Abstract
Background
Octreotide is used off-label in infants for treatment of chylothorax, congenital hyperinsulinism, and gastrointestinal bleeding. The safety profile of octreotide in hospitalized infants has not been described; we sought to fill this information gap.Methods
We identified all infants exposed to at least 1 dose of octreotide from a cohort of 887,855 infants discharged from 333 neonatal intensive care units managed by the Pediatrix Medical Group between 1997 and 2012. We collected laboratory and clinical information while infants were exposed to octreotide and described the frequency of baseline diagnoses, laboratory abnormalities, and clinical adverse events (AEs).Results
A total of 428 infants received 490 courses of octreotide. The diagnoses most commonly associated with octreotide use were chylothorax (50%), pleural effusion (32%), and hypoglycemia (22%). The most common laboratory AEs that occurred during exposure to octreotide were thrombocytopenia (47/1000 infant-days), hyperkalemia (21/1000 infant-days), and leukocytosis (20/1000 infant-days). Hyperglycemia occurred in 1/1000 infant-days and hypoglycemia in 3/1000 infant-days. Hypotension requiring pressors (12%) was the most common clinical AE that occurred during exposure to octreotide. Necrotizing enterocolitis was observed in 9/490 (2%) courses, and death occurred in 11 (3%) infants during octreotide administration.Conclusion
Relatively few AEs occurred during off-label use of octreotide in this cohort of infants. Additional studies are needed to further evaluate the safety, dosing, and efficacy of this medication in infants.Free full text

Safety of octreotide in hospitalized infants
Abstract
Background
Octreotide is used off-label in infants for treatment of chylothorax, congenital hyperinsulinism, and gastrointestinal bleeding. The safety profile octreotide in hospitalized infants has not been described; we sought to fill this information gap.
Methods
We identified all infants exposed to at least 1 dose of octreotide from a cohort of 887,855 infants discharged from 333 neonatal intensive care units managed by the Pediatrix Medical Group between 1997 and 2012. We collected laboratory and clinical information while infants were exposed to octreotide and described the frequency of baseline diagnoses, laboratory abnormalities, and adverse events (AEs).
Results
A total of 428 infants received 490 courses of octreotide. The diagnoses most commonly associated with octreotide use were chylothorax (50%), pleural effusion (32%), and hypoglycemia (22%). The most common laboratory AEs that occurred during exposure to octreotide were thrombocytopenia (47/1000 infant-days), hyperkalemia (21/1000 infant-days), and leukocytosis (20/1000 infant-days). Hyperglycemia occurred in 1/1000 infant-days and hypoglycemia in 3/1000 infant-days. Hypotension requiring pressors (12%) was the most common clinical AE that occurred during exposure to octreotide. Necrotizing enterocolitis was observed in 9/490 (2%) courses, and death occurred in 11 (3%) infants during octreotide administration.
Conclusion
Relatively few AEs occurred during off-label use of octreotide in this cohort of infants. Additional studies are needed to further evaluate the safety, dosing, and efficacy of this medication in infants.
1. Introduction
Octreotide is a somatostatin analog that inhibits the release of growth hormone, glucagon, and insulin [1]. It decreases pancreatic secretion, gallbladder contraction, and gastrointestinal tract motility, and reduces intestinal blood flow by vasoconstriction of the splanchnic vessels [2,3]. Through these effects on the gastrointestinal tract, octreotide reduces fat absorption and lymphatic flow in the thoracic duct [2].
In adults, it is labeled for use in the treatment of acromegaly, carcinoid tumors, and vasoactive intestinal peptide tumors [4]; additionally, octreotide is often used off-label for several other indications, including acute esophageal variceal bleeding, tumor growth stabilization, tumor linkage, treatment of idiopathic pulmonary fibrosis, and acute pancreatitis [5–9]. Although octreotide is not labeled for use in children, case reports, case series, and cohort studies document the use of octreotide in children for several indications, including pancreatitis, chylothorax, and gastrointestinal bleeding [10–19]. In infants, the most common indication is treatment of congenital chylothorax, chylothorax secondary to thoracic surgery, congenital hyperinsulinism, and gastrointestinal bleeding [12–14]. Evidence of octreotide efficacy in this population is limited to small case series and retrospective studies [12,15,20].
