Abstract
Free full text

Transient gamma-secretase inhibition accelerates and enhances fracture repair likely via Notch signaling modulation
Associated Data
Abstract
Approximately 10% of skeletal fractures result in healing complications and non-union, while most fractures repair with appropriate stabilization and without pharmacologic intervention. It is the latter injuries that cannot be underestimated as the expenses associated with their treatment and subsequent lost productivity are predicted to increase to over $74 billion by 2015. During fracture repair, local mesenchymal stem/progenitor cells (MSCs) differentiate to form new cartilage and bone, reminiscent of events during skeletal development. We previously demonstrated that permanent loss of gamma-secretase activity and Notch signaling accelerates bone and cartilage formation from MSC progenitors during skeletal development, leading to pathologic acquisition of bone and depletion of bone marrow derived MSCs. Here, we investigated whether transient and systemic gamma-secretase and Notch inhibition is capable of accelerating and enhancing fracture repair by promoting controlled MSC differentiation near the fracture site. Our radiographic, microCT, histological, cell and molecular analyses reveal that single and intermittent gamma-secretase inhibitor (GSI) treatments significantly enhance cartilage and bone callus formation via the promotion of MSC differentiation, resulting in only a moderate reduction of local MSCs. Biomechanical testing further demonstrates that GSI treated fractures exhibit superior strength earlier in the healing process, with single dose GSI treated fractures exhibiting bone strength approaching that of un-fractured tibiae. These data further establish that transient inhibition of gamma-secretase activity and Notch signaling temporarily increases osteoclastogenesis and accelerates bone remodeling, which coupled with the effects on MSCs likely explains the accelerated and enhanced fracture repair. Therefore, we propose that the Notch pathway serves as an important therapeutic target during skeletal fracture repair.
Introduction
Fracture healing is a multistage repair process that involves the complex integration of numerous cell types, growth factors, and the extracellular matrix, eventually resulting in the repair and restoration of function without forming a fibrous scar [1]. Long bones such as the tibia heal primarily via endochondral ossification, whereby bone formation occurs through a cartilage intermediate. Alternatively, in a biomechanically rigid and stable environment, bone forms directly from differentiated osteoblasts in a process termed intramembranous ossification [2]. Several signaling pathways including BMP, TGFβ, and WNT/β-catenin [3-9] have been well studied in their regulation of fracture repair, thus providing clues into pharmacologic management. However, the role of the Notch pathway, which has recently been identified as crucial regulator of cartilage and bone development [10-16], has gone largely unexplored during the process of fracture repair.
The Notch signaling pathway is known to regulate cell proliferation, differentiation, cell fate determination, and stem/progenitor cell self-renewal in both embryonic and adult organs [17-19]. In mammals, Notch signaling is primarily initiated when one of the Notch ligands (JAG1-2; DLL1,3,4; DLK1-2; MAGP1-2; DNER; and NB2) activates a cell surface Notch receptor (NOTCH 1–4), leading to a series of receptor cleavage events, mediated by ADAM proteases and proteins of the gamma-secretase complex. Cleavage of the receptors results in the release of the Notch intracellular domain (NICD) where it translocates to the nucleus and binds the transcriptional regulators RBPjκ and MAML, creating a transcriptionally active complex. NICD–RBPjκ–MAML ternary complexes drive expression of downstream Notch target genes, such as the Hes/Hey family of basic helix–loop–helix (bHLH) transcriptional factors [20].
It has been long suspected that fracture repair, which primarily occurs through endochondral ossification, recapitulates a series of spatiotemporal cellular and signaling events that occur during skeletal development [21,22]. Recently, genetic studies using Notch gain-(GOF) and loss-of-function (LOF) approaches have identified the Notch signaling pathway as a critical genetic regulator of skeletal progenitor cell differentiation, chondrocyte proliferation and maturation, and osteoblast proliferation and maturation during cartilage and bone development [10-16]. Specifically, previous studies have demonstrated that Notch activation in early progenitors, either mesenchymal stem/progenitor cells (MSCs) or bipotentialed chondro-osteoprogenitors (COPs) leads to an inhibition of differentiation and maintenance of the progenitor pool [10,11], whereas loss of Notch signaling in MSCs enhances chondrogenesis and osteogenesis [10,12]. Additionally, using both in vivo and in vitro GOF and LOF studies, our prior work revealed that cartilage-specific Notch signaling promotes both the onset and progression of chondrocyte maturation, while suppressing chondrocyte proliferation throughout cartilage development [13]. The Notch pathway also coordinates osteoblastogenesis, via both regulation of osteoblast differentiation from MSCs and regulating proliferation and differentiation of committed osteoblasts. Work by Hilton et al. demonstrated that loss of Notch signaling in MSCs significantly enhanced trabecular bone mass in adolescent mice at the expense of depleting the bone marrow MSC (BMSC) pool [12]. Ina complementary study, Notch gain-of-function in early osteoblast progenitors (over-expression of NICD driven by a 3.6-kb Col1a1 promoter) results in low bone mass or severe osteopenia due to a decreased number of mature osteoblasts [14]. Together these results support a model wherein Notch signaling normally acts to maintain the progenitor pool by suppressing osteoblast differentiation. Other genetic studies in which the Notch pathway was specifically activated in committed osteoblasts (over-expression of NICD driven by a 2.3-kb Col1a1 promoter) [15,16] demonstrated an induction of osteoblast proliferation and inhibition of terminal differentiation, leading to an osteosclerotic phenotype in mutant mice. Collectively, these data suggest that the Notch pathway plays a context dependent function in different cell types and at different time points within a cell linage, thus the timing at which Notch signals are removed or activated in the skeletal linage differentially affects endochondral bone development.
