Abstract
Free full text

Ubiquitylation of Nuclear Receptors: New Linkages and Therapeutic Implications
Abstract
The nuclear receptor superfamily is a group of transcriptional regulators that control multiple aspects of both physiology and pathology, and are broadly recognized as viable therapeutic targets. While receptor-modulating drugs have been successful in many cases, the discovery of new drug targets is still an active area of research, because resistance to nuclear receptor-targeting therapies remains a significant clinical challenge. Many successful targeted therapies have harnessed the control of receptor activity by targeting events within the nuclear receptor signaling pathway. In this review, we explore the role of nuclear receptor ubiquitylation and discuss how the expanding roles of ubiquitin might be leveraged to identify additional entry points to control receptor function for future therapeutic development.
Brief overview of NR signaling and drug targets
Nuclear receptors (NRs) comprise a family of transcriptional regulators that control multiple physiological processes including growth, development, reproduction and metabolism through the control of gene expression (Di Croce et al. 1999; Aranda & Pascual 2001). The founding member of the family, estrogen receptor-α (ERα), was identified via its high affinity binding to radiolabeled ligand, estradiol (Toft & Gorski 1966; Jensen et al. 1967). Following the cloning of the gene encoding the glucocorticoid receptor (GR, NR3C1) (Miesfeld et al. 1984; Hollenberg et al. 1985), numerous other nuclear receptors were identified and combined into a superfamily composed of a total of 48 receptors in mammals including estrogen receptors α (ERα; NR3A1) and β (ERβ, NR3A2), thyroid hormone receptor (TR, NR1A1), progesterone receptor (PR, NR3C3), androgen receptor (AR, NR3C4), retinoic acid receptor (RAR, NR1B1-3), retinoic X receptor (RXR, NR2B1-3), Vitamin D receptor (VDR, NR1I1), Peroxisome proliferator-activated receptors (PPAR, NR1C1-3), and a number of orphan receptors with no known ligands (Evans & Mangelsdorf 2014). The receptors share a similar architecture consisting of an intrinsically disordered N-terminus, which in some receptors encodes a ligand-independent transactivation domain, a central DNA binding domain (DBD) containing two zinc finger motifs, and a C-terminal ligand binding domain (LBD). The LBD mediates multiple receptor functions including ligand binding, dimerization, co-regulator interactions, and ligand-dependent transcriptional activation function. It is no surprise then that research has focused largely on the LBD and the modulation of receptor actions through both endogenous and synthetic ligands (Gronemeyer et al. 2004; McDonnell & Wardell 2010).
The dissection of the molecular events that regulate receptor function have greatly advanced the NR field and contributed significantly to the drug discovery tool box. Originally, NRs were considered to participate in a relatively simple signal transduction pathway in which activated receptors directly mediated a responses in the nucleus through direct DNA binding and transcriptional activation. Though fundamentally correct, the broadening knowledge of components in the nuclear receptor activation mechanism has greatly expanded the model and simultaneously expanded the opportunity to control receptor function. In the contemporary model, ligands bind to receptors in the cytoplasm or nucleus or, in some cases, plasma membrane bound receptors. Ligand-binding triggers a series of intracellular events, including release of inactive receptors from heat shock protein complexes, changes to receptor protein conformation, mobilization, dimerization, and recruitment of multi-protein transcriptional complexes. The activated NR transcriptional complexes include co-regulators (activators and repressors), chromatin modifying and remodeling complexes, and components of the basal transcriptional machinery. To date, over 300 NR co-regulators have been identified (Jung et al. 2005; Malovannaya et al. 2011; www.nursa.org). Ligand activation of membrane receptors couples receptor activation to intracellular signaling cascades (Hammes & Levin 2011). Additionally, NRs can be activated indirectly through ligand-independent mechanisms by growth factors. The complexity of NR function and regulation is further expanded by the addition of a temporal component to receptor transcriptional complexes (Métivier et al. 2003; Nagaich et al. 2004). Collectively, the elucidation of this activation cascade form the basis for identification of agents targeting receptors at multiple levels including co-activator interactions (Norris et al. 1999; Parent et al. 2008; Gunther et al. 2009), dimerization, subcellular localization (Tran et al. 2009) and DNA binding (Wang et al. 2006; Mao et al. 2008; Andersen et al. 2010; Caboni & Lloyd 2013).
Post-translational modifications (PTM) are another regulatory mechanism governing NR function. PTMs represent an important cross-talk mechanism by which other signaling pathways interface with NR activation. In the case of ERα, all domains of the receptor can be phosphorylated in response to ligand and/or growth factor cascades (Ali et al. 1993; Le Goff et al. 1994; Bunone et al. 1996; Weis et al. 1996; Chen et al. 1999; Yudt et al. 1999; Clark et al. 2001; Michalides et al. 2004; Held et al. 2012). Studies in breast cancer cell models have demonstrated that phosphorylation can impact multiple aspects of receptor function including protein stability, dimerization, DNA binding, and co-activator preferences (Arnold et al. 1995; Tzeng & Klinge 1996; Chen et al. 1999; Henrich et al. 2003; Sheeler et al. 2003; Calligé et al. 2005; Valley et al. 2005; Likhite et al. 2006; Bhatt et al. 2012). ERα is also subject to other modifications including acetylation (Kim et al. 2006), methylation (Le Romancer et al. 2008; Subramanian et al. 2008), SUMOylation (Sentis et al. 2005; Hilmi et al. 2012), and palmitoylation (Acconcia et al. 2005). Readers are referred to a recent comprehensive review of ERα post-translational modifications (Le Romancer et al. 2011). Importantly, ERα and other NRs are targets of ubiquitylation, a PTM that couples receptor protein turnover and transcriptional function at multiple levels of the receptor signaling pathway.
