Abstract
Objective
We assessed whether type 1 diabetes (T1D) can be diagnosed earlier using a new approach based on prediction and natural history in autoantibody-positive individuals.Research design and methods
Diabetes Prevention Trial-Type 1 (DPT-1) and TrialNet Natural History Study (TNNHS) participants were studied. A metabolic index, the T1D Diagnostic Index60 (Index60), was developed from 2-h oral glucose tolerance tests (OGTTs) using the log fasting C-peptide, 60-min C-peptide, and 60-min glucose. OGTTs with Index60 ≥2.00 and 2-h glucose <200 mg/dL (Ind60+Only) were compared with Index60 <2.00 and 2-h glucose ≥200 mg/dL (2hglu+Only) OGTTs as criteria for T1D. Individuals were assessed for C-peptide loss from the first Ind60+Only OGTT to diagnosis.Results
Areas under receiver operating characteristic curves were significantly higher for Index60 than for the 2-h glucose (P < 0.001 for both DPT-1 and the TNNHS). As a diagnostic criterion, sensitivity was higher for Ind60+Only than for 2hglu+Only (0.44 vs. 0.15 in DPT-1; 0.26 vs. 0.17 in the TNNHS) OGTTs. Specificity was somewhat higher for 2hglu+Only OGTTs in DPT-1 (0.97 vs. 0.91) but equivalent in the TNNHS (0.98 for both). Positive and negative predictive values were higher for Ind60+Only OGTTs in both studies. Postchallenge C-peptide levels declined significantly at each OGTT time point from the first Ind60+Only OGTT to the time of standard diagnosis (range -22 to -34% in DPT-1 and -14 to -27% in the TNNHS). C-peptide and glucose patterns differed markedly between Ind60+Only and 2hglu+Only OGTTs.Conclusions
An approach based on prediction and natural history appears to have utility for diagnosing T1D.Free full text

A New Approach for Diagnosing Type 1 Diabetes in Autoantibody-Positive Individuals Based on Prediction and Natural History
Associated Data
Abstract
OBJECTIVE
We assessed whether type 1 diabetes (T1D) can be diagnosed earlier using a new approach based on prediction and natural history in autoantibody-positive individuals.
RESEARCH DESIGN AND METHODS
Diabetes Prevention Trial–Type 1 (DPT-1) and TrialNet Natural History Study (TNNHS) participants were studied. A metabolic index, the T1D Diagnostic Index60 (Index60), was developed from 2-h oral glucose tolerance tests (OGTTs) using the log fasting C-peptide, 60-min C-peptide, and 60-min glucose. OGTTs with Index60 ≥2.00 and 2-h glucose <200 mg/dL (Ind60+Only) were compared with Index60 <2.00 and 2-h glucose ≥200 mg/dL (2hglu+Only) OGTTs as criteria for T1D. Individuals were assessed for C-peptide loss from the first Ind60+Only OGTT to diagnosis.
RESULTS
Areas under receiver operating characteristic curves were significantly higher for Index60 than for the 2-h glucose (P < 0.001 for both DPT-1 and the TNNHS). As a diagnostic criterion, sensitivity was higher for Ind60+Only than for 2hglu+Only (0.44 vs. 0.15 in DPT-1; 0.26 vs. 0.17 in the TNNHS) OGTTs. Specificity was somewhat higher for 2hglu+Only OGTTs in DPT-1 (0.97 vs. 0.91) but equivalent in the TNNHS (0.98 for both). Positive and negative predictive values were higher for Ind60+Only OGTTs in both studies. Postchallenge C-peptide levels declined significantly at each OGTT time point from the first Ind60+Only OGTT to the time of standard diagnosis (range −22 to −34% in DPT-1 and −14 to −27% in the TNNHS). C-peptide and glucose patterns differed markedly between Ind60+Only and 2hglu+Only OGTTs.
CONCLUSIONS
An approach based on prediction and natural history appears to have utility for diagnosing T1D.