While several Notch signaling components are up-regulated during both endochondral and intramembranous bone healing [23], whether fracture repair can be enhanced or accelerated through manipulation of the Notch pathway remains to be determined. In this study, we reasoned that transient inhibition of Notch signaling may promote BMSC/MSC differentiation and enhance bone formation during fracture repair. Therefore, we systemically administered a few regimens of the gamma-secretase/Notch inhibitor, DAPT, to treat murine tibia fractures. DAPT treated mice demonstrated enhanced bony callus size, accelerated bone remodeling, an earlier and increased expression of chondrocyte and osteoblast-specific genes, and better biomechanical properties, with the single dose DAPT treated fracture group exhibiting the most robust fracture repair. Bone marrow stromal cell cultures from DAPT treated fractures underwent a more rapid differentiation without depleting resident BMSCs. Additional in vivo data also indicated that the effects of transient DAPT administration on fracture repair are primarily due to the early enhancement of BMSC/MSC differentiation, but likely involves other stimulatory effects on osteoclastogenesis during fracture repair. Altogether, our findings establish for the first time that the gamma-secretase and Notch pathways likely serve as important targets in the development of novel skeletal repair and regeneration therapies.
Materials and methods
Experimental animals
All animal studies were done in accordance with approval of the University of Rochester Committee on Animal Resources. Wild-type C57BL/6J male mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA).
Tibia fracture model
Open mid-shaft tibial fractures were created unilaterally in 8–10-week-old wild-type C57BL/6J mice. Briefly, a 6 mm long incision was made in the skin on the anterior side of the tibia. A sterile 26 G needle was inserted into the tibial marrow cavity from the proximal end, temporarily withdrawn to facilitate transection of the tibia with a scalpel at mid-shaft, and then reinserted. The incision was closed with 5-0 nylon sutures. The gamma-secretase inhibitor (GSI) DAPT (Enzo Life Sciences, Farmingdale, NY, USA) (50 mg/kg/day) or DMSO (vehicle) was delivered two days following fracture via i.p. injections for one day or four consecutive days. Healing of the fractured tibiae was confirmed immediately after surgery and monitored during fracture repair using digital radiographs, which were obtained at 0, 10, 14, 21 and 28 days post fracture (dpf) under anesthesia using a Faxitron Cabinet X-Ray System (N ≥ 9 per group per time point) (Faxitron X-Ray Corporation, Lincolnshire, IL, USA).
Histological analysis of fracture calluses
The fractured tibiae were collected at 7, 10, 14, 21 and 28 dpf for detailed analyses. Excess muscle and soft tissue were excised. Four to five specimens in each group at each time point were fixed in 10% neutral buffered formalin. These specimens were decalcified for 7–10 days in formic acid or 14% EDTA (pH 7.2), processed and embedded in paraffin, and sectioned at a thickness of 3 μm. Sections were stained using alcian blue/hematoxylin/orange-g (ABH/OG) and TRAP in order to analyze the cartilage composition and osteoclast formation in the fracture callus tissues. In situ hybridization (ISH) was performed as previously described [24] using 35S-labeled riboprobes for TRAP. Cartilage area, bone area, mesenchyme area, and osteoclast surface per bone surface (OC. S./BS) were quantified on ABH/OG, TRAP, and ISH for TRAP stained sections using the Osteomeasure Analysis System (Osteometrics, Decatur, GA, USA). Immunohistochemistry (IHC) for OPG (Abcam, ab73400) and RANKL (LS-Bio, LS-B1425) was performed on sections following the traditional antigen retrieval and colorimetric development methodologies.
MicroCT assessment of the mineralized callus and biomechanical torsion testing
After careful dissection and removal of the intramedullary pin, repaired tibiae from days 10, 14, 21, and 28 (five to seven mice per group per time point) were imaged using a micro-computed tomography (microCT) system (VivaCT 40, Scanco Medical, Wayne, PA, USA), with an integration time of 300 ms, a current of 145 mA, and an energy setting of 55 kV. The threshold was chosen using 2D evaluation of several slices in the transverse anatomic plane so that mineralized callus was identified but surrounding soft tissue was excluded. Quantification for the volumes of the bony calluses was determined as previously described using the Scanco analysis software [25]. 3D images were generated using a constant threshold of 275 for metaphyseal trabecular bone of the contralateral un-fractured tibiae. After the microCT imaging of the fracture calluses, the specimens were moistened with PBS and frozen at − 20 °C until being thawed for biomechanical testing as previously described [26].
Briefly, specimens were potted in poly(methyl methacrylate) (PMMA) bone cement (DePuy Orthopaedics, Inc., IN, USA) in square aluminum tube holders and allowed to rehydrate in PBS at room temperature for 1–2 h. Specimens were tested in torsion using an EnduraTec TestBench system (200 N·mm torque cell; Bose Corporation, MN, USA) at 1°/s until failure. The torque data was plotted against the rotational deformation to determine the maximum torque, torsional rigidity, and energy to maximum.
RNA isolation, cDNA synthesis, and quantitative gene expression analyses
In mice administered tibial fractures, at 2, 4 and 7 dpf, tibiae were removed and dissected free from muscle and soft tissue, and intramedullary pins were removed. Four-millimeters of fractured calluses were excised, flushed of marrow, and flash frozen in liquid nitrogen for mRNA extraction. Frozen fracture calluses were homogenized using the TissueLyser II system, and mRNA was extracted using a RNeasy kit (Qiagen, Valencia, CA, USA). mRNA was then reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) and cDNAs were analyzed by real-time qPCR using primers for Notch target gene (Hes1, Hey1, HeyL), chondrogenic genes (Sox9, Col2a1, Col10a1), osteogenic genes (Col1a1, Bsp, Oc), and osteoclastogenic genes (Opg and Rankl) (N = 4 per group per time point).
CFU-F assays
Bone marrow stromal cells were isolated from one day DAPT and vehicle treated mice at 4 and 7 dpf. Femurs and tibiae were removed and bone marrow cells were flushed from the marrow cavity. Cells were plated at a density of 1 × 106 cells/well in 6-well tissue culture plates for 14 days without change of mouse MSC medium (StemCell Techonologies, Vancouver, BC, Canada). On day 14 after plating, cells were fixed for crystal violet and alkaline phosphatase (ALP) staining. For CFU-F assays, we scored type I colonies (CFU-F) as previously described [12] (N = 5 per group per time point).
Statistics
Results are presented as the mean ± SEM. Statistical analyses were performed by Student's t test and two-way ANOVA followed by Dunnett's test; a p value <0.05 was considered significant.
