Abstract
Aim
To determine the expressions of inducible nitric oxide synthase (iNOS) and matrix metalloproteinase-9 (MMP-9) in hepatocellular carcinoma (HCC) and to investigate the relationship between iNOS and MMP-9 expression and their effects on angiogenesis and progression of HCC.Methods
In this study, we examined iNOS, MMP-9, and CD34 expression in specimens surgically removed from 32 HCC patients and 7 normal liver tissues by immunohistochemical staining. Meanwhile, microvessel density (MVD) was determined as a marker of angiogenesis by counting CD34-positive cells.Results
The positive rates of iNOS and MMP-9 expression were 71.88% (23/32) and 78.13% (25/32) in HCC. MMP-9 expression was significantly correlated with tumor size, capsule status, TNM stage, and risk of HCC recurrence (P = 0.032, P = 0.033, P = 0.007, and P = 0.001, respectively). There was also a significant relationship between iNOS expression and capsule status and risk of HCC recurrence (P = 0.049 and P = 0.004, respectively), but no correlation between iNOS expression and tumor size and TNM stage. There was a positive association between MVD and TNM stage and risk of HCC recurrence (P = 0.037 and P = 0.000, respectively). The count of MVD was significantly different in different iNOS and MMP-9 immunoreactivity groups (F = 17.713 and 17.097, P = 0.000 and P = 0.000, respectively). The examination of Spearman's rank correlation coefficient showed that there was a significant positive correlation between MVD and iNOS, MMP-9 immunoreactivity (r = 0.754 and 0.751, P = 0.000 and P=0.000, respectively). There was also a significant association between MMP-9 and iNOS expression in HCC (P = 0.010).Conclusion
Nitric oxide (NO) produced by iNOS could modulate MMP-9 production and therefore contribute to tumor cell angiogenesis and invasion and metastasis in HCC. The strong expression of iNOS and MMP-9 in HCC may be helpful in evaluating the recurrence of HCC, predicting poor prognosis. For patients with strong expression of MMP-9 and iNOS, the optimal treatment scheme needs to be selected.Free full text

Expressions of inducible nitric oxide synthase and matrix metalloproteinase-9 and their effects on angiogenesis and progression of hepatocellular carcinoma
Abstract
AIM: To determine the expressions of inducible nitric oxide synthase (iNOS) and matrix metalloproteinase-9 (MMP-9) in hepatocellular carcinoma (HCC) and to investigate the relationship between iNOS and MMP-9 expression and their effects on angiogenesis and progression of HCC.
METHODS: In this study, we examined iNOS, MMP-9, and CD34 expression in specimens surgically removed from 32 HCC patients and 7 normal liver tissues by immunohistochemical staining. Meanwhile, microvessel density (MVD) was determined as a marker of angiogenesis by counting CD34-positive cells.
RESULTS: The positive rates of iNOS and MMP-9 expression were 71.88% (23/32) and 78.13% (25/32) in HCC. MMP-9 expression was significantly correlated with tumor size, capsule status, TNM stage, and risk of HCC recurrence (P = 0.032, P = 0.033, P = 0.007, and P = 0.001, respectively). There was also a significant relationship between iNOS expression and capsule status and risk of HCC recurrence (P = 0.049 and P = 0.004, respectively), but no correlation between iNOS expression and tumor size and TNM stage. There was a positive association between MVD and TNM stage and risk of HCC recurrence (P = 0.037 and P = 0.000, respectively). The count of MVD was significantly different in different iNOS and MMP-9 immunoreactivity groups (F = 17.713 and 17.097, P = 0.000 and P = 0.000, respectively). The examination of Spearman’s rank correlation coefficient showed that there was a
significant positive correlation between MVD and iNOS, MMP-9 immunoreactivity (r = 0.754 and 0.751, P = 0.000 and P=0.000, respectively). There was also a significant association between MMP-9 and iNOS expression in HCC (P = 0.010).
