Abstract
Free full text

PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro.
Abstract
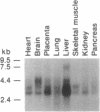
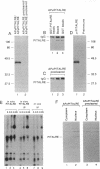
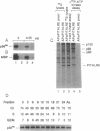
Members of the cell division cycle 2 (CDC2) family of kinases play a pivotal role in the regulation of the eukaryotic cell cycle. In this communication, we report the isolation of a cDNA that encodes a CDC2-related human protein kinase temporarily designated PITALRE for the characteristic Pro-Ile-Thr-Ala-Leu-Arg-Glu motif. Its deduced amino acid sequence is 47% identical to that of the human cholinesterase-related cell division controller (CHED) kinase, which is required during hematopoiesis, and 42% identical to the Saccharomyces cerevisiae SGV1 gene product, a putative kinase involved in the response to pheromone via its guanine nucleotide-binding protein alpha subunit. PITALRE expression is ubiquitous, but its expression levels are different in various human tissues. PITALRE is an approximately 43-kDa protein that associates with three cellular polypeptides of 80, 95, and 155 kDa. PITALRE is localized primarily to the nucleus. In addition, we have identified a retinoblastoma protein kinase activity associated with PITALRE immunocomplexes that cannot phosphorylate histone H1, suggesting that the target phosphorylation site of PITALRE differs from that of CDC2 kinase. Interestingly, the retinoblastoma kinase activity associated with PITALRE does not oscillate during the cell cycle.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Nasmyth K. Control of the yeast cell cycle by the Cdc28 protein kinase. Curr Opin Cell Biol. 1993 Apr;5(2):166–179. [Abstract] [Google Scholar]
- Fang F, Newport JW. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. [Abstract] [Google Scholar]
- Tsai LH, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [Abstract] [Google Scholar]
- Tsai LH, Harlow E, Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. [Abstract] [Google Scholar]
- Hanks SK. Homology probing: identification of cDNA clones encoding members of the protein-serine kinase family. Proc Natl Acad Sci U S A. 1987 Jan;84(2):388–392. [Europe PMC free article] [Abstract] [Google Scholar]
- Elledge SJ, Spottswood MR. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. [Europe PMC free article] [Abstract] [Google Scholar]
- Ninomiya-Tsuji J, Nomoto S, Yasuda H, Reed SI, Matsumoto K. Cloning of a human cDNA encoding a CDC2-related kinase by complementation of a budding yeast cdc28 mutation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9006–9010. [Europe PMC free article] [Abstract] [Google Scholar]
- Lapidot-Lifson Y, Patinkin D, Prody CA, Ehrlich G, Seidman S, Ben-Aziz R, Benseler F, Eckstein F, Zakut H, Soreq H. Cloning and antisense oligodeoxynucleotide inhibition of a human homolog of cdc2 required in hematopoiesis. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):579–583. [Europe PMC free article] [Abstract] [Google Scholar]
- Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. EMBO J. 1992 Aug;11(8):2909–2917. [Europe PMC free article] [Abstract] [Google Scholar]
- Okuda T, Cleveland JL, Downing JR. PCTAIRE-1 and PCTAIRE-3, two members of a novel cdc2/CDC28-related protein kinase gene family. Oncogene. 1992 Nov;7(11):2249–2258. [Abstract] [Google Scholar]
- Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10867–10871. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, Sherr CJ. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. [Abstract] [Google Scholar]
- Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. [Abstract] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. [Abstract] [Google Scholar]
- Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993 Apr;210(1):179–187. [Abstract] [Google Scholar]
- Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. [Abstract] [Google Scholar]
- Giordano A, Whyte P, Harlow E, Franza BR, Jr, Beach D, Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. [Abstract] [Google Scholar]