Results
DAPT treatment following fracture inhibits Notch signaling and enhances callus formation
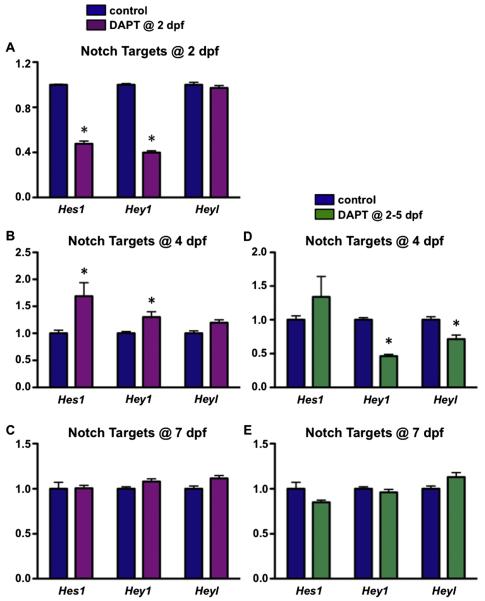
To determine if fracture repair can be enhanced and accelerated by manipulating Notch signaling, we performed open mid-shaft tibia fractures on C57BL/6J male mice and treated the mice transiently with DAPT, a small molecule inhibitor of the gamma-secretase complex that blocks all Notch signaling. Specifically, DAPT or DMSO (vehicle) was delivered via i.p. injections for one or four consecutive days. Treatment began two days following fracture to ensure normal initiation of MSC recruitment and proliferation at the fracture site. The half-life of DAPT in vivo is approximately 6 h [27], and our short-term, relatively high dose of DAPT treatment strategy appeared to have no notable side effects on mouse survival, body weight, or GI tract microarchitecture (data not shown). As a first step to assess Notch inhibition, we isolated RNA from callus tissues of DAPT treated and control mice at 2, 4, and 7 dpf. Real-time qPCR was performed for Hes1, Hey1, and Heyl (Fig. 1), since each of these RBPjk-dependent Notch targets has been shown to play a role in bone and cartilage development or maintenance [28,29]. The efficacy of Notch inhibition was confirmed by demonstrating a significant reduction in Hes1 and Hey1 6 h after DAPT treatment in mice treated with a single dose of DAPT at 2 dpf, while Heyl gene expression appeared to be unaffected. Interestingly, at day 4 Hes1 and Hey1 were elevated in DAPT treated mice as compared to controls, a “rebound” phenomenon that is common with transient Notch inhibition, most likely due to the accumulation of un-cleaved Notch fragments that are subsequently cleaved by the gamma-secretase complex following GSI clearance [30]. Additionally, systemic Notch inhibition was also observed in mice treated with DAPT from 2–5 dpf and reflected by reduced Hey1 and Heyl expression at 4 dpf. Surprisingly, Hes1 expression was unaffected at this point. By day 7, all Notch target genes showed comparable levels of expression to that of the controls in both DAPT treated groups. These data demonstrate that both single day and four consecutive day treatments with systemic DAPT inhibited local Notch signaling within callus tissues in a transient manner over the course of fracture healing.
To assess the effects on fracture repair from transient inhibition of gamma-secretase activity and Notch signaling, we performed longitudinal assessments of healing on DAPT treated and control mice using plain radiography. Digital radiographs were taken from fractured tibiae at 0, 10, 14, 21, 28 dpf to assess the presence of bony callus formation and bone union (Fig. 2A). At day 0 immediately after fracture radiographs demonstrated the presence of comparable, uncomplicated tibia fractures in all DAPT and vehicle treated mice. Newly calcified bony callus was apparent at 10 dpf in both DAPT treated fracture groups as compared to the vehicle treated group. At days 14 and 21 post-fracture, both DAPT treated groups exhibited larger bony calluses and radio-graphic evidence of bone union as compared to vehicle treated mice. Finally, by 28 dpf a near complete mineralized repair was evident in both DAPT treated and untreated mice. These data suggest that both DAPT treatments enhance early stages of callus formation and bone union as compared to controls, although the time it takes for bone callus remodeling appeared similar in all groups as assessed by autoradiography.
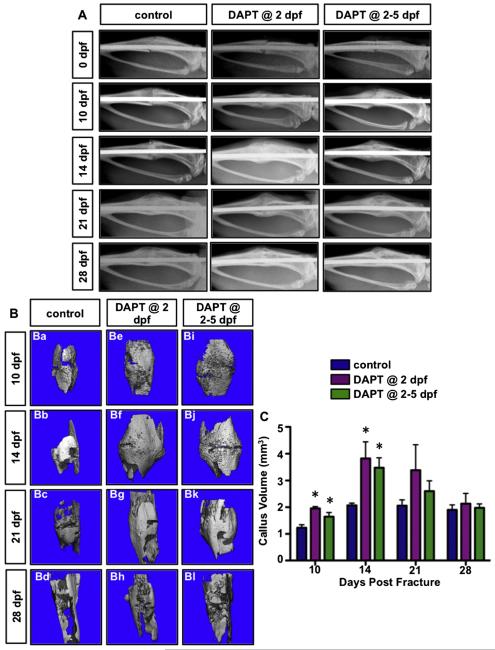
Transient inhibition of gamma-secretase activity and Notch signaling following fracture leads to enhanced and accelerated callus mineralization and bone union during early stages of fracture repair. (A) Real-time radiographic analysis of 3 representative fractured tibiae in vehicle treated, one day DAPT treated and four consecutive day DAPT treated mice at 0, 10, 14, 21, and 28 dpf. (B) Representative reconstructions of mineralized callus in vehicle treated, one day DAPT treated and four consecutive day DAPT treated mice at 10, 14, 21, and 28 dpf. (C) Quantifications of the mineralized callus volume indicate significant increase in either DAPT treated group relative to control group at 10 and 14 dpf. N ≥ 5, *, p < 0.05 versus control.