CONCLUSION: Nitric oxide (NO) produced by iNOS could modulate MMP-9 production and therefore contribute to tumor cell angiogenesis and invasion and metastasis in HCC. The strong expression of iNOS and MMP-9 in HCC may be helpful in evaluating the recurrence of HCC, predicting poor prognosis. For patients with strong expression of MMP-9 and iNOS, the optimal treatment scheme needs to be selected.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a highly vascular tumor characterized by a propensity for vascular invasion and distant metastasis. Angiogenesis is a prerequisite for tumor growth and metastasis[1]. In the process of invasion and metastasis of tumor cells, destruction of the extracellular matrix (ECM) is an essential initial step. Among the enzymes responsible for ECM degradation, several studies have shown a critical role played by matrix metalloproteinases (MMPs)[2]. MMP-9 (gelatinase B, 92-ku type IV collagenase) is capable of degrading the main components of the ECM, types IV and V collagen and gelatin[3-6]. Thus, its activities are closely related to the angiogenesis and invasiveness and metastasis of tumor cells[7,8]. However, it is secreted by cells as an inactive form, proMMP-9, which is then activated through an enzymatic cascade involving the generation of plasmin by urokinase-type plasminogen activator (u-PA)[9-12].
Nitric oxide (NO) is an important bioactive agent and a signaling molecule and may contribute to the pathogenesis of cancer[13]. Three distinct isoforms of NOS catalyze the formation of NO. Endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) are constitutively expressed in different tissues, whereas inducible nitric oxide synthase (iNOS) is related to a high-output pathway and is responsible for various pathological processes[14]. Although overexpression of iNOS has been demonstrated in various human neoplasms, such as colorectal cancer[15], pancreatic cancer[16], and breast cancer[17], its exact function in tumor biology is complex and remains to be fully defined. During the initiation of tumor growth, natural killer cells and macrophages kill tumor cells by a NO-mediated mechanism[18]. However, NO may also suppress the antitumor defense, promote tumor angiogenesis and blood flow in the tumor neovasculature, and enhance tumor growth, invasion and metastasis[18]. Recently, investigators have tended to attribute NO-mediated stimulation of invasiveness to an alteration in the balance between the productions of MMPs and TIMPs[19-21]. Since NO has been assessed for the ability to upregulate u-PA in endothelial cells of post capillary venules during the process of NO-mediated stimulation of angiogenesis[22], the u-PA can convert plasminogen to plasmin, which can activate numerous MMPs.
There is evidence that NO produced by iNOS enhances the activity of MMP-9[19-21]. Both iNOS and MMP-9 expression have been reported to be increased in bone and joint disease and cardiovascular disease[23-25]. However, the relationship between them in tumors has been very seldom studied. To determine the role of iNOS and MMP-9 in HCC, we compared their expressions by the immunohistochemical method.
MATERIALS AND METHODS
Materials
Thirty-two patients (24 males and 8 females, ranging in age from 16 to 69 years, mean 53 years) who had undergone curative hepatectomy for HCC at the Second Hospital, Jilin University, and Tumor Hospital, Jilin Province, between May 2001 and October 2002 were involved in this study. As controls, seven normal adult liver specimens were wedge biopsies obtained during surgeries from patients with rupture of liver and cavernous hemangioma of liver. Of the 32 patients, 21 were positive for hepatitis B surface antigen (HBsAg), while 11 were infected with neither hepatitis C virus nor HBV. Five patients showed tumor thrombi in the portal vein, 3 patients showed lymph node metastasis in the porta hepatis, 14 patients showed microsatellite nodules. The patients were regarded as a high-risk group for HCC recurrence, if any one of the following factors existed: lymph node metastasis of the porta hepatis; tumor thrombi in the portal vein; surrounding the primary tumor two or more microsatellite tumor nodules were present in the same lobe of liver, or the microsatellite tumor nodules scattered over the two or more liver lobes. Eighteen cases were in high recurrence-risk group and 14 cases in low recurrence-risk group. The histological grade of malignancy was classified according to the criteria of World Health Organization. There were 17 cases of G1, 9 cases of G2, and 6 cases of G3. The degree of TNM classification was decided according to the general rules for the clinical and pathological study of primary liver tumor. One patient was staged I, 15 patients were staged II, and 16 patients were staged III. None of our patients received preoperative treatments, such as chemotherapy or embolization therapy. The resected surgical specimens were fixed in 10% buffered formalin and embedded in paraffin. Four-micrometer-thick sections were prepared for H&E and immunohistochemical staining.