Nuclear receptor degradation by the ubiquitin proteasome pathway
The first studies investigating the mechanisms of NR protein turnover pointed to the role of proteasomes and subsequently ubiquitylation in targeting receptors to the degradation pathway. Multiple groups demonstrated that proteasome inhibitors disrupted estrogen-induced decreases in ERα protein levels (Alarid et al. 1999; El Khissiin & Leclercq 1999; Nawaz et al. 1999a). Subsequently, it was observed that retinoic acid receptor γ2 (RARγ2) and retinoic acid receptor α (RARα) were down-regulated in response to their ligand, all-trans-retinoic acid, and the down-regulation was blocked by proteasome inhibitors, MG132 and lactacystin (Zhu et al. 1999; Kopf et al. 2000). Thyroid hormone receptor (TR), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR) were also found to be downregulated in response to ligand binding via a similar pathway (Dace et al. 2000; Wallace & Cidlowski 2001; Yokota et al. 2004). These studies established the proteasome pathway as a key regulator of NR protein stability (Alarid et al. 2006) (Fig. 1). A caveat, however, is that proteasome inhibitor studies also disrupt NR motility and transcription (Lonard et al. 2000; Reid et al. 2003; Elbi et al. 2004; Stavreva et al. 2004). Proteasome inhibitors can also affect NR gene expression and indirectly lead to downstream changes in NR target gene regulation (Powers et al. 2010; Prenzel et al. 2011). Further, the proteasome pathway is not selective for NRs, and inhibition of such a vital cellular function can lead to both inhibition and activation of other signaling pathways, production of reactive oxygen species, and induction of apoptosis (Shinohara et al. 1996; Emanuele et al. 2002; Cirit et al. 2012). Recent studies also implicate lysosomes in the degradation of a number of nuclear receptors including ERα, AR, and GR (He et al. 2011; Totta et al. 2014). Due to the confounding effects of proteasome inhibitors and the complex regulation of NR protein stability, the study of NR proteolysis shifted to better understanding the role and mechanisms of ubiquitylation in targeting NRs to proteasome-mediated degradation.
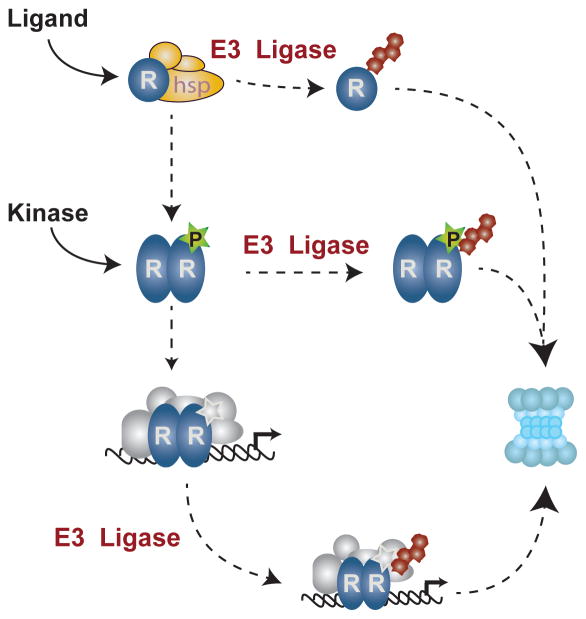
The primary function ascribed to ubiquitin in NR signaling is targeting receptors to the proteasome. Shown is a simplified NR signaling pathway in which receptor (R) is activated either by ligand or by kinases from growth factor or membrane bound NRs. Receptors held bound by heat shock proteins can be targeted for degradation by the 26S proteasome following ubiquitylation by E3 ligases, such as CHIP. Upon binding ligand, receptors undergo dimerization (homo- and heterodimerization are not distinguished here) and can be decorated by multiple post-translational modifications including phosphorylation, shown as a star. Phosphorylated receptors can be directly recognized by E3 ligases, ubiquitylated, and directed to the proteasome for degradation. Alternatively, post-translationally modified receptors can be incorporated into active transcriptional complexes of variable co-regulator components, represented by a grey multiprotein-complex. The make-up of the co-regulator/receptor complexes can recruit E3 ligases that direct ubiquitylation and degradation of the transcriptional complex. In the degradative pathways, post-translational modifications and co-regulator complexes guide E3 targeting that allows subpopulations of receptors to be selectively degraded in cells.
Ubiquitin is a 76-residue protein that can modify target substrates by covalent attachment of its C-terminal carboxyl group to a lysine residue on the target substrate in a catalytic process involving three classes of enzymes (Hershko & Ciechanover 1998; Pickart 2001; Komander 2009). The first class of enzymes, known as E1 activating enzymes, bind ubiquitin through a catalytic cysteine residue in an ATP-dependent mechanism, creating a high-energy thioester bond. The E1 enzyme is loaded with a second ubiquitin molecule and then recruits the second class of enzymes, E2 conjugating enzymes. The E1-Ub complex then transfers the ubiquitin to a conserved catalytic cysteine residue of the E2 enzyme forming a thioester linked E2-Ub complex, in a process known as transthioesterification (Lee & Schindelin 2008). Lastly, the third classes of enzymes, known as E3 ubiquitin ligases, facilitate the transfer of ubiquitin from the E2 conjugating enzyme, directly or indirectly, to a lysine on the substrate forming an isopeptide bond. To date, two ubiquitin-specific E1 activating enzymes (UBA1, UBA6), ~35 E2 conjugating enzymes, and over 600 E3 ligases have been reported in humans (Bernassola et al. 2008; Deshaies & Joazeiro 2009; Markson et al. 2009; Schulman & Harper 2009; Ye & Rape 2009; van Wijk & Timmers 2010).