Introduction
Diagnostic criteria for both type 1 diabetes (T1D) and type 2 diabetes include fasting glucose values ≥126 mg/dL and 2-h glucose values ≥200 mg/dL (1). Those glucose values were selected primarily because they coincide with observed thresholds for the occurrence of diabetic retinopathy (1). Since they were essentially derived from findings in adults, they might not always be appropriate for pediatric populations. This is particularly relevant to T1D, which commonly occurs in children. Moreover, current criteria do not take into account the evidence that the pathogenesis of T1D begins years before it is diagnosed with standard glucose criteria (2–4). This suggests that other criteria can be used to diagnose T1D at earlier stages of disease.
We previously performed a study of Diabetes Prevention Trial–Type 1 (DPT-1) and TrialNet Natural History Study (TNNHS) participants, all autoantibody-positive relatives of T1D patients, showing that once a Diabetes Prevention Trial Risk Score (DPTRS) (5,6) of 9.00 is exceeded, the 2-year risk of T1D is very high (7). (The DPTRS is a validated predictor of T1D that is based on the log fasting C-peptide, glucose, and C-peptide sums from 30-, 60-, 90-, and 120-min values of 2-h oral glucose tolerance tests [OGTTs], log BMI, and age.) Moreover, when that threshold was exceeded, there was a substantial decline in insulin secretion. As a result of those findings, we have explored another approach for diagnosing T1D in autoantibody-positive relatives. Specifically, we have assessed whether T1D could be diagnosed in these individuals when a very high risk threshold of a metabolic index is exceeded along with a concomitant marked decline in insulin secretion. Since the analyses pertain to autoantibody-positive individuals, we are not advocating the replacement of standard diagnostic criteria with a metabolic index. Rather we use the index to show how another approach based on prediction and natural history might be helpful in the future for diagnosing T1D at an earlier stage of pathogenesis.
Research Design and Methods
Subjects
Data from pancreatic autoantibody-positive relatives of patients with T1D who participated in DPT-1 or the TNNHS were analyzed. Those from DPT-1 were positive for islet cell autoantibodies (ICA), whereas those from the TNNHS were positive for at least one of the following autoantibodies: GADA, IA-2A, mIAA, and ICA (very few had ICA alone). DPT-1 consisted of a low-dose parenteral insulin trial and an oral insulin trial. These trials have been described (8,9); neither intervention showed an overall effect. The TNNHS is an observational rather than an intervention study; that study has also been described (10). Both studies were approved by institutional review boards at all participating sites, and written informed consent and assent as appropriate were obtained in both studies.
Procedures
Participants were followed in both DPT-1 and the TNNHS for the development of T1D with OGTT surveillance at 6 (±3)-month intervals. Fasting samples for measurements of glucose and C-peptide were obtained, followed by the ingestion of a 1.75 g/kg oral glucose dose (maximum 75 g of carbohydrate). Samples were then obtained at 30-min intervals for measurements of glucose and C-peptide. When a fasting glucose level exceeded ≥126 mg/dL and/or a 2-h glucose level exceeded ≥200 mg/dL, an OGTT was repeated for confirmation. If either the fasting or the 2-h glucose threshold was exceeded again at confirmatory testing, T1D was diagnosed. Participants who did not exceed either threshold on the confirmatory OGTT continued to be followed at 6-month intervals. According to the protocols, the time of diagnosis was assigned to the date of the first OGTT in DPT-1, whereas the time of diagnosis was assigned to the date of the confirmatory OGTT in the TNNHS. For consistency in these analyses, we designated the date of diagnosis as the date of the first OGTT in both studies. Diagnoses were also made from clinical disease presentations. The glucose oxidase method was used to measure the plasma glucose. C-peptide was measured by radioimmunoassay (RAI) in DPT-1 and by the Tosoh assay in the TNNHS. In a prior analysis, 564 individuals had C-peptide measurements by both the Tosoh assay and the RAI used in DPT-1 (r = 0.961; Tosoh = 0.96 × RAI + 0.1). Undetectable fasting C-peptide values were assigned a value of one-half the limit of detection.