Methods
Immunohistochemistry Immunostaining was performed according to the avidin-biotin-peroxidase complex method. Sections were dewaxed in xylene, and dehydrated in ethanol, and then heated at 98 °C in EDTA-retrieved solution for the antigens. Endogenous peroxidase was blocked by 3% hydrogen peroxide for 60 min at room temperature. The samples were incubated for 20 min at room temperature with a protein-blocking solution containing 5% normal horse serum and 1% normal goat serum. Subsequently, slides were incubated with primary anti-iNOS rabbit polyclonal antibody (Boster Bio, Wuhan) at 1:200 dilution for 60 min at room temperature, mouse anti-human MMP-9 mAb (Maixin Bio, Fuzhou) at 1:200 dilution overnight at 4 °C, rat anti-mouse CD34 mAb (Maixin Bio, Fuzhou) at 1:100 dilution for 2 h at room temperature. Slides were then treated with a biotin-conjugated secondary antibody for 30 min followed by incubation with peroxidase-conjugated streptavidin for 60 min at room temperature. All steps were followed by washing in PBS. Finally, the sections were stained with a freshly prepared diaminobenzidine solution for 5 min and then counterstained with Mayer’s hematoxylin. For CD34 staining, counterstaining was not done. As a negative control, PBS was used instead of the primary antibody. Known immunostaining-positive slides were used as positive controls.
Evaluation of iNOS and MMP-9 expression
Two independent observers screened all sections as semiquantitative evaluation of iNOS and MMP-9 immunostaining. The immunoreactive score was determined by the sum of intensity and extension of staining[26]. The intensity of staining was scored on a scale of 0-3, in which 0, negative staining; 1, weakly positive staining; 2, moderately positive staining; and 3, strongly positive staining. The extent of distribution of positive cells was estimated on a scale of 0-3, in which 0, negative or positive staining in 1-5% of cells; 1, positive staining in 6-25% of cells; 2, positive staining in 26-50% of cells; 3, positive staining in 51-100% of cells. The combined staining score (intensity+extension) ³2 was considered as positive staining. The diagnosed grades accorded to the sum of two scores, -: 0-1; +: 2; ++: 3-4; and +++: >5.
Quantification of MVD
Microvessel density (MVD) was evaluated by two independent observers who were blinded to the patients’ clinicopathologic data after sections were immunostained with anti-CD34 antibodies according to the procedure described by Weidner et al[27]. In all tumors collected, the density of microvessels was higher in the peripheral tumor tissue close to the margin than in the central areas. Therefore, the peripheral tissue sections were used for counting of microvessels. Any brown-stained endothelial cells or endothelial cell clusters that was clearly separated from adjacent microvessels, tumor cells, or other stromal cells were counted as one microvessel. For MVD estimation, at low power magnification (×40 and ×100), the tissue sections were screened and five areas with the highest vessel density (hot spots) were selected. Microvessel counts of these areas were performed at a high power field (×200, the surface area of every vision field being 0.708 mm2) and the maximum microvessel count of the five richest vascular areas was taken as the MVD.