Substrate selectivity is primarily guided by E3 ubiquitin ligases, which belong to three subtypes, RING (Really Interesting New Gene), HECT (Homologous to E6-AP C-terminus) and RBR (RING-between-RING) (Berndsen & Wolberger 2014). These ligases are classified based on the corresponding motifs (RING, HECT and RBR) required for E3 activity as well as the distinct mechanisms involved. RING E3 ligases can function as a monomer, dimer (homo or hetero), or multi-protein complex with the RING domain binding to specific E2s and a distinct region of the ligase (or ligase complex) binding to specific substrates. RING ligases promote the transfer of ubiquitin from ubiquitin-charged E2 without itself forming an intermediate thioester with a ubiquitin molecule. In contrast, HECT ligases accept ubiquitin from ubiquitin-charged E2s to its catalytic cysteine, which is then transferred to the substrate. RBR ligases have a combination of RING and HECT mechanisms whereby one of the RING domains binds to E2s and the other contains a catalytic cysteine that accepts a ubiquitin from ubiquitin-charged E2s and then transfers it to substrates. Table 1 lists the ubiquitin E3 ligases that participate in NR ubiquitylation which are organized by NR types and in a general chronological order of discovery in NR regulation.
Table 1
E3 ligases involved in NR ubiquitylation.
| Nuclear Receptor | E3 Ligase | Class of Ligase | Type of Ub | References |
|---|---|---|---|---|
| Estrogen Receptor α | E6AP | HECT | poly | (Nawaz et al. 1999b; Li et al. 2006; Sun et al. 2012; Rajbhandari et al. 2014) |
| CHIP | RING (U-box) | poly | (Fan et al. 2005) | |
| Mdm2 | RING | poly | (Duong et al. 2007) | |
| BRCA1/BARD1 | RING | mono | (Eakin et al. 2007) | |
| EFP(TRIM25) | RING | poly K48 | (Nakajima et al. 2007) | |
| SPOP | RING (Cullin) | poly | (Byun and Jung 2008) | |
| RBCK1 | RING (RBR) | ? | (Gustafsson 2010) | |
| CUEDC2 | ?* | ? | (Pan et al. 2011) | |
| Skp2 | RING (F-box) | poly | (Bhatt et al. 2012) | |
| VHL | RING | poly | (Jung et al. 2012) | |
| RNF31 | RING (RBR) | mono | (Zhu et al. 2014) | |
|
| ||||
| Estrogen Receptor β | CHIP | RING (U-box) | poly K48 | (Tateishi et al. 2006) |
| E6AP | HECT | poly | (Picard et al. 2008) | |
| Mdm2 | RING | poly | (Sanchez et al. 2013) | |
|
| ||||
| Androgen Receptor | Mdm2 | RING | poly | (Lin et al. 2002) |
| CHIP | RING (U-box) | poly | (Cardozo et al. 2003) | |
| NEDD4 | HECT | (Li et al. 2008) | ||
| RNF6 | RING | poly-K6 or -K27 | (Xu et al. 2009b) | |
| Siah2 | RING | poly K48 | (Qi et al. 2013) | |
| UBR1 | RING | ? | (Sultana et al. 2013) | |
| Skp2 | RING | poly | (Li et al. 2014) | |
|
| ||||
| Glucocorticoid Receptor | Hdm2 | RING | poly | (Sengupta and Wasylyk 2001) |
| CHIP | RING (U-box) | poly | (Connell et al. 2001) | |
| FBXW7 | RING (F-box) | poly | (Malyukova et al. 2013) | |
| UBR1 | RING | ? | (Sultana et al. 2013) | |
|
| ||||
| Progesterone Receptor | CUEDC2 | ?* | ? | (Zhang et al. 2007) |
| BRCA1/BARD1 | RING | poly | (Calvo and Beato 2011) | |
|
| ||||
| Retinoic Acid Receptor α | FLRF (Rnf41) | RING | ? | (Jing et al. 2008) |
|
| ||||
| Retinoic X Receptor | RNF8 | RING | ? | (Takano et al. 2004) |
|
| ||||
| Mineralocorticoid Receptor | CHIP | RING (U-box) | poly | (Faresse et al. 2010) |
|
| ||||
| PPARγ | Siah2 | RING | poly | (Kilroy et al. 2012) |
| MKRN-1 | RING | poly | (Kim et al. 2014) | |
|
| ||||
| Estrogen-Related Receptors | Parkin | RING (RBR) | poly | (Ren et al. 2011) |
Abbreviations: BARD1, BRCA1-associated RING domain protein 1; BRCA1, breast cancer type 1 susceptibility protein; CHIP, C-terminus of Hsc70-interacting protein; CUEDC2, CUE domain containing 2; E6AP, E6-associated protein; EFP, estrogen-responsive finger protein; FBXW7, F-box/WD repeat-containing protein 7; FLRF, fetal liver ring finger; Hdm2, Human double minute 2; HECT, Homologous to the E6-AP Carboxyl Terminus; Mdm2, Mouse double minute 2; MKRN-1, Makorin Ring Finger Protein 1; NEDD4, Neural Precursor Cell Expressed, Developmentally Down-Regulated 4; PPARγ, Peroxisome proliferator-activated receptor gamma; RBCK1, RanBP-Type and C3HC4-Type Zinc Finger Containing 1; RING, Really Interesting New Gene; RNF6, Ring Finger Protein 6; RNF8, Ring Finger Protein 8; RNF31, Ring Finger Protein 31; Rnf41, Ring Finger Protein 41; Siah2, Seven in Absentia Homolog 2; Skp2, S-phase kinase-associated protein 2; SPOP Speckle-Type POZ Protein; Ub, Ubiquitin; UBR1, Ubiquitin Protein Ligase E3 Component N-Recognin 1; VHL, Von Hipple-Lindau Tumor Suppressor.