Data Analysis
We developed a metabolic index, the T1D Diagnostic Index60 (Index60), for the purpose of this study. Index60 includes log fasting C-peptide, 60-min glucose, and 60-min C-peptide values from OGTTs. It differs from the DPTRS, which includes log fasting C-peptide, glucose, and C-peptide sums from 30-, 60-, 90-, and 120-min values; log BMI; and age. An Index60 threshold was chosen for a diagnostic criterion rather than the DPTRS, since, in contrast to the sum of the 30- to 120-min values used for the DPTRS, the 60-min values are independent of the 120-min glucose, which is a diagnostic criterion. In addition, Index60 does not rely upon age and BMI, nonmetabolic measures, for diagnosis. The intent of the analysis was to determine whether an Index60 threshold of ≥2.00 could be used as an additional diagnostic criterion for T1D.
Index60 was developed from the DPT-1 database using a proportional hazards regression model. Glucose and C-peptide values at 60 min were found to be strong univariate predictors of T1D (P < 0.001); they were more strongly predictive than the respective 2-h glucose and C-peptide values (Supplementary Table 1). The log fasting C-peptide was also predictive (P = 0.03), and its inclusion in the model appreciably enhanced overall prediction. Fasting glucose values did not contribute significantly to the model. The log fasting C-peptide, 60-min glucose, and 60-min C-peptide were all highly predictive of T1D within the Index60 model. The equation for the Index60 model is:

More information pertaining to the Index60 model is included in Supplementary Table 2. An Index60 threshold of ≥2.00 corresponded (by linear regression) to a high DPTRS value: 8.02. The development of the DPTRS and its conversion to a risk estimate has previously been described (5).
Those with an Index60 value ≥2.00 at baseline were excluded, as were those with fasting glucose levels ≥126 mg/dL or 2-h glucose levels ≥200 mg/dL at baseline. We did not perform analyses pertaining to the ≥126 mg/dL fasting glucose threshold since it is uncommon without the presence of 2-h glucose values ≥200 mg/dL in autoantibody-positive individuals.
Hereafter, OGTTs will be characterized in the following manner:
Index60 value ≥2.00 = Ind60+
Index60 value <2.00 = Ind60−
2-h glucose value ≥200 mg/dL = 2hglu+
2-h glucose value <200 mg/dL = 2hglu−
Comparisons of diagnostic criteria were based on whether T1D was diagnosed during follow-up and whether the diagnostic criteria were exceeded (after baseline) prior to or at diagnosis. 2hglu+ OGTTs triggered the performance of a confirmatory OGTT, whereas Ind60+ OGTTs were not considered in the protocols. This likely resulted in a bias in favor of 2hglu+ OGTTs over Ind60+ OGTTs, which should be considered in the interpretation of the findings.
Since there was no procedure for confirming Ind60+ OGTTs in the protocols, we designated the confirmatory OGTT for that threshold as the next OGTT performed within a 9-month interval. This interval was chosen because it corresponded to the upper limit of the standard 6 ± 3-month window for the next visit in DPT-1 and the TNNHS. If the next OGTT occurred after the 9-month interval, it was not considered for confirmation of the prior Ind60+ OGTT.
Receiver operating characteristic (ROC) curves were compared to assess prediction accuracy. The sensitivity indicates the proportion of individuals positive for a test criterion among those who were ultimately diagnosed with T1D. The specificity indicates the proportion of individuals negative for a test criterion among those who were not diagnosed with T1D. The positive predictive value indicates the proportion of individuals who were ultimately diagnosed with T1D among those positive for a test criterion. The negative predictive value indicates the proportion of individuals who were not diagnosed with T1D among those negative for a test criterion. t tests were used for comparisons within individuals and between groups. χ2 tests were also used for group comparisons. Kaplan-Meier estimation was used to assess the occurrence of T1D. The SAS 9.1.3 and 9.2 versions were used. The P values are two-sided.