Statistical analysis
Qualitative data were expressed as the number of cases and quantitative data as mean±SD. These data were analyzed with the SPSS (version11.5) software. The qualitative data were compared using χ2 test and Fisher’s exact test, and the quantitative data were compared using one-way analysis of variance and independent sample t-test. Correlation between factors was evaluated using the Spearman’s rank correlation coefficient. P<0.05 was considered statistically significant.
RESULTS
Correlation between MMP-9 and clinicopathologic parameters in HCC
The incidence of positive MMP-9 expression in cases with their tumor being more than 5 cm, their tumor capsules being not integral or their tumor without capsules was significantly higher than that in those with their tumor measured either less than or equal to 5 cm in diameter and their tumor capsules being integral (χ2=5.283 and χ2=5.258, P=0.032 and P=0.033, respectively). The incidence of MMP-9 expression was also significantly higher in high-risk group for HCC recurrence and TNM III stage group than in low-risk group for HCC recurrence and TNM I-II stage group (χ2=11.160 and χ2=8.680, P=0.001 andP=0.007, respectively). At the same time no significant correlation was demonstrated between the expression of MMP-9 and other parameters, such as sex, age, ascites, hepatitis B, cirrhosis of liver, value of AFP, and grade of histology (Table (Table11).
Table 1
Correlation between clinicopathologic parameters and MMP-9, iNOS expression and MVD in HCC
| Variables | n (n=32) | MMP-9 | P | iNOS | P | MVD | P | ||
| +1 (n=25) | – (n=7) | + (n=23) | – (n=9) | (mean±SD) | |||||
| Sex | |||||||||
| Male | 24 | 20 | 4 | NS2 | 17 | 7 | NS | 68.50±17.38 | NS |
| Female | 8 | 5 | 3 | 6 | 2 | 80.64±29.51 | |||
| Age (yr) | |||||||||
| >50 | 19 | 17 | 2 | NS | 16 | 3 | NS | 76.11±18.86 | NS |
| ≤50 | 13 | 8 | 5 | 7 | 6 | 64.86±23.35 | |||
| Size of HCC (cm) | |||||||||
| >5.0 | 21 | 19 | 2 | 0 | 17 | 4 | NS | 74.48±18.16 | NS |
| ≤5.0 | 11 | 6 | 5 | 6 | 5 | 62.91±20.63 | |||
| Tumor capsule | |||||||||
| Present integrally | 15 | 9 | 6 | 0 | 8 | 7 | 0.05 | 67.73±25.72 | NS |
| Absent or not integral | 17 | 16 | 1 | 15 | 2 | 74.88±16.31 | |||
| HbsAg3 | |||||||||
| + | 21 | 18 | 3 | NS | 16 | 5 | NS | 75.48±19.02 | NS |
| – | 11 | 7 | 4 | 7 | 4 | 64.00±23.96 | |||
| Cirrhosis | |||||||||
| + | 19 | 16 | 3 | NS | 15 | 4 | NS | 72.79±16.30 | NS |
| – | 13 | 9 | 4 | 8 | 5 | 64.46±15.95 | |||
| Ascites | |||||||||
| + | 6 | 4 | 2 | NS | 3 | 3 | NS | 64.83±22.73 | NS |
| – | 26 | 21 | 5 | 20 | 6 | 73.08±20.99 | |||
| Serum AFP4 (µg/L) | |||||||||
| ≤400 | 13 | 10 | 3 | NS | 11 | 2 | NS | 68.84±21.77 | NS |
| >400 | 19 | 15 | 4 | 12 | 7 | 75.46±20.53 | |||
| Histological grade | |||||||||
| G1 | 17 | 12 | 5 | NS | 11 | 6 | NS | 65.94±19.14 | NS |
| G2 | 9 | 7 | 2 | 6 | 3 | 73.56±20.97 | |||
| G3 | 6 | 6 | 0 | 6 | 0 | 84.33±24.48 | |||
| TNM stage | |||||||||
| I–II | 16 | 9 | 7 | 0 | 9 | 7 | NS | 63.81±21.78 | 0.037 |
| III | 16 | 16 | 0 | 14 | 2 | 79.25±18.08 | |||
| Risk of recurrence | |||||||||
| High-risk group | 18 | 18 | 0 | 0 | 17 | 1 | 0 | 82.56±17.29 | 0 |
| Low-risk group | 14 | 7 | 7 | 6 | 8 | 57.36±17.19 | |||
Correlation between iNOS and clinicopathologic parameters in HCC
The positive immunohistochemical staining for iNOS in cases with their tumor capsules being not integral or their tumors without capsules was significantly higher than that in those with their tumor capsules being integral (χ2=4.652, P=0.049). The iNOS immunoreactivity was significantly higher in high-risk group for HCC recurrence than in low-risk group (χ2=10.043,P=0.004). But there was no significant difference between iNOS expression and each of the parameters such as sex, age, value of AFP, TNM stage, tumor size, grade of histology, hepatitis B, cirrhosis of liver, and ascites in HCC (Table (Table11).