The safety profile of octreotide has not been described in infants. In adults, the most frequent side effects are gastrointestinal events, glucose regulation disorders, hypothyroidism, and biliary tract abnormalities [4,21]. Based on small studies in children, side effects associated with octreotide include hyperglycemia, hypoglycemia, hypertension, hyperbilirubinemia, diarrhea, abdominal cramping and pain, and necrotizing enterocolitis (NEC) [12,15,16,22,23]. In the present study, we describe the safety profile of octreotide in a cohort of hospitalized infants.
2. Methods
2.1. Study design and setting
We identified all infants discharged from 333 neonatal intensive care units managed by the Pediatrix Medical Group from 1997 to 2012 who were exposed to at least one dose of octreotide. We used a database that prospectively captures information from daily progress notes, laboratory results, admission and discharge notes, and maternal information. Notes are generated by clinicians using a computer-assisted tool on all infants cared for by the Pediatrix Medical Group. For this study, we captured demographic data, discharge data, daily medications, dosing information, laboratory values, and diagnoses.
2.2. Definitions
Octreotide exposure was defined as any day an infant was receiving the drug. Exposure was evaluated at the day level and at the course level. An octreotide course was defined as the uninterrupted interval from start day of octreotide to end day of octreotide. If the first day of the next octreotide course was >1 day after the last day of the previous course, the next interval was considered a new course.
We defined octreotide indication by a diagnosis recorded prior to or during the octreotide course that matched reported indications for octreotide in the available literature [14,19,20,24,25]. All concomitant medications administered were captured during octreotide exposure.
Available laboratory and clinical information was collected while infants were exposed to octreotide. An adverse event (AE) was attributed to octreotide if it occurred between the start of octreotide exposure through the end of exposure. Laboratory abnormalities were classified as an AE or a serious adverse event (SAE) based on pre-specified cut-off values. Diagnoses included as AEs were NEC, focal intestinal perforation, intraventricular hemorrhage, periventricular leukomalacia, seizure, hypotension requiring pressors, and rash.
2.3. Statistical analysis
Standard summary statistics were used to describe demographic characteristics. Laboratory AEs were described on both the course level (number and percentage of courses with at least one abnormal laboratory result) and the infant-day level (number of days with abnormal labs per 1000 infant-days on octreotide); clinical AEs and concomitant medications were presented at the course level. At the course level, diagnoses, lab abnormalities, and concomitant medication that occurred more than once during the same course were only counted once. Octreotide use over time was described by the proportion of infants that received at least one course of octreotide out of the total number of infants in the database for each year during the study period. Dosing information was described if available.