Results
There were 633 DPT-1 participants (mean ± SD age, 14.1 ± 9.8 years; 56% male) and 1,717 TNNHS participants (mean ± SD age, 18.0 ± 13.1 years; 46% male) with Ind60− and nondiabetic-range OGTTs at baseline who had follow-up. Of the 633 in DPT-1, 203 (32%) were diagnosed with T1D, whereas of the 1,717 in the TNNHS, 221 (13%) were diagnosed with T1D. In DPT-1, 127/633 (20%) had at least one Ind60+Only OGTT, whereas in the TNNHS 83/1,717 (5%) had at least one Ind60+Only OGTT.
Comparison Between Index60 and 2-h Glucose for Predicting T1D
Individuals with glucose levels in the nondiabetic range and with Index60 values <2.00 at baseline were assessed for the accuracy of prediction of T1D by Index60 and the 2-h glucose. The area under the ROC curve (Supplementary Fig. 1), an indicator of accuracy, was significantly higher for Index60 than for the 2-h glucose in both DPT-1 (0.75 vs. 0.66; P < 0.001; n = 633) and the TNNHS (0.78 vs. 0.66; P < 0.001; n = 1,717).
Comparison Between Ind60+ and 2hglu+ as Diagnostic Criteria for T1D
To assess the accuracy of Ind60+ as a diagnostic criterion for T1D, we compared the first OGTTs that were Ind60+Only (i.e., Ind60+ and 2hglu−) with the first OGTTs that were 2hglu+Only (i.e., Ind60− and 2hglu+). Sensitivities, specificities, and predictive values are shown for Ind60+Only and 2hglu+Only OGTTs in Table 1. The sensitivities were higher for Ind60+Only OGTTs than for 2hglu+Only OGTTs in both DPT-1 and the TNNHS. Although the specificity was somewhat higher for 2hglu+Only OGTTs in DPT-1, there was no difference in the TNNHS. All specificities were >0.90. The positive and negative predictive values all tended to be higher for Ind60+Only OGTTs than for 2hglu+Only OGTTs in both studies.
Table 1
Comparison of performance of Ind60+ with 2hglu+ as diagnostic criteria for T1D
| DPT-1 | TNNHS | |||
|---|---|---|---|---|
| Ind60+Only | 2hglu+Only | Ind60+Only | 2hglu+Only | |
| Sensitivity | 0.44 (90/203) | 0.15 (31/203) | 0.23 (51/221) | 0.17 (38/221) |
| Specificity | 0.91 (393/430) | 0.97 (415/430) | 0.98 (1,464/1,496) | 0.98 (1,467/1,496) |
| Positive predictive value | 0.71 (90/127) | 0.67 (31/46) | 0.61 (51/83) | 0.57 (38/67) |
| Negative predictive value | 0.78 (393/506) | 0.71 (415/587) | 0.90 (1,464/1,634) | 0.89 (1,467/1,650) |
Ind60+Only OGTTs at the Last Visit Among Individuals Not Diagnosed
There were 21 DPT-1 participants not diagnosed whose last OGTT was Ind60+Only. The mean ± SD DPTRS value at that visit was 8.70 ± 0.67 (n = 20 due to one missing value), which corresponds to a calculated 3-year risk of 0.97 in DPT-1. Of those in the TNNHS not diagnosed, there were 14 with Ind60+Only OGTTs at the last visit. The mean ± SD DPTRS value for those individuals was also very high: 8.77 ± 0.89.