In the following sections, we highlight specific ligases that appear to have generalized activities on NRs and that are implicated in distinct aspects of NR signaling pathway with the goal of highlighting processes where interference of NR ubiquitylation may be leveraged as part on on-going drug development targeting the ubiquitin system.
Control of unliganded NR protein stability by CHIP
The control of NRs by Carboxyl-terminus of Hsc70-interacting protein (CHIP) represents specific ligase activity at early stages in NR signaling, including controlling basal NR expression and receptor availability prior to ligand binding. NRs are held stable in their unliganded state by chaperone complexes which include heat shock proteins (Hsp), Hsp70 and Hsp90 (Smith & Toft 1993). The Hsp interaction guides appropriate folding of NR protein and stabilizes the ligand binding pocket (Bresnick et al. 1989; Smith 1993; Stancato et al. 1996; Pratt 1997). Disruption of the Hsp90-NR complex using a chemical inhibitor, geldanamycin, was shown to cause down-regulation of ERα, PR, GR and AR in a proteasome-dependent manner (Whitesell & Cook 1996; Pratt & Toft 1997; Bagatell et al. 2001; Connell et al. 2001; Lee et al. 2002; Vanaja et al. 2002; Fan et al. 2005). CHIP is a RING E3 ligase that contains a tetratricopeptide repeat (TPR), which binds and decreases the ATPase activity of heat shock proteins. This association ultimately decreases the efficiency of the chaperone and impairs its function, leading to misfolding and subsequent degradation of its substrate by proteasomes (Ballinger et al. 1999; Connell et al. 2001). CHIP interacts with Hsp90 and incorporates itself into the NR-Hsp90 heterocomplex, causing remodeling that favors degradation of the NR. In the case of GR, CHIP can ubiquitylate GR both in vitro and in vivo and directly target it for degradation by interacting with the S5a subunit of the 26S proteasome (Connell et al. 2001). CHIP was also found to preferentially associate with unliganded ERα, increasing ubiquitylation and degradation of the receptor. Other NRs also appear to be ubiquitylated by CHIP, including ERβ, AR, GR, and MR, suggesting that ubiquitylation of NRs by CHIP is a conserved mechanism (Cardozo et al. 2003; Wang & DeFranco 2005; Tateishi et al. 2006; Faresse et al. 2010). Hence, CHIP ligase is a major regulator of unliganded NR protein expression (Fig. 1), which has relevance in scenarios in breast and prostate cancer whereas therapies such as aromatase inhibitors and abiraterone act by decreasing hormone production yet maintain the unliganded receptor.
Regulated NR ubiquitylation by phosphorylation and coactivator complexes
Beyond control of basal NR protein levels, ligand-induced turnover of NRs revealed additional layers of complexity in E3 ligase action in NR signaling. As described above, ligand binding to NRs triggers a series of events associated with the transcriptional activation mechanism, including phosphorylation and the recruitment of multi-protein transcriptional complexes. Ubiquitylation is integrated within this activation mechanism through both phosphorylation and the protein complexes recruited to NRs (Fig. 1). For example, the RING E3 ligase mouse double minute 2 (Mdm2) is implicated in the turnover for many NRs, including AR, ERα, ERβ, and GR (Table 1, references therein). The targeting of NRs by Mdm2 is triggered by at least two levels of regulation, NR phosphorylation and the composition of the NR transcriptional complex. In the case of AR, phosphorylation of AR on Ser 515 by Cyclin-dependent kinase 7 (Cdk7), a component of the basal transcriptional machinery, is critical for recruitment of Mdm2, and the subsequent ubiquitylation and degradation of AR by the proteasome (Chymkowitch et al. 2011). Ubiquitylation of AR by Mdm2 can also be signaled following phosphorylation of AR by Akt on Ser 210 and Ser 790 (Lin et al. 2002). Mutations of Ser 210 and Ser 790, or Ser 515, to alanine prevents recruitment of Mdm2 and ubiquitylation of AR. Like AR, Mdm2 is also recruited to ERα and ERβ complexes when the corresponding ER is phosphorylated (Valley et al. 2005; Picard et al. 2008; Sanchez et al. 2013). However, degradation of ERα upon Mdm2 over-expression provides an example wherein specific NR protein complexes are also a requirement for this response, in this case, a complex with p53 (Duong et al. 2007). Similarly, GR degradation following dexamethasone treatment involves the formation of a GR complex containing p53 and Hdm2 (Sengupta & Wasylyk 2001). In the case of ERβ, Mdm2 works in concert with a different coregulator, CREB-Binding Protein (CBP), to form a complex that results in ubiquitylation and ultimate degradation of ERβ (Sanchez et al. 2013). Interestingly, unlike ERβ, the Mdm2-CBP complex was unable to target ERα for degradation. These observations suggest that Mdm2 is recruited to NRs as part of larger multi-protein complexes which impart specificity of Mdm2 action in controlling ubiquitylation and degradation. Given that receptor complexes are dynamic (Métivier et al. 2003), this protein complex specificity, in addition to phosphorylation events, could impart temporal and context-specific regulation on the NR ubiquitylation, stability and associated functions.
