Abstract
Free full text

Polymorphisms in Dopamine Transporter (SLC6A3) are Associated with Stimulant Effects of d-Amphetamine: An Exploratory Pharmacogenetic Study Using Healthy Volunteers
Abstract
Individuals vary in their subjective responses to stimulant drugs, and these differences are believed to be partially genetic in origin. We evaluated associations between mood, cognitive and cardiovascular responses to d-amphetamine and four polymorphisms in the dopamine transporter (SLC6A3): rs460000, rs3756450, rs37022 and rs6869645. Healthy Caucasian male and female volunteers (N = 152) participated in a double-blind, crossover design study in which they received placebo, 10 and 20 mg of d-amphetamine. We measured self-reported rating of mood, performance on the Digit Symbol Substitution Task, blood pressure and heart rate. Individuals with the C/C genotype at rs460000 (N = 83) reported approximately twofold higher ratings of stimulation and euphoria relative to the A/A+A/C (N = 69) genotype group, at both the 10 and 20 mg doses. No other responses or SNPs showed significant effects. rs460000 is in perfect LD with rs463379 (CEU: D′ = 1; r2 = 1), which was not studied here, but has been associated with etiology of Attention Deficit Hyperactivity Disorder (ADHD). These findings suggest a pleiotropic effect of this polymorphic locus on both ADHD and sensitivity to the subjective effects of amphetamine.
Introduction
Individuals vary in their therapeutic and acute behavioral responses to stimulant drugs. This variability has been observed in clinical populations, such as patients treated for Binge Eating Disorder (Davis et al. 2007), Attention-Deficit Hyperactivity Disorder (Spencer etal. 1996) and narcolepsy (Mitler et al. 1993). Variability has also been reported in healthy volunteers, who receive acute doses of stimulants in laboratory studies (Brauer and de Wit 1996; Lott et al. 2005; de Wit et al. 1986; Gabbay 2003). In particular, healthy volunteers differ in subjective ratings of drug-induced mood effects, including mood effects that are linked to the drugs’ potential for abuse. One possible source of individual variability is in the dopamine transporter (SLC6A3), which is a direct target of amphetamine. Through its actions on the dopamine transporter, amphetamine blocks the reuptake of dopamine and causes reverse transport of intracellular dopamine into the synapse (Jones et al. 1998; Di Chiara and Imperato 1988, Schiffer et al. 2006). In humans, positron emission tomography studies demonstrate that cocaine-induced euphoria is correlated with the extent to which cocaine binds the dopamine transporter (Volkow et al. 2004). In animals, knockout mice lacking SLC6A3 show a reduced locomotor response to amphetamine (Spielewoy et al. 2001).
SLC6A3 is located on chromosome 5p15.33 and consists of 15 exons. The most commonly studied polymorphism in this gene is the variable number of tandem repeat polymorphism in the 3′ untranslated region of this gene (3′-UTR VNTR). This polymorphism has been associated with both Attention Deficit Hyperactivity Disorder (ADHD) and response to stimulants (Cook et al. 1995; Waldman et al. 1998; Daly et al. 1999; Bakker et al. 2005; Langley et al. 2005; Feng et al. 2005; Purper-Ouakil et al. 2005; Li et al. 2006; Lott et al. 2005). Recent studies indicate that single marker polymorphisms (SNPs) or haplotypes of SLC6A3 in the 5′ region may be also associated with ADHD and related phenotypes (Friedel et al. 2007; Brookes et al. 2006; Lasky-Su et al. 2006). These newly identified SNPs are not in linkage disequilibrium with the 3′-UTR VNTR, suggesting that there are other important polymorphisms within this gene.
In this study we evaluated four SNPs in SLC6A3. The first SNP, rs3756450, has been implicated in risk for schizophrenia (Talkowski et al. 2008). The second SNP, rs460000, is in perfect linkage disequilibrium with a number of neighboring SNPs that have been associated with ADHD and related haplotypes (Friedel et al. 2007; Lasky-Su et al. 2006). The remaining two SNPs have not been previously associated with any phenotypes. These SNPs may provide some insight into the role of variability in this gene, specifically on genetic variability related to response to amphetamine. We examined the relationship between these SNPs and measures of the subjective, cognitive and physiological response to amphetamine.