The Decline in C-Peptide After the Occurrence of Ind60+Only OGTTs
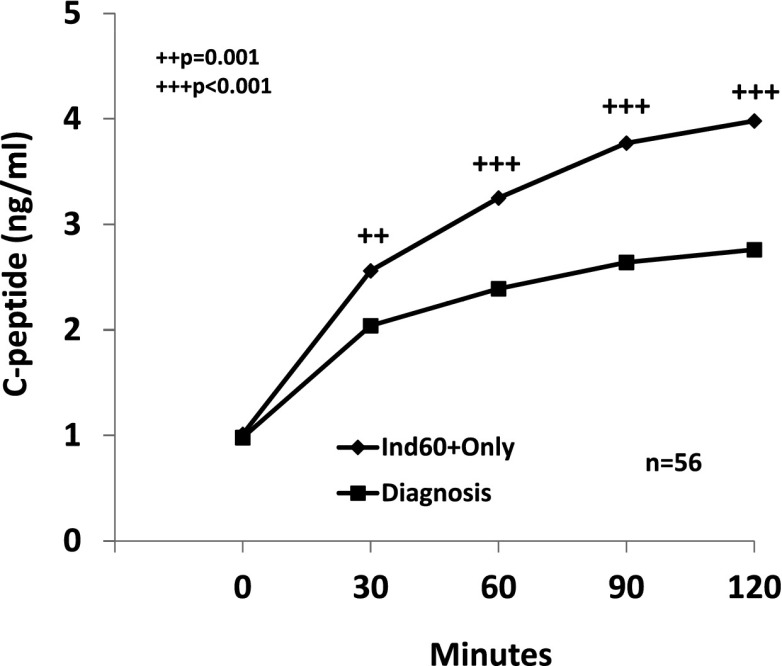
The change in C-peptide levels from the first Ind60+Only OGTT to diagnosis was examined among 56 individuals who had OGTTs at diagnosis in DPT-1 (Fig. 1). There was a marked decline in the postchallenge C-peptide measures (P = 0.001 or P < 0.001) over a period of 0.99 ± 0.66 years. The median percentage of change for the C-peptide values at the postchallenge OGTT time points ranged from −22 to −34%. The number analyzed was much smaller in the TNNHS (n = 17), but all of the differences were significant (P = 0.02 for 30 min and P ≤ 0.01 for the other postchallenge time points). The median percentage of decline ranged from −14 to −27% over an interval of 1.38 ± 1.25 years.
Comparisons of OGTT Patterns Between Ind60+Only and 2hglu+Only OGTTs
We compared metabolic patterns of Ind60+Only OGTTs (n = 115) with 2hglu+Only (n = 34) OGTTs. (Individuals with the alternate pattern on a subsequent OGTT were excluded from the analysis.) The C-peptide values (Fig. 2A) were lower (P < 0.01) at each time point during the Ind60+Only OGTTs than the values during the 2hglu+Only OGTTs. While postchallenge glucose values (Fig. 2B) were higher at 30 and 60 min during the Ind60+Only OGTTs, glucose values were higher at 90 and 120 min during the 2hglu+Only OGTTs.
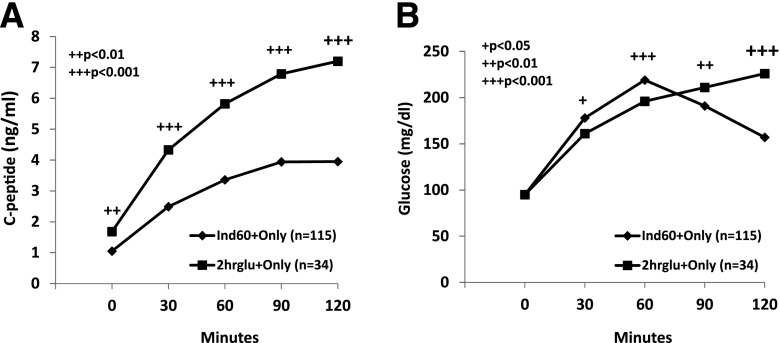
Panel A shows mean C-peptide levels for time points of Ind60+Only OGTTs and 2hglu+Only OGTTs in DPT-1. The C-peptide levels of the Ind60+Only OGTTs were much lower. Panel B shows mean glucose values for time points of Ind60+Only OGTTs and 2hglu+Only OGTTs in DPT-1. The glucose values tended to be higher at the earlier time points in the Ind60+Only OGTTs and higher at the later OGTT time points in the 2hglu+Only OGTTs.