CD34 expression and its relationship to clinicopathologic parameters in HCC
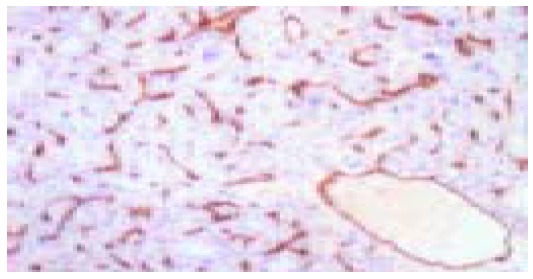
No CD34 staining was found in the sinusoids of seven healthy liver tissues. In HCC tissues from 32 cases, sinusoid-like tumor vessels reacted intensively with anti-CD34 (mean±SD, 71.53±21.19; range, 31-126), and the positive staining of CD34 distributed with a diffuse pattern or an intensive cluster pattern and was defined as the buffy zonale staining on the cell membrane (Figure (Figure1).1). In nonmalignant tissue, anti-CD34 stainings were confined to vessels of the portal triad with weak staining in few of the sinusoids at the periportal area in case of cirrhosis or chronic hepatitis. There was a significant difference in MVD between TNM III stage group (79.25±18.08) and TNM I-II stage group (63.81±21.78, t=2.182, P=0.037). There was also a significant difference in MVD between high-risk group for HCC recurrence (82.56±17.29) and low-risk group (57.36±17.19, t=4.100, P=0.000). But no significant association was noted between MVD and other clinicopathologic features (Table (Table11).
Relationship between iNOS, MMP-9 and MVD in HCC
As shown in Table Table2,2, tumors were divided into three groups based on the degree of immunoreactivity. Along with the increase of immunoreactivity grades, the incidence of iNOS and MMP-9 expression enhanced gradually. The count of MVD was significantly different in different iNOS and MMP-9 immunoreactivity groups (F=17.713 and 17.097, P=0.000 and P=0.000, respectively). The examination of Spearman’s rank correlation coefficient showed that there was a significant positive correlation between iNOS, MMP-9 immunoreactivity and MVD (r=0.754 and 0.751, P=0.000 and P=0.000, respectively).