Methods
Participants
Healthy Caucasian male and female volunteers (N = 152), aged 18–35 years, were recruited by posters, advertisement and word-of-mouth referrals. In order to reduce variability experienced from tolerance and withdrawal from nicotine or caffeine, we excluded subjects who reported smoking more than 10 cigarettes per week or consumed more than three cups of coffee per day. All subjects underwent a semi-structured psychiatric screening interview based on selected modules from the Diagnostic and Statistical Manual (First et al. 1995), and completed a psychiatric symptom checklist (SLC-90; Derogatis 1983), the Michigan Alcoholism Screening Test (MAST; Selzer 1971), and a health questionnaire with a detailed section on current and lifetime drug use. Subjects who had any current medical condition requiring medication, any Axis I psychiatric disorder, any treatment for a substance use disorder or a history of personal or legal problems related to drug use, or any current or past medical condition considered to be a contraindication to d-amphetamine (such as abnormal EKG or hypertension) were excluded from the study. Candidates had to speak English and have at least high school education. Their body mass index (BMI) had to be in the range between 19 and 26 kg/m2. Because women show a dampened response to d-amphetamine during the luteal phase of the menstrual cycle (White et al. 2002), female participants were scheduled to be in the study during the follicular phase only. Women who were pregnant or lactating, or planning to become pregnant during the study were excluded from participating in the study.
Design
This within-subject design study consisted of three 4-h sessions, separated by at least 48 h. Subjects received placebo, d-amphetamine 10 mg and d-amphetamine 20 mg in random order under double-blind conditions. A subset of subjects also received a 5 mg dose, but these data are not included to maximize the power of the analysis. d-amphetamine (Mallinkrodt, MO) was placed in size 00 capsules with dextrose filler. Placebo capsules contained dextrose only. d-amphetamine and placebo were administered in randomized order and under double-blind conditions. The study was approved by The University of Chicago Institutional Review Board and was performed in accordance with the Helsinki Declaration of 1975.
Volunteers first completed an orientation session in which the study procedures were explained. They signed the consent form and provided a blood sample for DNA extraction. They completed self-questionnaires and practiced computerized tests used in the study. Subjects were instructed to abstain from taking drugs, including alcohol, caffeine and nicotine, 24 h before each session and to fast from midnight the night before the sessions. The three experimental sessions were conducted from 09:00 to 13:00 h. Before the start of every session, subjects gave urine and breath samples to ensure their compliance with non-use of alcohol and other drugs. They received a light breakfast and at 9:00 h their baseline ratings of mood, blood pressure and heart rate were taken. Subjects were tested individually, and remained in a comfortably furnished room with television and reading material for the 4-h session. They could watch emotionally neutral movies and read during the sessions when measurements were not being taken, but they were not allowed to study. At 09:30 h, subjects ingested a capsule containing d-amphetamine (10 or 20 mg) or placebo with a glass of water. For blinding purposes, they were informed the capsule might contain a stimulant, sedative, or placebo. Self-reported drug effect questionnaires, heart rate and blood pressure were obtained 30, 60, 90, 150, and 180 min after ingestion of the capsule. A cognitive performance measure was administered once at 120 min after capsule injection At 13:00 h subjects left the laboratory. After completing all three sessions subjects were debriefed and paid.
Dependent measures
The Addiction Research Center Inventory (Martin et al. 1971) is a 49-item questionnaire consisting of five scales corresponding to typical effects of psychoactive drugs. The five scales are: (1) stimulation (ARCI A—Amphetamine scale), (2) euphoria (ARCI MBG—Morphine-Benzedrine Group scale), (3) intellectual efficacy and energy (ARCI BG—Benzedrine Group scale), (4) sedation (ARCI—PCAG Pentobarbital-Chlorpromazine-Alcohol Group scale) and (5) dysphoria (ARCI LSD—Lysergic Acid Diethylamide scale). The ARCI scales stimulation, euphoria, and intellectual efficacy and energy were selected as primary outcome measures to capture the prototypic effects of d-amphetamine.