To confirm the radiographic findings, we next examined the mineralized calluses by microCT analysis (Figs. 2B–C). Consistent with the results from radiographic analyses, at 10 dpf both DAPT treated groups exhibited large calcified calluses that mostly bridged the fracture gap, while vehicle treated fractures still exhibited a defined fracture gap with smaller calcified calluses surrounding the injury site (Fig. 2B). At 14 and 21 dpf, DAPT treated fractures revealed more pronounced bony callus volumes as compared to the control fractures. Of note, bone in the fracture site from DAPT treated mice at day 21 almost returned to mature, lamellar shape, whereas some persistence of immature woven bone was still observed in the vehicle treated fractures. The microCT images showed comparable patterns, structure, and the degree of mineralization of the bony callus in all animals by 28 dpf (Fig. 2B), suggesting that fracture repair was nearly complete at this time point. Reconstruction and statistical analyses of the microCT data demonstrate that both DAPT treated groups have significantly greater mineralized callus volumes at 10 and 14 dpf, although trends of increased callus volume were observed at 21 dpf in DAPT treated animals (Fig. 2C). However, single dose DAPT treatments did not exhibit a bone anabolic effect when assessing trabecular bone mass of the contralateral un-fractured tibiae at 14 dpf, reflected as no significant changes in BV/TV, trabecular number, spacing, or thickness (Supplemental Fig. 1). These data indicate that transient inhibition of gamma-secretase activity and Notch signaling following fracture results in enhanced and accelerated callus mineralization during early stages of fracture repair with a timely resolution of the bony callus.
Transient inhibition of gamma-secretase activity and Notch signaling promotes cartilaginous and bony callus formation and remodeling
To further characterize the effects of transient Notch inhibition on fracture repair, we analyzed fracture callus tissue sections at 7, 10, 14, 21, and 28 dpf via alcian blue/hematoxylin/orange-g (ABH/OG) staining. Histological analyses revealed an enhanced fracture repair by transient inhibition of Notch signaling following fractures (Fig. 3A). By 7 dpf, the callus tissue sections showed robust chondrogenic and osteogenic differentiation from MSCs at the fracture site. This was observed in the callus tissue sections via an increase in cartilage and bone formation from DAPT treated mice as compared to control treated mice. Such differences were more remarkable at 10 dpf. Interestingly, a central area of cartilage tissue persisted in the four consecutive day DAPT treated fracture calluses at 14 dpf (Fig. 3Am), whereas cartilage tissue was essentially absent in both the control and one day DAPT treated fracture groups. This is likely due to the fact that loss of Notch signaling in committed chondrocytes promotes chondrocyte proliferation, while suppressing terminal maturation of chondrocytes [13]. The persistent cartilage at 14 dpf in the four dose treatment group was entirely replaced by primary bone formation at 21 dpf (Fig. 3An). Of note, both DAPT treated fracture groups exhibited more robust new bone formation at 14 and 21 dpf, resulting in larger callus areas in these mice as compared to control mice. Closer examination of callus morphology at 28 dpf demonstrated that the bone formed near the proximal and distal sides of fracture junction in DAPT treated groups was more consolidated and restored to the original lamellar structure (Fig. 3Aj, Ao), although the size of the calluses was comparable in all groups.
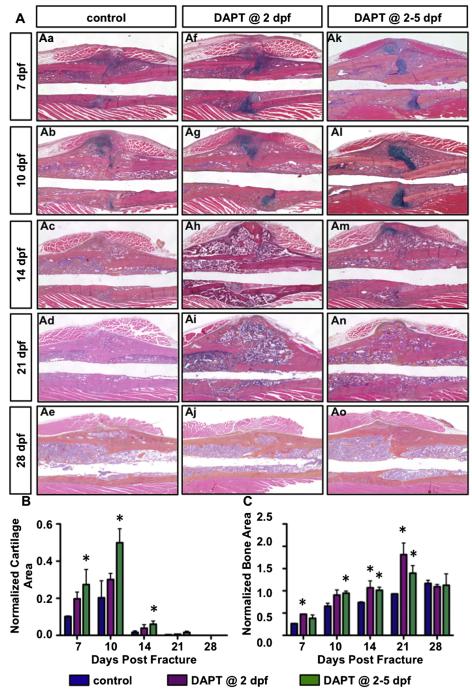
Transient inhibition of gamma-secretase activity and Notch signaling following fracture enhances the fracture healing process by generating a more robust cartilaginous and bony callus. (A) Representative histology from vehicle treated (Aa–Ae), one day DAPT treated (Af–Aj) and four consecutive day DAPT treated (Ak–Ao) mice at 7, 10, 14, 21, and 28 dpf. (B) Histomorphometric quantification identifies significantly increased cartilage, and persistence of cartilage at 14 dpf in four dose treatment group. (C) Significant increase in bony callus relative to control exists in both DAPT treated groups at 7, 10, 14, and 21 dpf. N ≥ 4, *, p < 0.05 versus control.
Quantitative histomorphometry performed on ABH/OG stained fracture callus sections revealed cartilaginous callus areas beginning within a week following fracture and peaking at 10 dpf followed by rapid resolution, while bone area progressively increased until 21 dpf. Both DAPT treatment regimens gave rise to increased cartilage callus areas at 7 and 10 dpf, while bone area was significantly increased at most time-points throughout the healing process (7, 10, 14, and 21 dpf) (Figs. 3B, C). Consistent with the persistent cartilage observed in ABH/OG stained histology, there was a significantly increased area of residual cartilage in the four day DAPT treatment group as compared with vehicle treated mice at 14 dpf (Fig. 3B). No significant changes were observed in mesenchyme area upon DAPT treatment (data not shown), indicating that MSC recruitment and proliferation in the fracture site were not significantly affected by DAPT treatment beginning at two days post-fracture. These data collectively suggest that transient inhibition of gamma-secretase activity and Notch signaling following fracture enhances the fracture healing process by generating a more robust cartilaginous and bony callus.