- Rosenblatt J, Gu Y, Morgan DO. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2824–2828. [Europe PMC free article] [Abstract] [Google Scholar]
- Giordano A, Lee JH, Scheppler JA, Herrmann C, Harlow E, Deuschle U, Beach D, Franza BR., Jr Cell cycle regulation of histone H1 kinase activity associated with the adenoviral protein E1A. Science. 1991 Sep 13;253(5025):1271–1275. [Abstract] [Google Scholar]
- Ashihara T, Baserga R. Cell synchronization. Methods Enzymol. 1979;58:248–262. [Abstract] [Google Scholar]
- Li YC, Ross J, Scheppler JA, Franza BR., Jr An in vitro transcription analysis of early responses of the human immunodeficiency virus type 1 long terminal repeat to different transcriptional activators. Mol Cell Biol. 1991 Apr;11(4):1883–1893. [Europe PMC free article] [Abstract] [Google Scholar]
- Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [Abstract] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. [Abstract] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. [Abstract] [Google Scholar]
- Irie K, Nomoto S, Miyajima I, Matsumoto K. SGV1 encodes a CDC28/cdc2-related kinase required for a G alpha subunit-mediated adaptive response to pheromone in S. cerevisiae. Cell. 1991 May 31;65(5):785–795. [Abstract] [Google Scholar]
- Garcia-Bustos J, Heitman J, Hall MN. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. [Abstract] [Google Scholar]
- Williams RT, Wu L, Carbonaro-Hall DA, Tolo VT, Hall FL. Identification of a novel cyclin-like protein in human tumor cells. J Biol Chem. 1993 Apr 25;268(12):8871–8880. [Abstract] [Google Scholar]
- Tyers M, Tokiwa G, Futcher B. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 1993 May;12(5):1955–1968. [Europe PMC free article] [Abstract] [Google Scholar]
- Molz L, Beach D. Characterization of the fission yeast mcs2 cyclin and its associated protein kinase activity. EMBO J. 1993 Apr;12(4):1723–1732. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.91.9.3834
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc43676?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.91.9.3834
Article citations
Novel meriolin derivatives potently inhibit cell cycle progression and transcription in leukemia and lymphoma cells via inhibition of cyclin-dependent kinases (CDKs).
Cell Death Discov, 10(1):279, 11 Jun 2024
Cited by: 0 articles | PMID: 38862521
CDK9-55 guides the anaphase-promoting complex/cyclosome (APC/C) in choosing the DNA repair pathway choice.
Oncogene, 43(17):1263-1273, 04 Mar 2024
Cited by: 0 articles | PMID: 38433256
Cyclin-dependent kinases: Masters of the eukaryotic universe.
Wiley Interdiscip Rev RNA, e1816, 17 Sep 2023
Cited by: 3 articles | PMID: 37718413 | PMCID: PMC10909489
Review Free full text in Europe PMC
Protocol to assess substrate dephosphorylation by serine/threonine phosphoprotein phosphatases in vitro.
STAR Protoc, 4(2):102148, 18 Apr 2023
Cited by: 1 article | PMID: 37074907 | PMCID: PMC10322857
Addressing Transcriptional Dysregulation in Cancer through CDK9 Inhibition.
Biochemistry, 62(6):1114-1123, 28 Feb 2023
Cited by: 4 articles | PMID: 36854448 | PMCID: PMC10035036
Go to all (142) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phosphorylation site specificity of the CDC2-related kinase PITALRE.
Biochem J, 320 ( Pt 3):983-989, 01 Dec 1996
Cited by: 21 articles | PMID: 9003389 | PMCID: PMC1218024
Cloning of murine CDK9/PITALRE and its tissue-specific expression in development.
J Cell Physiol, 177(2):206-213, 01 Nov 1998
Cited by: 35 articles | PMID: 9766517
CDC2-related kinase PITALRE phosphorylates pRb exclusively on serine and is widely expressed in human tissues.
J Cell Physiol, 172(2):265-273, 01 Aug 1997
Cited by: 27 articles | PMID: 9258347
The CDC2-related kinase PITALRE is the catalytic subunit of active multimeric protein complexes.
Biochem J, 319 ( Pt 1):293-298, 01 Oct 1996
Cited by: 42 articles | PMID: 8870681 | PMCID: PMC1217767