Some E3 ligases control NR function through both ligase activity-dependent and -independent mechanisms. A primary example of dual action E3 ligases is E6-Associated Protein (E6AP). The first studies to demonstrate ubiquitylation of endogenous NRs were done on ERα (Wijayaratne & McDonnell 2001). Subsequently, E6AP was found to be recruited to ERα in a calmodulin-dependent manner, leading to ubiquitylation and degradation of ERα (Li et al. 2006). In addition to calmodulin-dependent ubiquitylation, recruitment of E6AP to ERα as well as ERβ requires phosphorylation of the receptor (Picard et al. 2008; Rajbhandari et al. 2014). Consistent with the ligase function of E6AP, mammary and prostate glands of E6AP null mice show elevated levels of ERα (Gao et al. 2005). However, E6AP also functions as a coactivator for ERα, as well as other NRs such as PR, AR, and GR (Nawaz et al. 1999b; Ramamoorthy & Nawaz 2008). In these cases, the disruption of ubiquitin ligase activity as well as the HECT domain of E6AP by mutagenesis had no effect on NR coactivation activity (Nawaz et al. 1999b). Similar ligase-independent coregulator function has been noted in NR regulation by other HECT ligases, including NEDD4-1, Rsp5, and HACE1. For example, HACE1 was identified as an NR-interacting partner in a yeast two hybrid screen, and shown to interact with RARα, RARγ, ERα, and TRα. Mutation of critical cysteine residues in the HECT ligase domain had no effect on its transcriptional repressor activity toward RARs (Zhao et al. 2009). Likewise, the ligase activity of Rsp5 is not essential for Rsp5 coactivation of PR and GR transactivation (Imhof & McDonnell 1996). Among the HECT ligases, E6AP alone thus far has been directly shown to function as a key NR regulator via ubiquitin ligase-dependent and -independent mechanisms.
Current ubiquitin-targeting therapeutics
The examples provided above indicate the potential for targeting multiple steps (protein folding, coactivator interactions, transcriptional function) in the NR signaling pathway via control of ubiquitylation. Clinical approaches in cancer therapy have thus far focused on inhibiting the 26S proteasome (Teicher et al. 1999). Bortezomib (Velcade, PS-341) is a general proteasome inhibitor that is FDA approved for the treatment of multiple myeloma and mantle cell lymphoma. Second generation proteasome inhibitors have also been developed including Carfilzomib, which was approved in 2012 for multiple myeloma patients that are refractory to bortezomib therapy (Mitsiades et al. 2011). While the preclinical data supports the efficacy of proteasome inhibitors in other cancer types, the results outside of hematological malignancies have been disappointing (Yang et al. 2006). Hence, efforts to more specifically target the ubiquitylation machinery and their substrates are underway.
A glimpse into this potential with relevance to the NR field is provided by studies of SCF-Skp2 and p27kip1. Skp2 is an F-box protein and component of the Skp1-Cullin1-F-Box (SCF) RING ubiquitin E3 ligase complex. Skp2 ligase ubiquitylates and degrades ERα and high Skp2 expression in human tumors correlates with ERα-negative status (Bhatt et al. 2012). Skp2 is overexpressed in human cancers and deregulation of Skp2 is implicated in progression through loss of control of cell cycle control and transcription (Bloom & Pagano 2003; Kamata et al. 2005; Davidovich et al. 2008). Skp2 ligase activity was shown to be dependent on 17β-estradiol-induced phosphorylation, leading to ubiquitylation of p27kip1 (Lecanda et al. 2007; Huang et al. 2012). The loss of nuclear p27kip1 has been shown to occur in 17β-estradiol-induced type I endometrial carcinogenesis (Lecanda et al. 2007). Using a small molecule screen, specific agents have been identified that block Skp2-dependent ubiquitylation of p27kip1, thus preventing its degradation. Treatment of 17β-estradiol-induced endometrial carcinoma cell lines with these small molecules resulted in increased levels of p27kip1 along with decreased proliferation (Pavlides et al. 2013). These experiments demonstrate that alterations of E3 ligase activity using small molecule inhibitors could be a viable strategy for future therapeutic development. This possibility is further supported by a recent report of peptide and small molecule inhibitors of HECT ligases (Mund et al. 2014).
To date, three E3-targeting drugs have been approved by the FDA, and all three target the same enzyme, Cereblon (CRBN). CRBN is a part of the Cul4-Rbx1-DDB1-CRBN RING ubiquitin E3 ligase complex and the three drugs that target CRBN—thalidomide, lenalidomide, and pomalidomide; commonly referred to as IMiDs (immunomodulatory drugs)—bind to CRBN and promote the recruitment of substrates, including Ikaros (IKZF1) and Aiolos (IKZF3), which are subsequently ubiquitylated and degraded (Ito et al. 2010; Chamberlain et al. 2014; Fischer et al. 2014; Lu et al. 2014). Currently these drugs are approved for multiple myeloma therapy (Martiniani et al. 2012; Terpos et al. 2013).