To assess the effects of transient inhibition of gamma-secretase activity and Notch signaling on osteoclast differentiation, we first performed real-time qPCR for Opg and Rankl (Figs. 4A–D) from callus tissues of DAPT treated and control mice at 2, 4, and 7 dpf. Mice treated with a single dose of DAPT at 2 dpf demonstrated a significant reduction and an up-regulation in Opg and Rankl 6 h after DAPT treatment, respectively. Thus, the Opg/Rankl ratio was significantly reduced at this point. Mice treated with DAPT from 2–5 dpf also showed significantly reduced and enhanced Opg and Rankl expression at 4 dpf, respectively, also resulting in a significant decrease of the Opg/Rankl ratio. By day 7, both Opg and Rankl showed comparable levels of expression in all three groups. These data demonstrate that both single day and four consecutive day treatments with systemic DAPT enhanced osteoclastogenesis within callus tissues in a transient manner over the course of fracture healing. Furthermore, while transcript levels of Opg and Rankl were normalized by day 7, we did detect changes in OPG and RANKL protein at later time points that were more reflective of the time points at which our TRAP studies were conducted. Immunohistochemistry (IHC) for OPG demonstrated a clear down-regulation of protein in DAPT treated fractures at 21 dpf, while by 28 dpf the levels more closely resembled control staining (Fig. 4E). Interestingly, RANKL IHC staining (Supplemental Fig. 2) did not appear to be dramatically different than controls at most time points, which is likely reflective of the minimal changes observed at the transcriptional level for Rankl. Since the changes of OPG levels were observed at later time points and osteoclasts play a significant role in bone remodeling during later stages of fracture repair, we next performed in situ hybridization (ISH) for TRAP and TRAP staining on callus tissue sections at 21 and 28 dpf, respectively (Fig. 5). Interestingly, our results indicated a significant increase in the percentage of bone surface covered by osteoclasts (Oc. S./BS) within newly formed bone regions in the fracture calluses of both DAPT treated groups at 21 dpf (Figs. 5A, B), suggesting an enhanced or accelerated remodeling process at this time point. By day 28, a time point by which fracture repair is nearly complete, fewer TRAP positive osteoclasts were observed in the one day DAPT treated group, and Oc. S./BS, was significantly decreased, whereas the four consecutive day DAPT treatment group showed comparable levels of Oc. S./BS relative to control mice (Figs. 5A, C). Collectively, these data are consistent with a more rapid bone remodeling and fracture repair in DAPT treated mice as observed in both radiographic and histological examination.
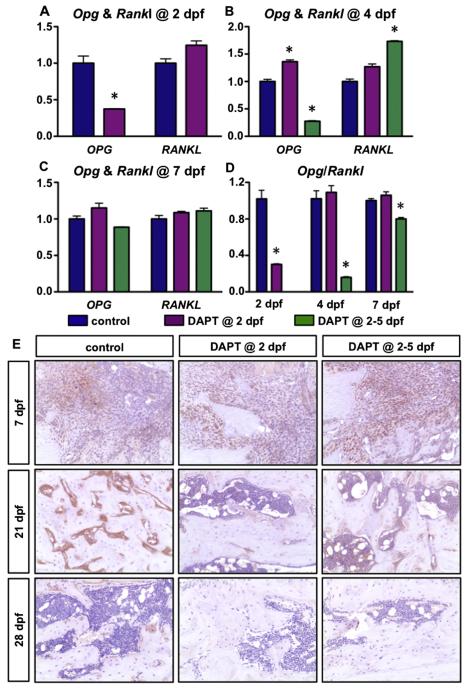
DAPT treated fractures have enhanced osteoclastogenesis. (A–D) Real-time qPCR for Opg and Rankl expression in the fracture site from vehicle treated, one day DAPT treated, and four consecutive day DAPT treated groups at 2 (A), 4 (B), 7 dpf (C) and the ratio of Opg/Rankl at indicated time-points (D). (E) IHC for OPG on fracture callus sections at 7, 21 and 28 dpf. N ≥ 4, *, p < 0.05 versus control.
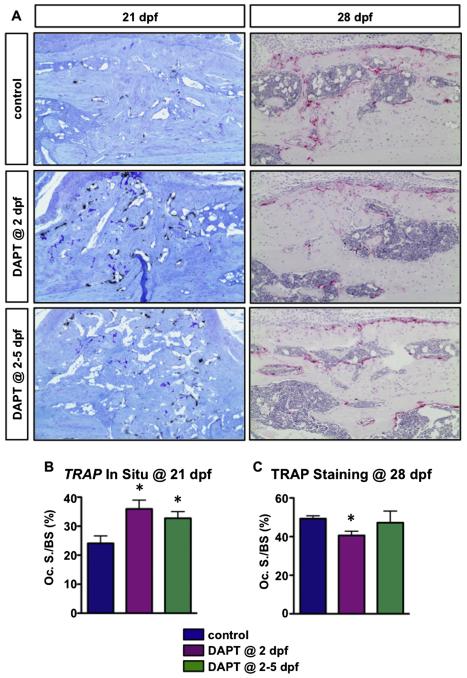
DAPT treated fractures have accelerated bone remodeling. (A) TRAP in situ (Aa–Ac) and TRAP staining (Ad–Af) of callus sections from control, one day DAPT treated and four consecutive day DAPT treated mice at 21 and 28 dpf, respectively. (B) Histomorphometric quantification of the osteoclast surface per bone surface (Oc. S./BS) on TRAP in situ and TRAP stained sections. N ≥ 4, *, p < 0.05 versus control.
Transient inhibition of gamma-secretase activity and Notch signaling enhances the biomechanical properties of fractured tibiae
To evaluate the biomechanical properties of the newly formed bone, we performed biomechanical torsion testing to test the quality of the repaired tibiae from DAPT treated and control mice at 10, 14, 21 and 28 dpf (Fig. 6). As expected, the DAPT treated fractures, besides displaying greater volume of bony callus, exhibited significantly higher maximum torques and torsional rigidity at 14, 21, and 28 dpf as compared to vehicle treated mice (Figs. 6A, B), suggesting that transient gamma-secretase and Notch inhibition following fracture dramatically enhances the strength and stiffness of newly formed fracture callus. Furthermore, DAPT treated fractures at 28 dpf required higher energy to fail during torsion testing (Fig. 6C). At 21 and 28 dpf, single day DAPT treated tibiae essentially reached the strength of normal tibiae without fractures. In contrast, the maximum torque of vehicle treated fractures at 21 and 28 dpf was only about two thirds of the normal tibiae, indicating that single day treated fractures are significantly stronger than the vehicle treated controls (Fig. 6A). Moreover, 20% of failures occurred in the intact bone rather than within the original fracture line in both single day and four consecutive day DAPT treated groups at 21 dpf, whereas none of such failure occurred in vehicle treated fractures. By 28 dpf, the percentage of repaired fractures that fall into biomechanical stage 4, characterized by the failure during torsion testing occurring within intact bone rather than through the original fracture line [31], increased to 40% in single day DAPT treated mice (Supplemental Table 1). This group also displayed the most enhanced biomechanical properties among the three experimental groups. Collectively, these data suggest that transient inhibition of gamma-secretase activity and Notch signaling markedly enhances the biomechanical properties of newly formed bone during fracture repair, with single day DAPT treated fractures exhibiting the most enhanced biomechanical outcomes.