The Digit Symbol Substitution Test (DSST; Wechsler 1958) is a test of visuo-spatial and motor speed-of-processing that is also a sensitive measure of frontal lobe executive functions (Vilkki and Holst 1991; Parkin and Java 1999). The DSST is a pencil and paper test in which subjects are required to substitute a series of numbers and symbols within 90 s. The number of correct responses within 90 s is reported. One point is given for each correctly drawn symbol.
Physiological measures included heart rate and blood pressure and were measured at regular intervals using a CritikonDinamap 1846 SX/P Version 089 monitor.
Genotyping
Four SLC6A3 SNPs were included in the Addictions Array (Hodgkinson et al. 2008). The genotyping was based on the Illumina GoldenGate platform. Arrays were imaged using an Illumina Beadstation GX500 and the data analyzed using GenCall v6.2.0.4 and GTS Reports software v5.1.2.0 (Illumina). Criteria for sample exclusion and classification as genotyping failure were previously described (Hodgkinson et al. 2008). A panel of 186 ancestry informative SNPs were also included on the array, as previously described (Hodgkinson et al. 2008). We used Structure 2.1 (Pritchard et al. 2000) to confirm self-reported and experimenter observed Caucasian designations. All subjects were confirmed as Caucasian based on this analysis. Linkage disequilibrium (r2) values between the SNPs reported in this study were <0.5. Genotypiong error rate was <1% according to concordance between duplicate samples.
Statistical analysis
First we ensured that the genotypic groups for each SLC6A3 SNP were similar in terms of gender, BMI, education, age, current substance abuse and lifetime substance use, using ANOVA for continuous measures or χ2 tests for categorical measures. Age, BMI and gender were also assessed in relation to drug responses. If they were correlated with drug responses they were included as covariates in further analyses. Using these criteria, sex was included in the analysis of rs6869645 and body mass index was included for all analyses of diastolic blood pressure.
Area under the curve for subjective ratings of mood (stimulation, euphoria, and intellectual efficacy and energy), blood pressure and heart rate was calculated by multiplying the average of each pair of consecutive observations by the corresponding time interval and then summing all such values starting with the first time point and ending with the last, as described in Matthews et al. (1990).
The genotype-independent drug effects were assessed using repeated measure ANOVA’s separately for each outcome measure. To analyze the impact of genotypes on drug response, repeated measure ANOVAS or ANCOVAs (when covariates were included) were performed for each outcome measure. Genotype was used as a grouping factor and AUC for placebo, 10 and 20 mg d-amphetamine were used as within-subjects factors. Post hoc analyses were conducted when the interaction between genotype and dose was significant. The P-value was set at P < 0.05 (twotailed) for all analyses. Because we viewed this as a pilot study we chose not to apply a correction for multiple comparisons.
Results
Administration of d-amphetamine produced the expected responses on all 5 scales of the ARCI. It increased scores on the stimulation scale F(2,302) = 58.7, P ≤ 0.001,
Administration of d-amphetamine also produced the typical effects on the physiological measures blood pressure and heart rate and on the DSST. d-amphetamine increased systolic blood pressure F(2,302) = 110.92, P ≤ 0.001,
Table 1 shows the genotype frequencies for the 4 SNPs included in the study. The frequencies were similar to the frequencies reported in the HapMap project. Because all SNPs had a minor allele frequency of <0.1, we pooled the homozygotes of each minor allele with the heterozygotes; this approach precluded the detection of recessive effects associated with the rare allele; however, due to the sample size, we did not have sufficient power to detect such effects.