All analyses were performed using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC, USA). The study was approved by the Duke University Institutional Review Board.
3. Results
Of the 887,855 infants discharged during the study period, 428 (0.05%) received 490 courses of octreotide. Infants exposed to octreotide had a median birth weight of 2290 g (interquartile range; 1110, 3090), median gestational age (GA) of 33 weeks (28, 37), and 314 (74%) were <37 weeks GA. On the first day of octreotide exposure, the median postnatal age was 27 days (11, 63), 225 (53%) infants were receiving mechanical ventilation, and 68 (16%) infants were receiving inotropes (Table 1).
Table 1
| N = 428 | |
|---|---|
| Gestational age (weeks) | 33 (28, 37) |
| Birth weight (g) | 2290 (1110, 3090) |
| Delivery by Cesarean section, n (%) | 277 (66) |
| Male, n (%) | 233 (55) |
| Race/ethnicity, n (%) | |
 White White | 199 (49) |
 Black Black | 68 (17) |
 Hispanic Hispanic | 121 (30) |
 Other Other | 20 (5) |
| Inborn, n (%) | 269 (63) |
| Postnatal age (days) | |
 On first octreotide day On first octreotide day | 27 (11, 63) |
| Weight (g) | |
 On first octreotide day On first octreotide day | 3050 (2290, 3815) |
| Mechanical ventilation, n (%) | |
 On first octreotide day On first octreotide day | 225 (53) |
| Inotropes, n (%) | |
 On first octreotide day On first octreotide day | 68 (16) |
| Length of stay (days) | 62 (32, 115) |
| Any major congenital anomaly | 239 (56) |
| Congenital heart disease | 112 (26) |
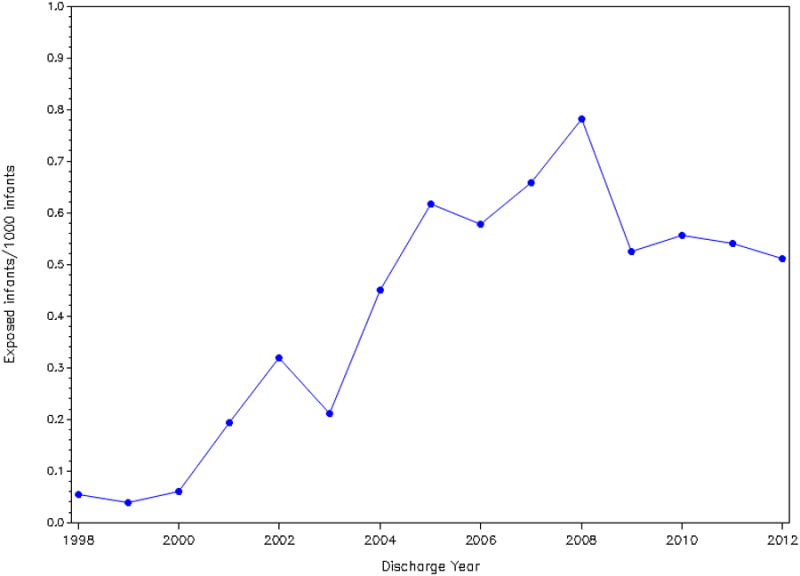
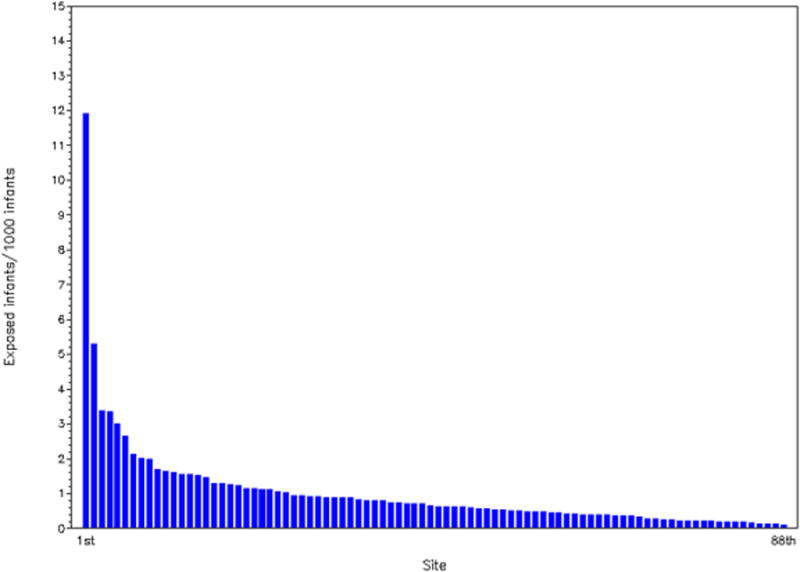
During the study period, the proportion of hospitalized infants given octreotide ranged from <0.1/1000 infants to 0.8/1000 infants (Figure 1). Octreotide use increased after 1998 but has remained relatively constant since 2005, with octreotide use ranging from 0.6/1000 infants to 0.8/1000 infants. Octreotide exposure varied greatly by site and ranged from 0.1 to 12/1000 infants (Figure 2).
The most common indications for octreotide use were chylothorax (245/490 [50%]), pleural effusion (158/490 [32%]), and hypoglycemia (110/490 [22%]) (Table 2). The most common concomitant medications included vancomycin (259/490 [53%]), gentamicin (220/490 [45%]), furosemide (217/490 [44%]), fentanyl (165/490 [34%]), and midazolam (154/490 [31%]) (Table 3).
Table 2
| Courses, n (%)* N = 490 | |
|---|---|
| Chylothorax | 245 (50) |
| Pleural effusion | 158 (32) |
| Hypoglycemia | 110 (22) |
| Gastrointestinal hemorrhage | 48 (10) |
| Bloody stools | 25 (5) |
| Pericardial effusion – acute | 13 (3) |
| Lymphangiectasia | 3 (1) |
| Congenital lymphedema | 1 (0.2) |
Table 3
| Courses, n (%) N = 490 | |
|---|---|
| Vancomycin | 259 (53) |
| Gentamicin | 220 (45) |
| Furosemide | 217 (44) |
| Fentanyl | 165 (34) |
| Midazolam | 154 (31) |
| Morphine | 133 (27) |
| Albumin | 112 (23) |
| Albuterol | 96 (20) |
| Ampicillin | 94 (19) |
| Dopamine | 93 (19) |
| Ranitidine | 81 (17) |
| Acetaminophen | 65 (13) |
Dosing information was available for 78/490 (16%) of the courses. Infants received the drug by continuous infusion in most of the courses (60/78 [77%]). The median dose by continuous infusion was 2 mcg/kg/hour (1, 6), and the median dose by intermittent administration was 7.5 mcg/kg/day (2, 20).