Regulation of NR function by E3 ligase-mediated monoubiquitylation
While the focus in the NR field and therapeutic approaches has largely been directed to the role of ubiquitin in degradation and stability of receptors, the scope of the ubiquitin field extends well beyond degradative mechanisms associated with the proteasome pathway which requires the attachment of a ubiquitin polymer (or chain) with 4 ubiquitin moiety or more (Thrower et al. 2000). For example, attachment of a single ubiquitin molecule (monoubiquitylation) on NRs by BRCA1 has been described. BRCA1, along with its partner BARD1, form a heterodimeric RING E3 ligase implicated in numerous cellular processes including DNA repair, cell cycle control, transcriptional regulation, apoptosis, and genomic stability (Deng 2006; Roy et al. 2012). BRCA1 ubiquitylation of both ERα and PR contributes to the their transcriptional function (Eakin et al. 2007; Calvo & Beato 2011). BRCA1/BARD1 monoubiquitylates ERα in vitro and in vivo (Eakin et al. 2007; Dizin & Irminger-Finger 2010; Ma et al. 2010; Zhu et al. 2014). This monoubiquitylation is dependent on BRCA1/BARD1 ligase activity as cancer pre-disposing BRCA1 mutations (C61G and C64G) affecting the ligase activity abolish the ability of BRCA1 to monoubiquitylate ERα. The site of monoubiquitylation on ERα was identified through mass spectrometry to be K302, however, the K302A ERα mutant was still monoubiquitylated in vitro. The adjacent lysine residue, K303, can be targeted for monoubiquitylation in lieu of K302 (Eakin et al. 2007). The precise function of ERα monoubiquitylation by BRCA1/BARD1 in vivo is still unclear, although it is hypothesized to play a role in inhibition of ERα transcriptional activity as well as 17β-estradiol-induced cell proliferation (Ma et al. 2010; La Rosa et al. 2011a). It should be noted, however, that BRCA1 mutant breast cancers are almost always ERα-negative and thus potential therapies targeting the interaction between ERα and BRCA1 would not be suitable in these cases (Karp et al. 1997; Loman et al. 1998). In the case of PR, BRCA1 induces ubiquitylation but whether PR ubiquitylation is polyubiquitylation (attachment of a ubiquitin chain on a lysine) or multi-monoubiquitylation (multiple single ubiquitin attachments on different lysine sites on the substrate) is unresolved (Calvo & Beato 2011).
In addition to BRCA1, RNF31—also known as HOIP or ZIBRA—can also monoubiquitylate ERα (Zhu et al. 2014). RNF31 is a RBR E3 ligase, and a component of the linear ubiquitin assembly complex (LUBAC). Studies by Zhu et al. show a positive correlation between RNF31 and ERα levels (Zhu et al. 2014). Further, manipulation of RNF31 by knockdown or overexpression decreases and increases ERα-mediated transcriptional activity, respectively. Importantly, the effects on receptor transcriptional function were shown to be dependent on RNF31 E3 ligase activity, supporting the idea that monoubiquitylation of ERα can regulate receptor transcriptional activation. The control of ERα by RNF31 and BRCA1/BARD1 suggests that there may be other NRs regulated in the same manner via monoubiquitylation. Moreover, given that RNF31 can act in the context of the LUBAC E3 ligase complex (see below), additional forms of ubiquitylation may also play regulatory roles in NR function.
Non-degradative ubiquitin code
Advances in the ubiquitin field have led to an emerging concept of the “ubiquitin code” (Kulathu & Komander 2012). Ubiquitin itself has 7 lysine residues - K6, K11, K27, K29, K33, K48 and K63 - each of which can serve as ubiquitylation sites to assemble ubiquitin chains connected via distinct internal lysine residues. The attachment of ubiquitin chains linked via lysine K48 marks the substrate for degradation by the 26S proteasome and, as mentioned above, a minimum of four ubiquitin molecules is needed for efficient recognition and degradation of substrates tagged with K48-linked polyubiquitin chains (Thrower et al. 2000). The aforementioned NR associated ligases form this type of ubiquitin chains. However, polyubiquitin chains linked by each of other ubiquitin lysines has been shown to be present in vivo through analysis by mass spectrometry (Xu et al. 2009a). Moreover, the amino group of the N-terminal methionine of ubiquitin can serve to assemble “M1-linked” polyubiquitin chains. Many substrates can also be multi-monoubiquitylated at multiple lysine sites as described above. Finally, different types of ubiquitin configurations could occur in a single substrate (i.e., “mixed” ubiquitin linkages). These varying ubiquitin chains significantly expanded the roles that ubiquitin plays in multitudes of molecular, cellular, physiological and pathological processes. The primary decoding of the information built into distinct types of ubiquitin chains is mediated by an array of ubiquitin binding domains (UBDs) or ubiquitin receptors (Dikic et al. 2009). For a more comprehensive overview of UBDs, we direct the reader to recently published reviews on UBDs (Dikic et al. 2009; Husnjak & Dikic 2012; Searle et al. 2012). The role for these less commonly studied forms of polyubiquitin linkages in NR regulation is a relatively untapped and emerging area of research. For example, ERα has recently been shown to contain a UBD in its AF-2 domain (Pesiri et al. 2013).
The study of RNF6 in AR biology provides a proof of principle that non-degradative ubiquitin chains can contribute to NR transcriptional function. Xu et al. discovered that AR was polyubiquitylated by the E3 ubiquitin ligase, RNF6, in prostate cancer cells (Xu et al. 2009b). Following identification as an interacting partner of AR in GST-pull down assays, the authors showed that RNF6 overexpression leads to an increase in polyubiquitylated AR without changes in AR protein levels. In vitro ubiquitylation assays revealed that RNF6 added K6- or K27-linked polyubiquitin chains to the AR. Overexpression of RNF6 increased the recruitment of co-factors, specifically ARA54, to androgen response elements (AREs), which suggests the possibility that specific ubiquitin linkages may contribute to recruitment and specificity of coregulator complexes on DNA. This may have implications in hormone-refractory prostate cancer where RNF6 has been shown to be overexpressed (Xu et al. 2009b).