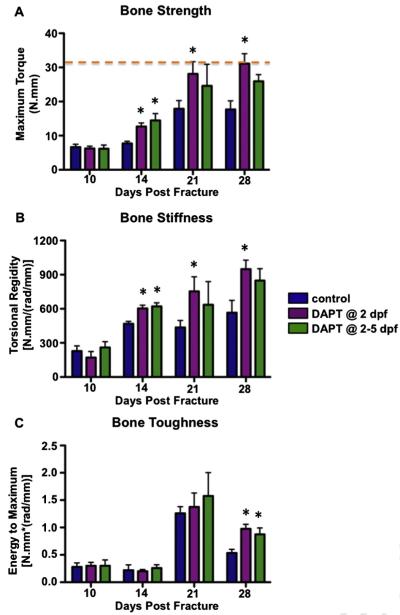
Transient inhibition of gamma-secretase activity and Notch signaling enhances biomechanical properties of the newly formed bone during fracture repair. Both one day DAPT treated and four consecutive day DAPT treated fractures are associated with increased (A) maximum torque, (B) torsional rigidity, and (C) energy to maximum of the newly formed callus. Orange dot line indicates the maximum torque of normal tibiae without fracture. N ≥ 5, *, p < 0.05 versus control. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Transient inhibition of gamma-secretase and Notch signaling promotes chondro-osteogenic gene expression and reduces clonal frequency of bone marrow stromal cells
To assess the early molecular changes in the fracture callus following DAPT treatment, we isolated RNA from the fracture calluses of DAPT treated and control mice at 4 and 7 dpf. Real-time qPCR was performed for chondrogenic and osteogenic gene expression in callus tissues. Consistent with the histological changes, both chondrogenic (Sox9, Col2a1, Col10a1) and osteogenic markers (Col1a1, Bsp, Oc) were significantly elevated in four consecutive day DAPT treated group at 4 dpf (~2–10 folds), while the expression levels of these markers were less significantly enhanced by 4 dpf within the one day DAPT treatment group (Figs. 7A, C). By 7 dpf, the expression levels of all the chondrogenic markers investigated were significantly up-regulated in both one day and four consecutive day DAPT treated calluses (~2-fold), while bone marker Bsp and Oc were significantly increased in four consecutive day DAPT treated calluses and one day DAPT treated calluses, respectively (~1.5-fold) (Figs. 7B, D). These data suggest that local MSCs in DAPT treated fractures are undergoing more rapid chondrogenic and osteogenic differentiation as compared to vehicle treated mice.
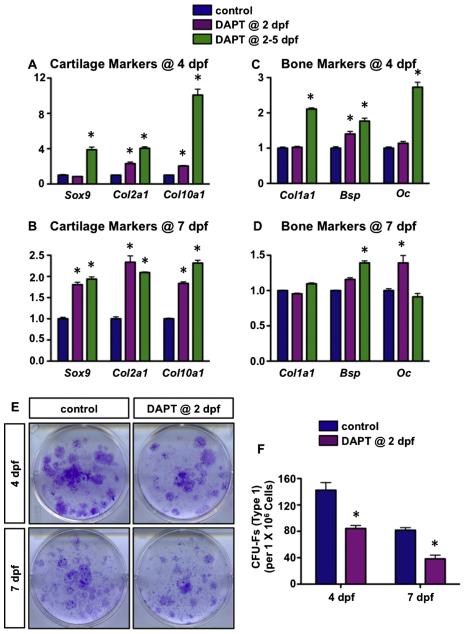
Transient inhibition of gamma-secretase activity and Notch signaling reduces CFU-Fs by promoting chondrogenic and osteogenic differentiation of local BMSCs. Real-time qPCR comparing cartilage marker (A, B) and bone marker (C, D) expression in the fracture site from vehicle treated, one day DAPT treated, and four consecutive day DAPT treated groups at 4 dpf and 7 dpf. N = 4. (E) CFU-F assays for bone marrow stromal cells from single day DAPT and control treated mice at 4 and 7 dpf. (F) The number of type I colonies (CFU-Fs) was quantified from CFU-F assays. N = 5, *, p < 0.05 versus control.
We next verified altered MSC differentiation following transient inhibition of gamma-secretase activity and Notch signaling by performing colony-forming unit fibroblast (CFU-F) assays using bone marrow stromal cells from DAPT treated and control mice. Bone marrow stromal cells extracted from one day DAPT treated and vehicle treated mice at 4 and 7 dpf were cultured for 14 days and crystal violet staining was performed (Fig. 7E). Crystal violet staining revealed fewer type I colonies (CFU-Fs) present in the DAPT treated bone marrow stromal cell cultures than those in vehicle treated mice (Fig. 7F). These data are consistent with those obtained in vivo in which the expression of chondro-osteogenic markers was significantly up-regulated following DAPT treatment, thus further proving that transient inhibition of gamma-secretase activity and Notch signaling promotes local BMSC differentiation in vivo. Taken together, these data demonstrate that the accelerated fracture healing is at least partially due to the early enhancement in local BMSC chondro-osteogenic differentiation and therefore a reduction in the number of clonal cells isolated from DAPT treated fractures as compared to vehicle treated fractures.
Discussion
Despite the demonstrated importance of Notch signaling during embryonic and postnatal skeletal development, the data provided here demonstrates for the first time that transient inhibition of gamma-secretase activity and Notch signaling via DAPT treatment following fracture enhances and accelerates skeletal fracture repair. This study establishes that DAPT treated fractures exhibit an early enhancement in local MSC differentiation and more rapid bone remodeling, resulting in accelerated fracture repair with improved biomechanical properties. Furthermore, our report suggests that the use of GSIs and/or direct NOTCH signaling inhibitors may have therapeutic potential for the treatment of human skeletal fractures.