The most common laboratory AEs that occurred during exposure to octreotide were thrombocytopenia (47/1000 infant-days), hyperkalemia (21/1000 infant-days), and leukocytosis (20/1000 infant-days). The most common laboratory SAEs during exposure to octreotide were elevated blood urea nitrogen (5/1000 infant-days), elevated direct bilirubin (5/1000 infant-days), elevated gamma-glutamyl transpeptidase (5/1000 infant-days), and leukocytosis (5/1000 infant-days). Hyperglycemia >250 mg/dl occurred in 1/1000 infant-days (1%) and hypoglycemia <40 mg/dl in 3/1000 infant-days (2% of octreotide courses) (Table 4).
Table 4
| Serum electrolytes | Adverse Events | Serious Adverse Events | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Courses, n (%) N=490 | Days (/1000 infant-days) N=7793 | Courses, n (%) N=490 | Days (/1000 infant-days) N=7793 | |||
| Hyperglycemia | > 250 mg/dL | 6 (1) | 7 (1) | > 400 mg/dL | 0 | 0 |
| Hypoglycemia | < 40 mg/dL | 11 (2) | 22 (3) | < 20 mg/dL | 3 (1) | 3 (0.4) |
| Hypernatremia | > 150 mmol/L | 15 (3) | 23 (3) | > 160 mmol/L | 1 (0.2) | 1 (0.1) |
| Hyponatremia | < 125 mmol/L | 19 (4) | 27 (4) | < 115 mmol/L | 1 (0.2) | 1 (0.1) |
| Hyperkalemia | > 6 mmol/L | 98 (20) | 161 (21) | > 7.5 mmol/L | 11 (2) | 15 (2) |
| Hypokalemia | < 3 mmol/L | 63 (13) | 102 (13) | < 2.5 mmol/L | 12 (2) | 19 (2) |
| Hypercalcemia | > 12.5 mg/dL | 2 (0.4) | 4 (1) | > 13.5 mg/dL | 2 (0.4) | 3 (0.4) |
| Renal dysfunction | ||||||
| Elevated BUN | > 70 mg/dL | 41 (8) | 126 (16) | > 100 mg/dL | 19 (4) | 42 (5) |
| Elevated creatinine | > 1.7 mg/dL | 15 (3) | 49 (6) | > 3.0 mg/dL | 7 (1) | 8 (1) |
| Liver dysfunction | ||||||
| Elevated AST | >500 U/L | 6 (1) | 9 (1) | > 1000 U/L | 1 (0.2) | 1 (0.1) |
| Elevated ALT | > 500 U/L | 2 (0.4) | 3 (0.4) | > 1000 U/L | 0 | 0 |
| Elevated GGT | > 100 U/L | 40 (8) | 91 (12) | > 200 U/L | 22 (4) | 39 (5) |
| Direct bilirubin | > 5 mg/dL | 49 (10) | 117 (15) | >10 mg/dL | 16 (3) | 39 (5) |
| Complete blood count | ||||||
| Leukocytosis | > 25,000/mm3 | 56 (11) | 152 (20) | > 40,000/mm3 | 15 (3) | 38 (5) |
| Leukopenia | < 5000/mm3 | 41 (8) | 80 (10) | < 2000/mm3 | 1 (0.2) | 1 (0.1) |
| Thrombocytopenia | < 100,000/mm3 | 88 (18) | 368 (47) | < 30,000/mm3 | 9 (2) | 11 (1) |
| Thrombocytosis | > 600,000/mm3 | 22 (5) | 35 (5) | > 1,000,000/mm3 | 2 (0.4) | 3 (0.4) |
BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase.
The most common clinical AE that occurred during octreotide exposure was hypotension requiring pressors in 57/490 (12%) courses (Table 5). Rash was present in 7/490 (1%) courses, and surgical NEC occurred in 1/490 (0.2%) course. There were 10/428 (2%) infants with gastrointestinal events: 9 with NEC, and one with focal intestinal perforation. Dosing information was available for 3 infants who had NEC. One infant received 10 mcg/kg/day from day of life (DOL) 71–79 and had NEC on DOL 141. The second infant received a continuous infusion of 10 mcg/kg/hour from DOL 6–17 and had NEC on DOL 17. The third infant received 1 mcg/kg/hour from DOL 1–55 and had NEC on DOL 50. A total of 79/428 (19%) infants ever exposed to octreotide died while in the hospital, and 11/428 (3%) died during octreotide use.