Table 2
Relationship between MVD and MMP-9, iNOS staining intensity in HCC (mean±SD)
| Degree of staining | n | iNOS | n | MMP-9 |
| – | 9 | 49.33±11.24 | 7 | 48.57±14.62 |
| +++ | 11 | 71.82±15.06b | 8 | 62.50±11.98 |
| +++ | 12 | 87.92±16.49b | 17 | 85.24±16.02bd |
Correlation between MMP-9 and iNOS expression in 32 HCC tissues
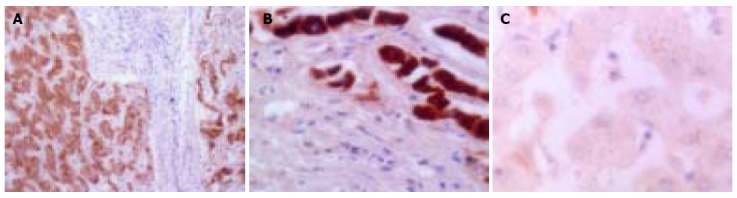
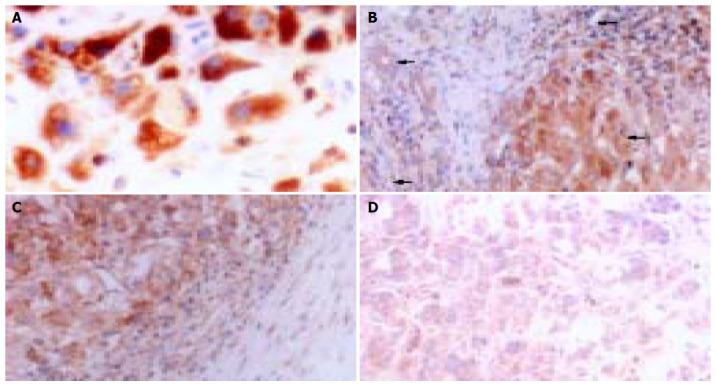
There was no MMP-9 staining in the normal liver tissue. MMP-9 immunoreactivity was detected in 25 (78.13%) of 32 HCCs. MMP-9 was mainly localized in cytoplasm and cytoplasmic membranes and its staining was detected in vascular endothelial cells, bile ducts, HCC cells, and stromal fibroblasts (Figures 2A and B). iNOS was expressed weakly in healthy liver tissue and mainly localized in tumor cytoplasm with a diffuse distribution pattern and a granular pattern. It was also found in cell membranes occasionally. There was no expression of iNOS in tumor nuclei or interstitial cells (Figure (Figure3A).3A). The common characteristics of MMP-9 and iNOS expression were that their stainings were more frequent in tumor cells localized in the anterior borders of invasion or neighboring capsules of tumor tissues (Figures (Figures2C2C and and3B)3B) than in those cells in central areas (Figures (Figures2D2D and and3C)3C) and that they displayed various degrees of intensities and inter-tumor heterogeneity. There was a significant correlation between MMP-9 and iNOS expression (χ2=8.052, P=0.010, Table Table33).
Immunohistochemical staining for MMP-9. A: MMP-9 in cytoplasm and cytoplasmic membranes in HCC (×400); B: MMP-9 in vascular endothelial cells, bile ducts, HCC cells, and stromal fibroblasts (arrow heads from top to bottom) within HCC (×200); C: MMP-9 expression in neighboring capsule of HCC (×200); and D: weak expression of MMP-9 in central areas of HCC tissues (×200).
Table 3
Correlation between MMP-9 and iNOS expression in 32 HCC tissues
| iNOS | MMP-9 | Total | P | |
| + | – | |||
| + | 21 | 2 | 23 | |
| – | 4 | 5 | 9 | 0.010 |
| Total | 25 | 7 | 32 | |
DISCUSSION
HCC is the fifth most common malignant disease in the world, causing almost one million deaths annually[28]. Surgical resection is the only proven cure for HCC, yet over 80%[29] of patients with HCC undergoing hepatectomy develop new tumors in the residual livers within 2 years. Further investigation of the pathological and biological factors of HCC in relation to recurrence and metastasis using molecular biology techniques will provide promising strategies for treatment and prevention of cancer invasion.