To begin to elucidate the potential involvement of the ubiquitin code in NR regulation, we briefly provide an example (NF-κB) where roles of distinct types of ubiquitylation is better understood (Chen & Chen 2013). NF-κB is a family of related dimeric transcription factors, which are held inactive in the cytoplasm by a class of inhibitor proteins, IκB (Inhibitor of κB). Cell signaling leads to the degradation of IκB, liberating NF-κB to the nucleus to initiate transcription. IκB degradation is mediated by phosphorylation of IκB, which creates a docking site for the β-TrCP (β-transducing repeat containing protein) RING ubiquitin E3 ligase complex that induces K48-linked polyubiquitylation of IκB and its degradation by the 26S proteasome. Degradation of IκB is analogous to the polyubiquitylation and degradation of NRs by the proteasome. Interestingly, the mechanism of activation of IKK (IκB kinase) complex that phosphorylates IκB involves non-degradative polyubiquitin chains, such as K63 and “M1” (linked via the N-terminal methionine of ubiquitin) chains, assembled by combinations of multiple E2’s and E3’s. In a simplified model, these polyubiquitin chains are recognized by UBD proteins, TAB2/3 (TAK1 binding proteins 2 and 3) and NEMO (NF-κB essential modulator), to induce TAK1-dependent IKK activation (Yamaoka et al. 1998) (Fig. 3, middle panel). Thus, NF-κB signaling highlights how non-degradative ubiquitin chains can serve as a novel scaffold to assemble multi-protein complexes. Similarly, non-degradative ubiquitin chains are used to assist in the assembly of protein complexes involved in DNA double strand break repair (Brown & Jackson 2015) (Fig. 3, right panel). It is conceivable that non-degradative ubiquitin chains could also be used to assist in assembly of NR transcriptional complexes (Fig. 3, left panel).
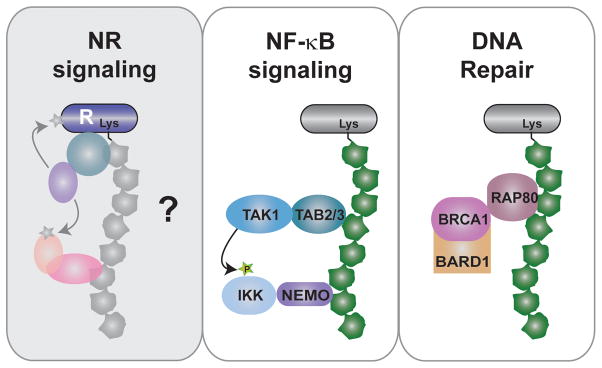
Non-degradative polyubiquitin chains can serve as protein assembly scaffolds. Examples shown in the middle and right panels are assemblages described in NF-κB signaling and DNA damage repair. Based on these models, we speculate the potential for non-degradative ubiquitin chains providing similar scaffolding in NR signaling, bringing together tertiary complexes with enzymatic activity to post-translationally modify receptor or other coregulator proteins to affect transcription. Such scaffolds could also bring other ubiquitin-binding domain (UBD) proteins into the NR complex without directly binding to NR.
A new frontier in ubiquitin regulation of NR
The RNF6 study in AR mentioned above suggests that non-degradative polyubiquitin chains may interface with NR activation mechanisms at the level of co-activators (Xu et al. 2009b). Indeed, an inspection of NR co-activators can identify several of them to contain RING finger domains (Table 2). Whether the RING domains in these coactivators contribute to their activities in NR signaling awaits further investigation. However, intriguingly, TIF1 assembles ternary coactivator complexes as part of AR transcriptional activation (Teyssier et al. 2006), and MAT1 is part of the assembly of TFIIH and Cdk-activating kinase complex involved in receptor phosphorylation and transcriptional synergy (Rochette-Egly et al. 1997; Bastien et al. 2000; Chen et al. 2000; Chymkowitch et al. 2011). It is tempting to speculate that the RING domains of these factors could produce ubiquitin-based assembly scaffolds similar to what is observed in NF-κB signaling. The limited studies of different ubiquitin linkages in biological contexts, including the NR field, could be due in part to the difficulty to detect and quantify proteins modified by specific ubiquitin linkages. Linkage specific antibodies are commercially available for the detection of K48, K63 or M1 linkages. These antibodies can be used in immunoprecipitation (IP)-Western analyses (Haglund & Dikic 2005; Emmerich et al. 2013; Jackson & Durocher 2013). This approach has been used to investigate the types of ubiquitin chains formed on ERα in response to 17β-estradiol (La Rosa et al. 2011b). Alternatively, overexpression and knockdown of specific E2 and E3s (e.g., Ubc13 for K63 chains or LUBAC subunits for M1 chains) (Kirisako et al. 2006; Tokunaga et al. 2009, 2011; Ikeda et al. 2011), or overexpression of ubiquitin mutants (K48R, K63R, etc), as well as replacement of endogenous ubiquitin with ubiquitin mutants (Xu et al. 2009c), has been applied to interrogate the role for specific ubiquitin chains. Finally, mass spectrometry techniques has also be used to map the ubiquitylation sites which are aided by the development of anti-di-Gly antibodies to enrich ubiquitylated peptide species following trypsin digestion (Kirkpatrick et al. 2005; Kim et al. 2011). Each of these approaches has limitations in cell systems (e.g. over-expression/knockdown can have effects on multiple substrates, background of endogenous ubiquitin in mM concentrations, multiple genes encoding ubiquitin, and effects on cell viability). Despite challenges, in combination these techniques have been instrumental in providing insight into the roles of alternative forms of ubiquitin linkages in cell signaling and regulation.