Through a combination of histological, molecular, and cellular-based assays we demonstrated that transient inhibition of gamma-secretase activity and/or Notch signals effectively and temporarily removes the “break” on MSC differentiation, and thereby allows MSCs to undergo more rapid chondro-osteogenic differentiation. Consistent with these data, several reports have indicated the ability of Notch signaling to regulate in vitro chondrogenic, osteogenic, and adipogenic differentiation from various progenitor and immature cell types [12,32,33]. Similar effects were also observed in our previous in vivo studies, such that RBPjk conditional LOF mutant embryos formed mesenchymal condensations earlier than control littermates and mesenchymal progenitors within condensations underwent a more rapid progression of chondrogenic and osteogenic differentiation [10,13]. It is also worth noting that transient inhibition of gamma-secretase activity and Notch signaling via DAPT treatment, which was applied two days following fracture, does not significantly impact MSC proliferation at the fracture site since the mesenchymal regions observed throughout the healing process were similar in all treatment and control groups. Although, Notch signaling has recently been implicated in the regulation of MSC proliferation in vivo [10] and in vitro [33,34]. Collectively, these data, along with our histological findings at early time-points, further support the notion that transient down-regulation of gamma-secretase activity and Notch pathway in MSCs would allow cells to more readily respond to differentiation cues during fracture repair, thereby resulting in more robust cartilaginous and bony callus formation at the fracture site.
The results observed in this study appear to be opposed by a report demonstrating via a genetic and permanent approach that systemic and prolonged down-regulation of Notch signaling delayed and impaired several phases of the fracture healing process [35]. These apparently conflicting reports suggest that the timing and duration of Notch inhibition is critical for appropriate bone fracture healing. Given the fact that MSC recruitment is promoted by the pro-inflammatory cytokines secreted at the injury site, which peak 24 h following fracture [36], we transiently inhibited gamma-secretase activity and Notch signaling by applying a small molecule GSI, DAPT, two days following fracture for a very short term. This GSI injection strategy starting at the conclusion of the acute inflammation phase and ending at the early chondrogenic healing phase would allow not only the normal initiation of MSC recruitment and proliferation in the fracture site prior to Notch inhibition, but also provide a model to understand the direct role of Notch signaling specifically during the early MSC differentiation phases of fracture repair. In contrast, prolonged Notch inhibition prior to fractures resulted in the persistence of inflammation throughout the entire healing process, eventually impairing later phases of fracture repair [35].
Remarkably, our biomechanical testing results showed that almost all of the biomechanical properties (strength, stiffness, and toughness) of DAPT treated fractures were significantly enhanced at 14 dpf and later stages of fracture repair, providing strong evidence of acceleration and enhancement of fracture repair. In particular, at 21 and 28 dpf, single day DAPT treated tibiae essentially reached the strength of normal tibiae without fractures. Interestingly, our biomechanical outcome measurements also revealed a discrepancy in the magnitude of bone strength enhancement observed in single day and four consecutive day DAPT treated fractures at 21 and 28 dpf. While four day DAPT treated mice only showed trends for increased strength as compared to control treated mice, single day DAPT treated fractures essentially reached the bone strength of normal, un-fractured tibiae from sex and age matched controls. There exist several possibilities to explain these varied healing effects on bone strength. First, it is of note that the compromised healing effects observed in fractures with four consecutive day DAPT treatments, which extends well into the early chondrogenic healing phase, could be caused by combined effects from the loss of Notch signaling in both the MSC population and committed chondrocytes in the cartilaginous callus. Our histological studies did identify un-resorbed cartilage persisting at the fracture junction in the four-day treatment group, while the cartilage was completely resolved in single day DAPT treated mice. This finding is not unexpected given the role of Notch signaling in regulating chondrocyte maturation, in which MSC specific [12], chondro-osteoprogenitor specific [11], and cartilage specific [13] permanent Notch LOF all lead to delayed progression of terminal chondrocyte maturation and cartilage matrix turnover. Additionally, the one day DAPT treated fractures exhibited an enhancement in osteoclast formation at 21 dpf, which subsequently decreased below control levels at 28 dpf, while the osteoclast formation in four day DAPT treated mice showed a less significant increase at 21 dpf that resolved only to control-treated levels by 28 dpf. The altered levels of osteoclast formation likely resulted in more rapid bone remodeling and may have lead to the superior biomechanical integrity in single day DAPT treated fractures. Therefore, collectively our data suggest that limited Notch inhibition following fracture appears to be preferential to longer term Notch inhibition in order to achieve an appropriate balance of enhanced MSC and osteoclast differentiation resulting in accelerated fracture repair with improved biomechanical properties.
The role of Notch signaling in osteoclastogenesis has been extensively investigated. Previous findings have shown that Notch negatively regulates osteoclastogenesis directly through expression in macrophage precursors in a cell-autonomous manner [37], or indirectly through expression in mature osteoblasts in a cell-non-autonomous manner [12,14,15]. Consistently, our data demonstrated that DAPT treated fracture calluses exhibited significant inhibition and induction of Opg and Rankl genes, respectively, as well as a down-regulation of OPG protein via IHC which results in a reduced OPG/RANKL ratio and ultimately enhanced osteoclastogenesis within fracture calluses in a transient manner. Combined these data suggest accelerated osteoclastogenesis and bone remodeling within fracture calluses of DAPT treated mice. It is interesting to note that Notch signaling is largely normalized by 7 dpf as indicated by Hes/Hey gene expression due to the short treatment regimen and half-life of DAPT, although persistent effects on OPG and osteoclasts are observed out to at least 21 dpf. We therefore speculate that transient inhibition of gamma-secretase activity and Notch signaling may result in longer-term changes within the callus by disrupting cellular physiology and the cellular components present throughout fracture healing.
Although the present study was not designed to be mechanistic-driven, we remain curious about the underlying mechanisms by which Notch signaling may regulate MSC differentiation at the fracture site. A recent study may provide some understanding of how transient Notch inhibition enhances chondrogenic and osteogenic differentiation from MSCs in the inflammatory setting of fracture repair. Zhang et al. [38] demonstrated that chronic inflammation increased the expression of non-canonical NF-κB proteins, which activated Notch signaling by binding to and promoting nuclear translocation of NICD. Additionally, this inflammation mediated Notch activation in MSCs suppressed osteogenic differentiation, which could be prevented by administration of the Notch inhibitor, DAPT. In the context of fracture repair, the acute inflammation phase that is required to initiate the repair cascade by promoting MSC recruitment to the fracture site [39], may also mediate temporary Notch activation through the mechanism illustrated by Zhang and colleagues. Indeed, Notch and NF-κB signaling components are up-regulated during the early phases of fracture repair [23]. Therefore, we speculate that our transient DAPT treatment inhibited the inflammation mediated Notch activation, thus freeing MSCs recruited to the fracture site to undergo differentiation into chondrocytes and osteoblasts, eventually resulting in accelerated and enhanced fracture repair. This concept is further highlighted by our results showing that DAPT treatment did not exhibit any bone anabolic effects on the trabecular bone of the contralateral un-fractured tibiae that does not have localized inflammatory response (Supplemental Fig. 1).