A plot of the C-peptide values against the glucose values (Supplementary Fig. 2) underscores the marked difference in the patterns between Ind60+Only OGTTs and 2hglu+Only OGTTs. Those with Ind60+Only OGTTs were younger than those with 2hglu+Only OGTTs, both in DPT-1 (12.9 ± 7.2 vs. 18.1 ± 10.2 years; P < 0.001) and the TNNHS (14.7 ± 11.2 vs. 23.9 ± 14.6 years; P < 0.001).
Two Scenarios in Which Ind60+Only OGTTs Could Be Used as a Diagnostic Criterion
Two scenarios were examined in which Ind60+ OGTTs could be useful for diagnosing T1D. We first assessed its utility in individuals with an Ind60+Only OGTT who were again Ind60+ (with either 2hglu+ or 2hglu−) at the next OGTT (<9 month interval). There were 54 such individuals in DPT-1, of whom, 50 (93%) were diagnosed with T1D. The maximum follow-up from the second OGTT of those not diagnosed was 1.0 year. In the TNNHS, 18/21 (86%) of those individuals were diagnosed. The maximum follow-up from the second OGTT of those not diagnosed was 0.6 years.
The second scenario involved individuals with OGTTs that were both Ind60+ and 2hglu+ who then had an OGTT for standard confirmation (<3-month interval) that was Ind60+Only. Thus they were negative for the 2-h glucose on the standard confirmatory OGTT, but Ind60+ on both OGTTs. In DPT-1, among those individuals, 28/30 (93%) were subsequently diagnosed. The two individuals not diagnosed had no follow-up. Among the individuals in the TNNHS who had an OGTT that was Ind60+ and 2hglu+ and then had an Ind60+Only confirmatory OGTT, 7/9 (78%) were subsequently diagnosed. As in DPT-1, the two not diagnosed had no follow-up. Thus all of those followed in both studies were ultimately diagnosed when the confirmatory OGTT was Ind60+Only. Table 2 summarizes the findings for the two scenarios from DPT-1.
Table 2
Summary of findings from scenarios for the use of Ind60+ OGTTs as an additional diagnostic criterion to 2hglu+ OGTTs in DPT-1
| First OGTT | Second OGTT | Diagnosed/total | ||
|---|---|---|---|---|
| Ind60 | 2hglu | Ind60 | 2hglu | |
| + | − | + | + or − | 50/54 (93%)* |
| + | + | + | − | 28/30 (93%)** |
Since Ind60+ OGTTs were not used for diagnosis in the DPT-1 and TNNHS protocols, those with an Ind60+ OGTT and a subsequent confirmatory Ind60+ OGTT continued to be followed. This means that some individuals were included in both scenarios. Still, a total of 67 would have been diagnosed earlier in DPT-1 if Ind60+ OGTTs had been used in addition to standard diagnostic criteria.
Conclusions
In contrast to the clinical diagnosis of T1D, the diagnosis in prevention trials is often made through OGTT surveillance in asymptomatic individuals. We have thus explored whether an additional criterion, Index60 values >2.00, might result in an earlier diagnosis in the autoantibody-positive individuals who typically participate in prevention trials. Although limited to those individuals, the findings suggest the possibility of developing diagnostic criteria based on prediction and natural history that could result in an earlier diagnosis in the general population. OGTT surveillance could become more common as the identification of at-risk populations improves, especially if acceptable therapies to slow the T1D disease process are identified and become available.