Table 1
Allele and genotype frequencies of the SLC6A3 SNP’s investigated in this study
| SNP | Reference allele | Other allele | Genotype | ||
|---|---|---|---|---|---|
| rs3756450 | T | C | T/T | T/C | C/C |
| 269(88.5) | 35(11.5) | 120(78.9) | 29(19.1) | 3(2.0) | |
| rs460000 | C | A | C/C | A/C | A/A |
| 227(75.0) | 77(25.0) | 83(54.6) | 61(40.1) | 8(5.3) | |
| rs37022 | A | T | A/A | A/T | T/T |
| 247(81.2) | 57(18.8) | 100(65.8) | 47(31.0) | 5(3.2) | |
| rs6869645 | C | T | C/C | C/T | T/T |
| 286(94.0) | 18(6.0) | 134(88.2) | 18(11.8) | 0(0) |
Values represent n (%)
Table 2 provides a summary of demographic characteristics and drug use histories. Most of the subjects were in their twenties with at least some college education. They were moderate caffeine and alcohol drinkers and low cigarette and marijuana smokers. The genotypic groups formed by the four SNPs in SLC6A3 did not differ on most demographic characteristics. However, for rs6869645, which consisted of 18 C/T and 134 C/C individuals, there were significantly more male subjects in the C/T group (males = 14, females = 4) than in the C/C group (males = 71, females = 63). As a result gender was included as a covariate for all analyses of rs6869645.
Table 2
Demographic characteristics and drug use histories of the participants (N = 152)
| Age (years; mean ± SD) | 22.8 ± 3.4 |
| Gender: Male/female | 85/67 |
| BMI (Mean ± SD) | 22.6 ± 2.2 |
| Education (n) | |
 High school High school | 2 |
 Some college Some college | 64 |
 College degree College degree | 68 |
 Advanced degree Advanced degree | 18 |
| Current substance use (Mean ± SD) | |
 Alcohol (drinks per week) Alcohol (drinks per week) | 4.6 ± 03.7 |
 Cigarettes (per week) Cigarettes (per week) | 0.7 ± 01.8 |
 Marijuana (uses per month) Marijuana (uses per month) | 0.9 ± 2.3 |
 Caffeine (cups per week) Caffeine (cups per week) | 7.3 ± 7.0 |
| Lifetime substance use (% ever used) | |
 Marijuana Marijuana | 60.0 |
 Stimulants Stimulants | 31.6 |
 Opiates Opiates | 27.0 |
 Tranquilizers Tranquilizers | 7.5 |
In the absence of drug administration there were no robust differences on the ARCI scores between SLC6A3 genotypic groups; however, subjects in the T/T genotype group for SNP rs3756450 scored slightly lower on the baseline stimulation scale (2.02 ± 1.03) compare to subjects in the C/T+C/C genotype group (2.53 ± 1.0; P = 0.013).
For the SNP rs460000 there were significant differences in the response to amphetamine. We identified a significant genotype x dose interaction for both the stimulation scale F(2,300) = 4.42, P = 0.015
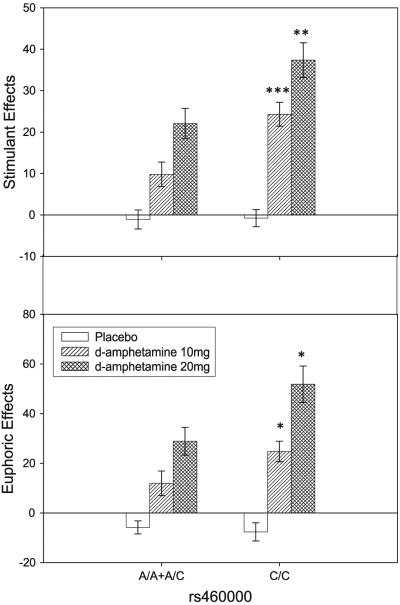
Mean area under the curve ± SEM for stimulation scale (top panel) and euphoria scale (bottom panel) for both rs460000 genotypic groups. The C/C group (N = 83) reported greater stimulant effects at both 10 mg (***) and 20 mg (**) doses, compared to the combined A/A+A/C group (N = 69). In addition, the C/C group (N = 83) reported greater euphoric effects at both 10 mg (*) and 20 mg (*) doses, compared to the combined A/A+A/C group (N = 69). * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001
Discussion
The main finding of this study is that subjects homozygous for the C allele of rs460000 exhibited a pattern of enhanced responsiveness to the stimulant and euphoric effects of acute amphetamine. The two genotypic groups (A/A+A/C and C/C) of rs460000 did not differ on the placebo session, but the CC group reported stimulation and euphoria about twofold higher than the combined A/A+A/C group after both doses of d-amphetamine (10 and 20 mg). This SNP did not influence physiological and cognitive measures in either the placebo or drug conditions. None of the other SNPs had significant effects on any of the measures studied.