Table 5
| Courses, n (%) N=490 | |
|---|---|
| Gastrointestinal | |
 Necrotizing enterocolitis–medical Necrotizing enterocolitis–medical | 8 (2) |
 Necrotizing enterocolitis–surgical Necrotizing enterocolitis–surgical | 1 (0.2) |
 Focal intestinal perforation Focal intestinal perforation | 1 (0.2) |
| Neurologic | |
 Intraventricular hemorrhage Intraventricular hemorrhage | 2 (0.4) |
 Periventricular leukomalacia Periventricular leukomalacia | 2 (0.4) |
 Seizure Seizure | 4 (1) |
| Cardiovascular | |
 Hypotension requiring pressors Hypotension requiring pressors | 57 (12) |
| Dermatologic | |
 Rash Rash | 7 (1) |
| Any clinical adverse event | 70 (14) |
4. Discussion
In our cohort of hospitalized infants, octreotide use was rare but increased over time. Octreotide use coincided with several AEs, and most AEs identified were laboratory abnormalities. AEs are defined by the U.S. Food and Drug Administration (FDA) as any untoward medical occurrence associated with the use of a drug in humans, whether or not considered drug-related [26]. AEs associated with octreotide use in our population could be related with the drug or with the underlying disease; however, we do not have the ability to determine causation from these data. Concomitant medications could also be responsible for the AEs, as well as the interaction between the different drugs.
Hypoglycemia and hyperglycemia are expected adverse reactions caused by octreotide action on insulin/glucagon balance [4]. Octreotide has a potent inhibitory effect on growth hormone secretion and a transient inhibitory effect on insulin and glucagon secretion [27]. Hyperglycemia and hypoglycemia are commonly described AEs in retrospective studies and case reports in children receiving octreotide [15,28–30]. However, in our cohort, hyperglycemia and hypoglycemia occurred in only 1/1000 and 3/1000 infant-days, respectively.
Thrombocytopenia was identified as an AE associated with octreotide in post-marketing reports [4]. In our study, thrombocytopenia was the most common laboratory AE, occurring in 47/1000 infant-days. Severe thrombocytopenia (<30,000/mm3) occurred in 1/1000 infant-days. Although our results could be a drug-related AE, thrombocytopenia is not uncommon in hospitalized, critically ill infants and is usually associated with septicemia [31]. Premature infants have higher incidence of thrombocytopenia (>10%), and 73% of infants in our cohort were <37 weeks GA [31]. The majority of infants in our cohort were critically ill; 52% were receiving mechanical ventilation, and 14% were receiving inotropes when the first use of octreotide occurred.
Biliary abnormalities (gallstones, sludge without gallstones, and biliary duct dilation) are AEs reported with octreotide use on the FDA label [4]. Gallbladder stasis is increased and intestinal transit is prolonged after octreotide use [32,33]. Octreotide also alters hepatic bile composition, decreasing bile pH and increasing the risk of cholesterol and calcium bilirubinate precipitation [33]. In children, previous single-center retrospective studies did not report biliary abnormalities associated with octreotide [30,34,35]. However, a case report described a 33-week-GA infant receiving octreotide for congenital hyperinsulinism who developed cholelithiasis after five weeks of treatment [36]. In our study, only one infant had cholelithiasis, which was diagnosed on DOL 113. This infant received octreotide from DOL 4–55.
Octreotide reduces intestinal blood flow by vasoconstriction of the splanchnic vessels, causing concern that it may increase the risk of NEC [3]. Infants with NEC requiring surgery have mortality higher than 50% [37]. Short- and long-term morbidities associated with NEC include intestinal failure, short bowel syndrome, intestinal strictures, and neurodevelopment impairment [23,38]. Two case reports described NEC occurring within 72 hours after octreotide initiation. The first was an infant with 2600 g birth weight who received octreotide for treatment of chylothorax diagnosed two weeks after a coarctation repair; the second, a 22-day-old, full-term infant who received octreotide for persistent hypoglycemia treatment [22,39]. However there is a concern that octreotide may be associated with NEC, we observed an incidence of only 2% (10/428) of NEC in our infants exposed to octreotide, which is similar to a previously described cohort of hospitalized infants (1.2% [6460/560,227]) [35].