Throughout this study we utilized the small molecule GSI, DAPT, which in addition to being a Notch inhibitor is known to have certain toxic effects in the intestine. Our DAPT treatments post fracture did not result in an altered number of intestinal goblet cells (data not shown), which is a hallmark of the toxic side effects from prolonged GSI use [40]. The gamma-secretase complex is also known to have numerous other protein substrates in addition to the Notch receptors [41], although there are no reported effects regarding the transient or permanent systemic inhibition of these proteins on fracture repair. Additionally, existing mouse genetic evidence suggests that systemic impairment in most of these alternate gamma-secretase substrates (APP, DCC, p75 NTR, Apo ER2, Syndecan-3, Nectin-1 α, CD44, LRP, and N-cadherin) results in no skeletal phenotypes and in some cases result in decreased bone formation, not increased bone formation as observed in all Notch inhibition and loss-of-function mutants. Based on the data presented here (accelerated osteoblast differentiation from MSCs, enhanced cartilage formation from MSCs and delayed cartilage turnover, and enhanced osteoclastogenesis), it is highly likely and completely consistent with the notion that the accelerated and enhanced fracture repair following GSI treatment is a direct result of transient Notch inhibition.
Similar to our observations, pharmacological disruption of Notch signaling using a GSI has proven to have beneficial effects on a variety of healing systems [42-44], and several GSIs are now being used in clinical trials primarily focused on various types of cancer and Alzheimer's disease [45-50]. Inhibitory antibodies are also being developed for both the NOTCH1 and NOTCH2 receptors and are currently under clinical investigations in similar disease settings as the GSIs [40]. Our findings presented here raise the possibility that transient Notch inhibition via systemic DAPT (or other similar GSI and possibly direct NOTCH inhibitors) administration during the early stages of fracture repair has promising clinical utility for enhancing and accelerating this process. As our work here suggests by the early and persistently increased biomechanical strength of bones due to a single day DAPT treatment, a quarter of the time required to resolve normal bone fracture repair could be saved. While the majority of fractures in patients repair with appropriate stabilization and without any pharmacologic intervention, the socioeconomic impact of these injuries cannot be underestimated, as expenses related to treating fractures and associated lost productivity are predicted to increase to over $74 billion by 2015 [51]. Therefore, novel therapies focused on transient gamma-secretase and Notch inhibition, which have been illustrated here to efficiently enhance and accelerate normal fracture healing, could have enormous health and socioeconomic benefits if applied to orthopedic trauma patients.
Supplementary Material
1
2
3
4
5
Acknowledgments
This work was supported in part by the following United States National Institute of Health grants: R01 grants (AR057022 and AR063071), R21 grant (AR059733 to MJH), a P50 Center of Research Translation grant (AR054041 to RJO), and a P30 Core Center grant (AR061307 to MJH and HA). We would like to gratefully acknowledge the technical expertise and assistance of Sarah Mack, Kathy Maltby, Ashish Thomas, and Jason Inzana within the Center for Musculoskeletal Research Histology, Biochemistry, and Molecular Imaging Core.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2014.12.007.
Disclosures
All authors state that they have no conflict of interests.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bone.2014.12.007
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4336841?pdf=render
Citations & impact
Impact metrics
Article citations
Vascular restoration through local delivery of angiogenic factors stimulates bone regeneration in critical size defects.
Bioact Mater, 36:580-594, 01 Jun 2024
Cited by: 1 article | PMID: 39100886 | PMCID: PMC11295624
Nanofiber-induced hierarchically-porous magnesium phosphate bone cements accelerate bone regeneration by inhibiting Notch signaling.
Bioact Mater, 37:459-476, 25 Apr 2024
Cited by: 1 article | PMID: 38698920 | PMCID: PMC11063995
Dnmt3b ablation affects fracture repair process by regulating apoptosis.
BMC Musculoskelet Disord, 25(1):180, 27 Feb 2024
Cited by: 0 articles | PMID: 38413962 | PMCID: PMC10900613
Directing cellular responses in a nanocomposite 3D matrix for tissue regeneration with nanoparticle-mediated drug delivery.
Mater Today Bio, 23:100865, 14 Nov 2023
Cited by: 0 articles | PMID: 38054034 | PMCID: PMC10694759
DNA methylation-mediated Rbpjk suppression protects against fracture nonunion caused by systemic inflammation.
J Clin Invest, 134(3):e168558, 05 Dec 2023
Cited by: 3 articles | PMID: 38051594 | PMCID: PMC10849763
Go to all (19) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
γ-Secretase inhibitor reverts the Notch signaling attenuation of osteogenic differentiation in aged bone marrow mesenchymal stem cells.
Cell Biol Int, 40(4):439-447, 04 Feb 2016
Cited by: 11 articles | PMID: 26801333
NOTCH signaling in skeletal progenitors is critical for fracture repair.
J Clin Invest, 126(4):1471-1481, 07 Mar 2016
Cited by: 66 articles | PMID: 26950423 | PMCID: PMC4811137
Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer's disease.
J Pharmacol Exp Ther, 331(2):598-608, 11 Aug 2009
Cited by: 87 articles | PMID: 19671883
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.
Cell Signal, 26(1):149-161, 16 Oct 2013
Cited by: 106 articles | PMID: 24140475
Review
Funding
Funders who supported this work.
NIAMS NIH HHS (5)
Grant ID: P50 AR054041
Grant ID: R21 AR059733
Grant ID: R01 AR063071
Grant ID: P30 AR061307
Grant ID: R01 AR057022
United States National Institute of Health (5)
Grant ID: AR063071
Grant ID: AR059733
Grant ID: AR057022
Grant ID: AR054041
Grant ID: AR061307