Findings like this with an individual SNP must be interpreted with caution because of problems with multiple testing. However, one reason to believe that the results of this analysis reflect a true difference in stimulant responses between the groups is their consistency with results of recent studies describing association of either SNPs or haplotypes of the SLC6A3 with ADHD and related phenotypes (Friedel et al. 2007; Brookes et al. 2006; Lasky-Su et al. 2006). ADHD, like response to stimulants, is related to dopamine function, and stimulant drugs are an effective treatment for ADHD. Friedel et al. (2007) found a significant association between ADHD and rs463379. Our SNP (rs460000) is in perfect linkage disequilibrium with rs463379 (both D′ and r2 = 1 for CEU). In addition, Lasky-Su et al. (2006) evaluated 35 SNPs in SLC6A3 in relation to substance use disorder in ADHD patients and found that six SNPs, including rs460000, were associated with that phenotype, although correction for multiple comparisons made the significance of rs460000 marginal. In addition, Brookes et al. (2006) found that the only SLC6A3 haplotype showing a trend towards significant association with ADHD included rs460000 (best nominal P = 0.075; see supplementary information). These results suggest that this locus pleiotropically influences subjective responses to stimulant drugs, ADHD and substance use in ADHD patients. These other observations lend support to the idea that our observations reflect a true biological effect.
Our results make sense in the context of the current understanding of the biological basis for the reinforcing effects of stimulants. It is widely believed that the reinforcing and addictive properties of stimulants depend on their ability to interact with the dopamine transporter, thereby increasing the extracellular concentration of the neurotransmitter dopamine within specific brain areas (Kuhar et al. 1991; Wise and Bozarth 1987). The increased extracellular dopamine is thought to mediate the drugs’ subjective (Broadbent et al. 1991) and reinforcing effects (Ritz et al. 1987; Bergman et al. 1989; Volkow et al. 1997). Although the functional consequences of rs460000 or SNPs in linkage disequilibrium with rs460000 are as yet unknown, it seems logical to postulate that the functional variant affects drug responses by altering expression of the dopamine transporter, which would be expected to alter synaptic dopamine levels.
This study had both strengths and limitations. We tested a reasonable number of subjects (N = 152) for this type of drug challenge study. Moreover, the two genotypic groups of rs460000 were of about equal size, allowing us to make the comparison between C/C and A/A+A/C groups with a maximum of power. On the other hand, our small sample size and the preliminary nature of the study did not provide enough power to support a stringent correction of multiple comparisons. If we had used the Bonferroni-corrected P value (.0014) to account for the multiple comparisons, the drug-genotype interaction on the measure of stimulation (both 10 and 20 mg condition) would remain significant, but the effects on euphoria would not. A further limitation of the study is that the selected SNPs were not tagSNPs and thus do not capture the bulk of the variability in this gene. As such, there may be other important alleles of this gene that we did not detect.
In summary, we showed that rs460000 predicts stimulant and euphoric effects of acute doses of d-amphetamine in healthy volunteers. Notably, the differences across the genotypic groups were apparent at both doses of d-amphetamine, suggesting that the effect is replicable. It is interesting that this locus appears to influence both ADHD and the response to amphetamine, a drug that is used to treat ADHD, thus providing a particularly interesting example of pleiotropy. The results of this research may aid in our understanding of individual variability in the response to drug of abuse and could help to predict individual susceptibility to stimulant drug abuse.
Acknowledgments
We would like to thank Ms. Patricia Kriegel and Ms. Margo Meverden for their skillful technical assistance. In addition, we acknowledge the generous support of David Goldman and Colin Hodgkinson for this project. Andrew Skol provided expert assistance with questions relating to statistics. This work was supported by DA024920, DA021336, DA02812 and RR00055.
