prominent role in the response to ragweed allergens, but IL-25 also plays a role in the lung response to this allergen (58). TSLP is also often detectable, and given its role in causing steroid resistance it would be interesting to determine if cotreatment of pimozide with steroids might also be more effective in inhibiting lung remodelling in steroid-resistant asthma.
This picture has recently become more complex, with data showing that the tumor necrosis factor family member TL1A (Tnfsf15) can also directly activate ILC2 to produce type-2 cytokines (65, 66). TL1A is expressed after activation of myeloid cells and endothelial cells at mucosal surfaces but can also be expressed by T cells that carry the cognate receptor DR3 (67). TL1A-DR3 signaling has been shown to play a role in a number of disease models, including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and allergic inflammation (68). Polymorphisms in the TNFSF15 gene, encoding TL1A, also associate with rheumatoid arthritis and inflammatory bowel disease. ILC2 express DR3, and treatment with TL1A resulted in ILC2 proliferation and their expression of IL-5 in vitro (65, 66) and in vivo (66). This response was enhanced in the presence of IL-7 but was an order of magnitude below that induced by IL-33. DR3−/− mice were used by both groups to analyze the effect of TL1A signaling in the response to intranasal papain. ILC2 numbers were reduced in the lungs and BAL of DR3−/− mice, as compared with control mice, and this correlated with decreased inflammation (65, 66). This effect was not due solely to the role of TL1A on T cells, because DR3−/−Rag2−/− mice also had fewer ILC2, but these data may also suggest that DR3 signaling in T cells is required to regulate ILC2 numbers (66). Notably, human ILC2 also express DR3 and respond to TL1A. However, like the mouse system, human TL1A is less potent than IL-33 and IL-25 but acts synergistically with these factors to drive an optimal ILC2 response. Thus TL1A regulates both adaptive immune cells and ILC2 and may represent a therapeutic target in asthma.
Asthma is now recognized as a heterogeneous syndrome, and only now are we starting to dissect it into its representative parts. Although ILC2 fit conveniently into an eosinophil-dominated allergic asthma phenotype, the elements regulating nonallergic asthma, often characterized by neutrophils, are less clear. Obesity is often associated with asthma, especially in nonatopic adults who show steroid resistance. It now appears that ILC3 may form part of the underlying etiology of this asthma phenotype (18). Mice fed a high-fat diet (HFD) developed increased airways hyperreactivity that was dependent on IL-17A produced by ILC3. These ILC3 were sensitive to the increased levels of IL-1β released by M1 macrophages in the lungs of obese mice, and blockade of the IL-1 signaling pathway using IL-1 receptor antagonist Anakinra was sufficient to prevent airway hyperresponsiveness in HFD-fed wild-type mice. ILC3-like cells were also identified in the BAL of patients with asthma, but larger patient cohorts will be necessary to determine whether there is a correlation between ILC3 numbers and specific asthma subtypes (18). It will also be important to determine how these responses may alter in the presence of allergen challenges.
The link between ILCs and obesity also extends to ILC2 that are found constitutively in the visceral adipose tissue and are associated with eosinophils and alternatively activated macrophages. In this context both IL-33 and IL-25 have been shown to increase ILC2 cell numbers and maintain a lean phenotype when mice are fed an HFD (17, 19). However, the mechanisms by which eosinophils and alternatively activated macrophages regulate glucose homeostasis and weight gain remain to be discovered.
Conclusions
Progress in our understanding of the biology of innate lymphoid cells continues apace. Committed progenitor and precursor populations have now been identified, and we have started to unearth the molecular cues that constitute the transcriptional programs that lead to specific ILC subsets. Notably, these innate cells are intimately involved not only in immune defense but also in metabolic regulation, and dietary changes can influence their differentiation and functional characteristics. However, how such factors alter disease states, such as asthma and allergy, is currently poorly understood. Significantly, recent reports have indicated that ILC2 not only play roles at the initiation of type-2 immunity but also can contribute to chronic pathology, such as fibrosis, and can impact on the priming of the adaptive T-cell response. Importantly, as these new avenues of research open up, novel therapeutic targets are being identified for further investigation.
Footnotes
Supported by MRC, Wellcome Trust, and American Asthma Foundation.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
Articles from Annals of the American Thoracic Society are provided here courtesy of American Thoracic Society
Full text links
Read article at publisher's site: https://doi.org/10.1513/annalsats.201403-097aw
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4298972?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1513/annalsats.201403-097aw
Article citations
New insights into allergic rhinitis treatment: MSC nanovesicles targeting dendritic cells.
J Nanobiotechnology, 22(1):575, 19 Sep 2024
Cited by: 0 articles | PMID: 39294599 | PMCID: PMC11411834
Failed Induction of the TH1 System in TH2 Dominant Patients: The Cancer-Permissive Immune Macroenvironment.
Integr Med (Encinitas), 23(2):24-35, 01 May 2024
Cited by: 0 articles | PMID: 38911450 | PMCID: PMC11193407
Progesterone amplifies allergic inflammation and airway pathology in association with higher lung ILC2 responses.
Am J Physiol Lung Cell Mol Physiol, 327(1):L65-L78, 23 Apr 2024
Cited by: 0 articles | PMID: 38651968
Omics approaches in asthma research: Challenges and opportunities.
Chin Med J Pulm Crit Care Med, 2(1):1-9, 02 Mar 2024
Cited by: 0 articles | PMID: 39170962 | PMCID: PMC11332849
Review Free full text in Europe PMC
Estrogen contributes to sex differences in M2a macrophages during multi-walled carbon nanotube-induced respiratory inflammation.
FASEB J, 38(1):e23350, 01 Jan 2024
Cited by: 2 articles | PMID: 38071600
Go to all (72) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regulation of group 2 innate lymphoid cells.
Cytokine, 87:1-8, 30 May 2016
Cited by: 28 articles | PMID: 27255596
Review
TH2, allergy and group 2 innate lymphoid cells.
Nat Immunol, 14(6):536-542, 01 Jun 2013
Cited by: 392 articles | PMID: 23685824
Review
[Function and modulation of type Ⅱ innate lymphoid cells and their role in chronic upper airway inflammatory diseases].
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 52(2):130-135, 01 Feb 2017
Cited by: 1 article | PMID: 28219179
Review
Group 2 innate lymphoid cells in lung inflammation.
Immunology, 140(3):281-287, 01 Nov 2013
Cited by: 27 articles | PMID: 23866009
Review
Funding
Funders who supported this work.
Medical Research Council (1)
Investigation of immune and haematopoietic disorders
Dr Andrew McKenzie, MRC Laboratory of Molecular Biology
Grant ID: MC_U105178805