Although octreotide use has increased over time (Figure 1), the frequency of its use is still relatively low, with <0.1% of hospitalized infants exposed to octreotide. This may be true not only because safety and efficacy studies are lacking but also because of the low incidence of the diagnoses for which octreotide is used. The diagnosis most commonly associated with octreotide use in our cohort was chylothorax, and, in our database, the overall incidence of chylothorax was 0.08%.
Our study has several strengths, including a relatively large sample size compared with previous reports in infants. We had information on a day-level for each infant receiving octreotide in our database. We were also able to collect information on concomitant medications and diagnoses, in addition to associated laboratory AEs. Limitations of this cohort study include our inability to infer causality with reported AEs and our inability to include a comparison group of infants given the uniqueness of infants who are exposed to octreotide. We also did not have access to cause of death.
5. Conclusion
Octreotide is an understudied drug used off-label in critically ill infants. Relatively few AEs occurred during off-label use of octreotide in this cohort of infants. Additional studies are needed to further define the safety, dosing, and efficacy of octreotide in this population.
Acknowledgments
Funding source: This work was funded under NICHD contract HHSN27500016 for the Pediatric Trials Network. This work was also supported by the American Recovery and Reinvestment Act, DHHS-1R18AE000028-01) (P.B.S). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement: P.B.S. receives salary support for research from the National Institutes of Health (NIH) and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Child Health and Human Development (HHSN2752010000031 and 1R01-HD081044-01) and the Food and Drug Administration (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). C.P.H. receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117).
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.earlhumdev.2015.04.008
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4450124?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.earlhumdev.2015.04.008
Article citations
Standardised practices in the networked management of congenital hyperinsulinism: a UK national collaborative consensus.
Front Endocrinol (Lausanne), 14:1231043, 30 Oct 2023
Cited by: 2 articles | PMID: 38027197 | PMCID: PMC10646160
Review Free full text in Europe PMC
Neutrophil-to-lymphocyte ratio is prognostic factor of prolonged pleural effusion after pediatric cardiac surgery.
JRSM Cardiovasc Dis, 10:20480040211009438, 01 Jan 2021
Cited by: 2 articles | PMID: 34262699 | PMCID: PMC8252915
Propranolol for the Treatment of Lymphatic Malformations in a Neonate - A Case Report and Review of Literature.
J Pediatr Pharmacol Ther, 25(2):155-162, 01 Jan 2020
Cited by: 6 articles | PMID: 32071591 | PMCID: PMC7025746
Medication-induced hyperglycemia: pediatric perspective.
BMJ Open Diabetes Res Care, 8(1):e000801, 01 Jan 2020
Cited by: 14 articles | PMID: 31958298 | PMCID: PMC6954773
Review Free full text in Europe PMC
Creation of a Multicenter Pediatric Inpatient Data Repository Derived from Electronic Health Records.
Appl Clin Inform, 10(2):307-315, 01 Mar 2019
Cited by: 10 articles | PMID: 31067576 | PMCID: PMC6506334
Go to all (10) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Safety of milrinone use in neonatal intensive care units.
Early Hum Dev, 91(1):31-35, 26 Nov 2014
Cited by: 13 articles | PMID: 25460254 | PMCID: PMC4302030
Octreotide use and safety in infants with hyperinsulinism.
Pharmacoepidemiol Drug Saf, 26(1):26-31, 02 Dec 2016
Cited by: 22 articles | PMID: 27910218 | PMCID: PMC5286465
Use of Propranolol in the Treatment of Chylous Effusions in Infants.
Pediatrics, 148(1):e2020049699, 29 Jun 2021
Cited by: 8 articles | PMID: 34187907
Octreotide therapy for chylothorax in infants and children: A brief review.
Pediatr Crit Care Med, 7(6):576-579, 01 Nov 2006
Cited by: 30 articles | PMID: 16878051
Review
Funding
Funders who supported this work.
ASPE HHS (1)
Grant ID: DDHHS-1R18AE000028-01
FDA HHS (2)
Grant ID: 1R18-FD005292-01
Grant ID: R18 FD005292
NCATS NIH HHS (2)
Grant ID: UL1 TR001117
Grant ID: UL1TR001117
NICHD NIH HHS (7)
Grant ID: HHSN275201000003I
Grant ID: R01 HD081044
Grant ID: 1R01-HD081044-01
Grant ID: HHSN275201000001Z
Grant ID: K24 HD058735
Grant ID: HHSN275201000001G
Grant ID: HHSN275201000003C