References
- Bakker SC, van der Meulen EM, Oteman N, Schelleman H, Pearson PL, Buitelaar JK, et al. DAT1, DRD4, and DRD5 polymorphisms are not associated with ADHD in Dutch families. Am J Med Genet B Neuropsychiatr Genet. 2005;132:50–52. [Abstract] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhumans primates. III. Selfadministration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [Abstract] [Google Scholar]
- Brauer LH, de Wit Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry. 1996;39:26–32. [Abstract] [Google Scholar]
- Broadbent J, Michael EK, Riddle EE, Appel JB. Involvement of dopamine uptake in the discriminative stimulus effects of cocaine. Behav Pharmacol. 1991;2:187–197. [Abstract] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11:934–953. [Abstract] [Google Scholar]
- Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–998. [Europe PMC free article] [Abstract] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4:192–196. [Abstract] [Google Scholar]
- Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis, et al. Dopamine transporter (DAT1) associated with appetite suppression to methylphenidate in a case-control study of binge eating disorder. Neuropsychopharmacology. 2007;32:2199–2206. [Abstract] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. [Abstract] [Google Scholar]
- Derogatis L. SLC-90-R manual II. Clinical Psychometric Research; Towson: 1983. [Google Scholar]
- Di Chiara G, Imperato Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. [Europe PMC free article] [Abstract] [Google Scholar]
- Feng Y, Wigg KG, Makkar R, Ickowicz A, Pathare T, Tannock, et al. Sequence variation in the 30-untranslated region of the dopamine transporter gene and attention-deficit hyperactivity disorder (ADHD) Am J Med Genet B Neuropsychiatr Genet. 2005;139:1–6. [Abstract] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part I: Description. J Pers Disord. 1995;9(2):83–91. [Google Scholar]
- Friedel S, Saar K, Sauer S, Dempfle A, Walitza Association and linkage of allelic variants of the dopamine transporter gene in ADHD. Mol Psychiatry. 2007;12:923–933. [Abstract] [Google Scholar]
- Gabbay FH. Variations in affect following amphetamine and placebo: markers of stimulant drug preference. Exp Clin Psychopharmacol. 2003;11:91–101. [Abstract] [Google Scholar]
- Hodgkinson C, Yuan Q, Xu K, Shen P-H, Heinz E, Lobos, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43(5):505–515. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. [Abstract] [Google Scholar]
- Langley K, Turic D, Peirce TR, Mills S, Van Den Bree MB, Owen MJ, et al. No support for association between the dopamine transporter (DAT1) gene and ADHD. Am J Med Genet B Neuropsychiatr Genet. 2005;139:7–10. [Abstract] [Google Scholar]
- Lasky-Su J, Biederman J, Doyle AE, Wilens T, Monuteaux M, Smoller JW, et al. Family based association analysis of statistically derived quantitative traits for drug use in ADHD and the dopamine transporter gene. Addict Behav. 2006;31:1088–1099. [Abstract] [Google Scholar]
- Li D, Sham PC, Owen MJ, He Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15:2276–2284. [Abstract] [Google Scholar]
- Lott D, Kim S, Cook E, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–609. [Abstract] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. [Abstract] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. [Europe PMC free article] [Abstract] [Google Scholar]
- Mitler MM, Hajdukovic R, Erman MK. Treatment of narcolepsy with methamphetamine. Sleep. 1993;16:306–317. [Europe PMC free article] [Abstract] [Google Scholar]
- Parkin AJ, Java RI. Deterioration of frontal lobe function in normal aging: Influences of fluid intelligence versus perceptual speed. Neuropsychology. 1999;13:539–545. [Abstract] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. [Europe PMC free article] [Abstract] [Google Scholar]
- Purper-Ouakil D, Wohl M, Mouren MC, Verpillat P, Ades J, Gorwood Meta-analysis of family-based association studies between the dopamine transporter gene and attention deficithyperactivity disorder. Psychiatr Genet. 2005;15:53–59. [Abstract] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. [Abstract] [Google Scholar]
- Schiffer WK, Fowler JS, Alexoff DL, Logan J, Dewey SC. Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse. 2006;59:243–251. [Abstract] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic 575 instrument. Am J Psychiatr. 1971;127:1653–1658. [Abstract] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. [Abstract] [Google Scholar]
- Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl) 2001;159:2–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17(5):747–758. [Europe PMC free article] [Abstract] [Google Scholar]
- Vilkki J, Holst Mental programming after frontal lobe lesions: results on digit symbol performance with self-selected goals. Cortex. 1991;27:203–211. [Abstract] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad MN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. [Abstract] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. [Abstract] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–1776. [Europe PMC free article] [Abstract] [Google Scholar]
- Wechsler D. Williams and Wilkins; Baltimore: 1958. The measure and appraisal of adult intelligence. [Google Scholar]
- White T, JusticeA JH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. [Abstract] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10519-009-9331-7
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4454285
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s10519-009-9331-7
Article citations
The effects of caffeine and d-amphetamine on spatial span task in healthy participants.