The findings suggest that the 2.00 Index60 value represents a point of transition from prediction to a virtual indication of T1D in autoantibody-positive relatives of T1D patients. The ROC curves showed that at baseline, Index60 was a much more accurate predictor of T1D than the 2-h glucose. Predictive values tended to be higher for Ind60+Only OGTTs than for 2hglu+Only OGTTs. Moreover, when the first Ind60+Only OGTTs during follow-up were exceeded, there was a concomitant substantial rate of decline in C-peptide levels. That decline is consistent with the C-peptide losses that occur in the perionset period of T1D (11–13). The use of the Ind60+ criterion represents a novel approach for diagnosing T1D because it is based on both prediction and natural history.
Since 2hglu+ was used as a diagnostic criterion in the DPT-1 and TNNHS protocols, there was potentially a bias in favor of 2hglu+ over Ind60+. Whereas 2hglu+Only OGTTs triggered the performance of confirmatory OGTTs for diagnosis, Ind60+Only OGTTs did not. It is thus possible that Ind60+ could have performed even better as a diagnostic criterion relative to 2hglu+. The higher predictive values for Ind60+Only OGTTs than for 2hglu+Only OGTTs indicate that the choice of an Index60 value of 2.00 as a threshold is reasonably conservative.
The scenarios showed potential utilities for Ind60+ OGTTs as an additional criterion for the diagnosis of T1D (Table 2). Very small percentages of those with confirmed Ind60+ OGTTs were not subsequently diagnosed (i.e., false positives). It is possible that the few false positives were individuals who ultimately would have developed T1D with longer follow-up. This is suggested by the very high DPTRS values of those not diagnosed who had Ind60+Only OGTTs at the last visit. Since Ind60+ would be used as an additional criterion to the standard criteria, there would be no false negatives.
The use of Ind60+ as an additional diagnostic criterion would help to shorten the duration of follow-up for a number of participants in prevention trials, with reductions in both inconvenience and cost. If the two scenarios presented above had been implemented, the addition of Ind60+ OGTTs as a criterion would have resulted in an earlier diagnosis of T1D in over one-fourth of those diagnosed in DPT-1.
The Ind60+ diagnostic criterion could be especially useful in children since individuals with Ind60+Only OGTTs were much younger than those with 2hglu+Only OGTTs in DPT-1 and the TNNHS. Moreover, the frequency of Ind60+Only OGTTs was higher in the younger DPT-1 cohort. It seems particularly worthwhile to assess the use of Ind60+ as an additional diagnostic criterion in both prevention trials and epidemiologic studies of children who are at risk for T1D. Confirmed Ind60+ OGTTs might also help to identify more individuals with “silent diabetes” (i.e., asymptomatic individuals diagnosed by metabolic testing); they have more insulin secretion capacity than those diagnosed after symptoms develop.
The marked differences in the patterns between Ind60+Only OGTTs and 2hglu+Only OGTTs, evident in Fig. 2, could be indicative of metabolic heterogeneity in the pathogenesis of T1D. The higher C-peptide levels in the 2hglu+Only OGTTs do not necessarily indicate better β-cell function, since they could be related to increased insulin resistance due to differences in age and pubertal status (14,15).
As indicated above, the findings only apply to autoantibody-positive relatives of T1D patients. However, since the autoantibody entry criteria differed between DPT-1 and the TNNHS, the similar findings suggest that Ind60+ OGTTs can be used across autoantibody-positive populations. Another limitation for using Index60 clinically is the need for standardization of C-peptide assays.
Sixty-minute glucose values have previously been used for diagnostic purposes. The National Diabetes Data Group criteria from 1979 included a glucose value ≥200 mg/dL between the fasting and 2-h values for diagnosis (16). A 60-min glucose value of ≥180 mg/dL is currently one of the criteria for gestational diabetes (1). C-peptide measures have not previously been used as diagnostic criteria for diabetes.