MMP-9 plays a key role in cancer invasion and metastasis by digesting native type IV collagen, which is a major structural component of basement membranes, since increased MMP-9 levels have been observed in various tumors such as HCC[30], bladder carcinoma[33], head, and neck cancer[19]. There is a trend toward a higher proportion of active MMP-9 with an increasing invasiveness of tumor cells. Our immunohistochemical study showed that the expression of MMP-9 was negative in the 7 normal liver tissues, and positive in 25 (78.13%) of 32 HCC tissues, suggesting that MMP-9 is produced by tumor cells. The strong staining in the marginal areas of tumor tissues suggests the close involvement of MMP-9 in the digestion of ECM. Moreover, positive staining of some fibroblasts and endothelial cells with anti-MMP-9 antibody suggests a participation of these cells in degradation of the ECM. The incidence of MMP-9 expression was significantly higher in high-risk group for HCC recurrence and TNM III stage group than in low-risk group and TNM I-II stage group, suggesting that overexpression of MMP-9 plays an important role in the progress of HCC, and that MMP-9 protein may serve as a marker for invasiveness and metastasis of HCC. These results are similar to the results reported by Arii et al[30] and Hayasaka et al[31]. In contrast to their study, there was also a significant correlation between MMP-9 expression and tumor size and capsule status of HCC. Both MMP-9 mRNA and activation of latent type of MMP-9 are significantly correlated with capsular infiltration of tumor[32]. Durkan et al[33] reported that MMP-9 staining is associated with tumor size. Therefore, the above data further indicate that MMP-9 plays an important role in the dispersion of HCC cells and may accelerate the growth of tumors by enhancing angiogenesis of tumors.
MVD is an important indicator to reflect the level of tumor angiogenesis[34]. The result of our study revealed that there was a positive association between the extent of neovascularization and the clinicopathologic characteristics related to aggressiveness, such as TNM stage and risk of recurrence, indicating that the MVD reflects the potentiality for HCC recurrence and metastasis. We also found that the MVD was statistically different in different MMP-9 immunoreactivity groups. There was a significant positive relationship between MMP-9 and MVD, the higher the MMP-9 expression, the higher the MVD. MMP-9 plays a vital role in the turnover of basement membrane collagen types IV and I, which exert a crucial effect on the formation of new capillary sprouts and neoangiogenesis. Based on the above facts, we speculate that MMP-9 expression may initiate angiogenesis and promote vascularization.
A large body of clinical and experimental data suggest that NO plays a promoting role in tumor progression and metastasis[35-37], though a few reports indicate that the presence of NO in tumor cells or their microenvironment may exert deleterious effects on the tumor cell survival and consequently their metastatic ability[13]. In the current study, we found that iNOS was expressed weakly in healthy liver tissues, suggesting that iNOS is associated with the physiological function of normal liver tissues. The expression of iNOS showed strong cytoplasmic staining in cancerous cells but not in tumor nuclei and interstitial cells, suggesting that NO is produced by HCC cells and directly or indirectly influences the fate of HCC cells. Here, we also demonstrated that the rate of iNOS expression in cases with their tumor capsules being not integral or their tumors without capsules was significantly higher than that in those with their tumor capsules being integral. The expression of iNOS in high recurrence-risk group was significantly higher than that in low recurrence-risk group, indicating that iNOS positivity is upregulated along with the biological aggressiveness of HCC lesions. It seems more likely that iNOS may increase the viability and infiltrative potential of HCC.
In this study, the count of MVD was statistically different in different iNOS immunoreactivity groups, the higher the iNOS expression the higher the MVD. NO has been implicated in all processes of angiogenesis in a manner consistent with a pre-angiogenic phenotype[38]. iNOS-transfected human colon adenocarcinoma DLD-1 cells have a higher vessel density and a higher growth rate in vivo than parental cells in mammary tumor model, suggesting that NO is a key mediator of C3L5 tumor-induced angiogenesis in NOS inhibitor-treated mice[39]. Therefore, the above data further indicate that local NO production by iNOS in HCC cells is able to enhance angiogenesis. It was reported that MMP-9 can also facilitate angiogenesis[40]. In this study, we found that there was a significant association between MMP-9 and iNOS. Both MMP-9 and iNOS positive expressions were more frequent in tumor cells localized in the anterior borders of invasion or neighboring capsules of tumor tissues and were associated with the capsule status and the risk of HCC recurrence, demonstrating that the effect of iNOS on angiogenesis can promote metastatic potential as well as tumor invasiveness by inducing HCC cells to produce MMP-9. To evaluate the mechanism, further investigations are needed.