PLoS One, 18(7):e0287538, 13 Jul 2023
Cited by: 0 articles | PMID: 37440493 | PMCID: PMC10343048
Systematic reviews of the acute effects of amphetamine on working memory and other cognitive performances in healthy individuals, with a focus on the potential influence of personality traits.
Hum Psychopharmacol, 38(1):e2856, 17 Oct 2022
Cited by: 2 articles | PMID: 36251504 | PMCID: PMC10078276
Review Free full text in Europe PMC
Shared Behavioral and Neurocircuitry Disruptions in Drug Addiction, Obesity, and Binge Eating Disorder: Focus on Group I mGluRs in the Mesolimbic Dopamine Pathway.
ACS Chem Neurosci, 10(5):2125-2143, 15 Apr 2019
Cited by: 10 articles | PMID: 30933466 | PMCID: PMC7898461
Review Free full text in Europe PMC
Amphetamine Enhances Gains in Auditory Discrimination Training in Adult Schizophrenia Patients.
Schizophr Bull, 43(4):872-880, 01 Jul 2017
Cited by: 14 articles | PMID: 27798224 | PMCID: PMC5472129
Neurocognitive enhancement or impairment? A systematic meta-analysis of prescription stimulant effects on processing speed, decision-making, planning, and cognitive perseveration.
Exp Clin Psychopharmacol, 24(4):269-284, 01 Aug 2016
Cited by: 23 articles | PMID: 27454675 | PMCID: PMC4968888
Review Free full text in Europe PMC
Go to all (20) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (5)
- (11 citations) dbSNP - rs460000
- (4 citations) dbSNP - rs3756450
- (2 citations) dbSNP - rs6869645
- (2 citations) dbSNP - rs37022
- (1 citation) dbSNP - rs463379
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dopamine transporter genotype and stimulant side effect factors in youth diagnosed with attention-deficit/hyperactivity disorder.
J Child Adolesc Psychopharmacol, 19(3):233-239, 01 Jun 2009
Cited by: 21 articles | PMID: 19519258 | PMCID: PMC2856973
Response to methylphenidate in adults with ADHD is associated with a polymorphism in SLC6A3 (DAT1).
Am J Med Genet B Neuropsychiatr Genet, 147B(2):201-208, 01 Mar 2008
Cited by: 40 articles | PMID: 17955457
Attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies.
Mol Psychiatry, 21(7):872-884, 24 May 2016
Cited by: 85 articles | PMID: 27217152 | PMCID: PMC5414093
Review Free full text in Europe PMC
Polymorphisms of the dopamine transporter gene: influence on response to methylphenidate in attention deficit-hyperactivity disorder.
Am J Pharmacogenomics, 4(2):83-92, 01 Jan 2004
Cited by: 32 articles | PMID: 15059031
Review
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: M01 RR000055
Grant ID: RR00055
NIDA NIH HHS (8)
Grant ID: F32 DA024920
Grant ID: F32 DA024920-02
Grant ID: DA024920
Grant ID: R01 DA021336
Grant ID: DA02812
Grant ID: R01 DA002812
Grant ID: F32 DA024920-01
Grant ID: DA021336