The findings strongly suggest that prediction and natural history can provide a basis for diagnosing T1D. They showed that a confirmed Index60 value of ≥2.00 has potential utility as an additional diagnostic criterion for T1D. It could also serve as a criterion for silent diabetes, and as an indicator of an impending rapid loss of insulin secretion. Whether Index60 or another measure is used, it appears that an approach based on prediction and natural history can aid in diagnosing individuals at an earlier stage of disease. This approach will become increasingly important as preventive treatments for T1D are assessed and ultimately implemented.
Article Information
Funding. This work was funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Research Resources, JDRF, and the American Diabetes Association.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.S. analyzed data and wrote the manuscript. J.S.S., L.A.D., J.P.K., C.J.G., L.E.R., D.M., K.C.H., and J.M. conducted the study and reviewed the manuscript. C.A.B. provided statistical support. D.B. performed programming and provided statistical support. J.P.P. conducted the study, reviewed the manuscript, and assisted in writing the manuscript. J.M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/10.2337/dc14-1813/-/DC1.
*A complete list of the Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups can be found in the Supplementary Data online.
References
Articles from Diabetes Care are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/dc14-1813
Read article for free, from open access legal sources, via Unpaywall:
https://care.diabetesjournals.org/content/diacare/38/2/271.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2337/dc14-1813
Article citations
Consensus guidance for monitoring individuals with islet autoantibody-positive pre-stage 3 type 1 diabetes.
Diabetologia, 67(9):1731-1759, 01 Sep 2024
Cited by: 1 article | PMID: 38910151 | PMCID: PMC11410955
Identification of type 1 diabetes risk phenotypes using an outcome-guided clustering analysis.
Diabetologia, 67(11):2507-2517, 06 Aug 2024
Cited by: 0 articles | PMID: 39103721
Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes.
Diabetes Care, 47(8):1276-1298, 01 Aug 2024
Cited by: 1 article | PMID: 38912694
Review
Comparisons of Metabolic Measures to Predict T1D vs Detect a Preventive Treatment Effect in High-Risk Individuals.
J Clin Endocrinol Metab, 109(8):2116-2123, 01 Jul 2024
Cited by: 1 article | PMID: 38267821 | PMCID: PMC11244203
Early Metabolic Endpoints Identify Persistent Treatment Efficacy in Recent-Onset Type 1 Diabetes Immunotherapy Trials.
Diabetes Care, 47(6):1048-1055, 01 Jun 2024
Cited by: 0 articles | PMID: 38621411
Go to all (49) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Index60 Identifies Individuals at Appreciable Risk for Stage 3 Among an Autoantibody-Positive Population With Normal 2-Hour Glucose Levels: Implications for Current Staging Criteria of Type 1 Diabetes.
Diabetes Care, 45(2):311-318, 01 Feb 2022
Cited by: 15 articles | PMID: 34853027 | PMCID: PMC8914436
Oral Glucose Tolerance Test Measures of First-phase Insulin Response and Their Predictive Ability for Type 1 Diabetes.
J Clin Endocrinol Metab, 107(8):e3273-e3280, 01 Jul 2022
Cited by: 4 articles | PMID: 35524749 | PMCID: PMC9282258
A comparison of the baseline metabolic profiles between Diabetes Prevention Trial-Type 1 and TrialNet Natural History Study participants.
Pediatr Diabetes, 12(2):85-90, 01 Mar 2011
Cited by: 11 articles | PMID: 20522170 | PMCID: PMC2955175
The development, validation, and utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS).
Curr Diab Rep, 15(8):49, 01 Aug 2015
Cited by: 14 articles | PMID: 26077017 | PMCID: PMC4795007
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIDDK NIH HHS (11)
Grant ID: U01 DK061042
Grant ID: U01 DK085461
Grant ID: P30 DK063720
Grant ID: U01 DK085499
Grant ID: U01 DK061010
Grant ID: U01 DK061058
Grant ID: UC4 DK106993
Grant ID: U01 DK085476
Grant ID: UC4 DK117009
Grant ID: P30 DK017047
Grant ID: UC4 DK097835

 1
1