In conclusion, there is a significant positive correlation between MMP-9 and iNOS expression, suggesting that NO produced by iNOS modulates MMP-9 production and therefore contributes to tumor cell angiogenesis, invasion, and metastasis in HCC.The strong expression of iNOS and MMP-9 in HCC may be helpful in evaluating the recurrence of HCC, predicting poor prognosis. For patients with strong expression of MMP-9 and iNOS, the optimal treatment scheme needs to be selected.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
Articles from World Journal of Gastroenterology are provided here courtesy of Baishideng Publishing Group Inc
Full text links
Read article at publisher's site: https://doi.org/10.3748/wjg.v11.i38.5931
Free to read at www.wjgnet.com
http://www.wjgnet.com/1007-9327/11/5931.asp
Free to read at www.wjgnet.com
http://www.wjgnet.com/abstract.asp?url=/1007-9327/11/5931
Free to read at www.wjgnet.com
http://www.wjgnet.com/downpdf.asp?url=/1007-9327/11/5931
Citations & impact
Impact metrics
Citations of article over time
Article citations
NOS2 and COX-2 Co-Expression Promotes Cancer Progression: A Potential Target for Developing Agents to Prevent or Treat Highly Aggressive Breast Cancer.
Int J Mol Sci, 25(11):6103, 01 Jun 2024
Cited by: 0 articles | PMID: 38892290 | PMCID: PMC11173351
Review Free full text in Europe PMC
Statin therapy: a potential adjuvant to immunotherapies in hepatocellular carcinoma.
Front Pharmacol, 15:1324140, 01 Feb 2024
Cited by: 2 articles | PMID: 38362156 | PMCID: PMC10867224
Review Free full text in Europe PMC
Effect of Percutaneous Biliary Drainage on Enzyme Activity of Serum Matrix Metalloproteinase-9 in Patients with Malignant Hilar Obstructive Hyperbilirubinemia.
Medicina (Kaunas), 59(2):336, 10 Feb 2023
Cited by: 0 articles | PMID: 36837539 | PMCID: PMC9958900
A correlative study between IVIM-DWI parameters and VEGF and MMPs expression in hepatocellular carcinoma.
Quant Imaging Med Surg, 13(3):1887-1898, 02 Jan 2023
Cited by: 2 articles | PMID: 36915336 | PMCID: PMC10006110
Transcriptional regulation of alcohol induced liver fibrosis in a translational porcine hepatocellular carcinoma model.
Biochimie, 182:73-84, 12 Jan 2021
Cited by: 7 articles | PMID: 33444661 | PMCID: PMC8356245
Go to all (39) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Expressions of inducible nitric oxide synthase and vascular endothelial growth factor and their relationship with microvessel density in hepatocellular carcinoma].
Ai Zheng, 24(1):99-103, 01 Jan 2005
Cited by: 2 articles | PMID: 15642211
Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer.
World J Gastroenterol, 8(4):591-595, 01 Aug 2002
Cited by: 93 articles | PMID: 12174362 | PMCID: PMC4656304
[Nitric oxide synthase and vascular endothelial growth factor expression in hepatocellular carcinoma and their relation to angiogenesis].
Zhonghua Zhong Liu Za Zhi, 22(4):301-303, 01 Jul 2000
Cited by: 3 articles | PMID: 11778555
Unveiling the significance of inducible nitric oxide synthase: Its impact on cancer progression and clinical implications.
Cancer Lett, 592:216931, 01 May 2024
Cited by: 0 articles | PMID: 38701892
Review