Table 2
NR coregulators that contain RING finger domains.
| Coregulator | Ring Finger Designation | Receptor | References |
|---|---|---|---|
| ARNIP | RNF199 | AR | (Beitel et al. 2002) |
| BRCA1 | RNF53 | ER PR | (Fan et al. 1999, Zheng et al. 2001, Kawai et al. 2002, Calvo & Beato, 2011) |
| EFP | RNF147 | ER | (Inoue et al. 1993, Nakajima et al. 2007) |
| MAT1 | RNF66 | ER PPARγ | (Talukder et al. 2003, Helenius et al. 2009) |
| RNF8 | RNF8 | RXR | (Takano et al. 2004) |
| RLIM | RNF12 | ER | (Johnsen et al. 2009) |
| TIF1 | RNF82, RNF96 | RXR, RAR ER, VDR, AR, TR | (von Baur et al. 1996, Thenot et al. 1997, Teyssier et al. 2006) |
| SNURF | RNF4 | AR, GR PR, ER | (Moilanen et al. 1998, Poukka et al. 2000, Saville et al. 2002) |
Abbreviations: RNF, Ring Finger protein; ARNIP, Androgen receptor N-terminal Interacting Protein; BRCA1, breast cancer type 1 susceptibility protein; EFP, Estrogen-responsive Finger Protein; MAT1, Menage-a trois homologue 1; RLIM, Ring finger protein, LIM Domain Interacting, TIF1, Transcriptional intermediary factor, SNURF, SNRPN Upstream Reading Frame, see references.
The role of non-degradative ubiquitin and the ubiquitin code in regulation of NR function is in its infancy and despite some of the current technical challenges, understanding how this protein modification regulates NR function may open new avenues of research and therapeutic design. There are many critical reagents being generated (e.g., antibodies that specifically detect different ubiquitin linkages (Newton et al. 2008; Matsumoto et al. 2012)), new techniques being developed (e.g., advanced, sensitive and quantitative MS analyses (Peng et al. 2003; Xu & Peng 2006; Phu et al. 2011)) and specific ubiquitin E2s, E3s and deubiquitinases (DUBs) that act on specific ubiquitin linkages are being identified (Komander et al. 2009; Ye & Rape 2009; Kar et al. 2012). These advances may accelerate the elucidation of the roles for non-degradative polyubiquitylation in regulation of the NR family of proteins. While capitalizing on the receptor ubiquitylation has yet to be tapped for clinical application, there is much to be gained by better understanding of the expanding role of ubiquitin in NR signaling. Just as the increased complexity of receptor genomic and non-genomic activities is providing new avenues of rationale design of therapeutics for NR-associated disease, the growing roles of ubiquitin in receptor protein control and transactivation provide an alternative to existing ligand-based therapies. The marriage of NR and ubiquitin fields presents an opportunity for both fields to explore fundamental biology of these important systems with high translational potential.

The different types of ubiquitylated species are represented as cartoons in the left-hand column. Polyubiquitin chains can be organized into a “closed” or “open” conformation based solely on the type of linkage that connects them. A (*) symbolizes that structural data is currently unavailable for these linkages; however, modeling of these structures predicts the conformation of each chain type. Cellular roles are determined based on the identification of each chain type in a specific cellular process. Currently the function of many of these chains is still unknown. This list is not meant to be comprehensive but rather to highlight the many diverse roles of ubiquitin.
Acknowledgments
Funding: This work was supported by NIH grants CA159578 (to E.T.A.), F31 CA186551 (to C.H.), and CA77474 and GM083681 (to S.M.).
We would like to acknowledge Dr. Eric Streiter for sharing expertise in ubiquitin linkages. We would also like to acknowledge the support of McArdle Laboratories for Cancer Research and University of Wisconsin Carbone Cancer Center.
Footnotes
Declaration of Interests: The authors declare no conflicts of interest.
Authors Contributions: K.T.H., C.H., S.M., and E.T.A. all contributed writing and editing of the manuscript.
References
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Molecular Biology of the Cell. 2005;16:231–237. 10.1091/mbc.E04-07-0547. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Alarid ET, Bakopoulos N, Solodin N. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Molecular Endocrinology. 1999;13:1522–1534. 10.1210/mend.13.9.0337. [Abstract] [CrossRef] [Google Scholar]
- Alarid ET, Preisler-Maskek MT, Solodin NM. Lives and Times of Nuclear Receptors. Molecular Endocrinology. 2006;20:1972–1981. 10.1210/me.2005-0481. [Abstract] [CrossRef] [Google Scholar]
- Ali S, Metzger D, Bornert JM, Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. The EMBO Journal. 1993;12:1153–1160. [Europe PMC free article] [Abstract] [Google Scholar]
- Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung J-K, Watt K, Tam T, Yang YC, Bañuelos CA, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–546. 10.1016/j.ccr.2010.04.027. [Abstract] [CrossRef] [Google Scholar]
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiological Reviews. 2001;81:1269–1304. [Abstract] [Google Scholar]
- Arnold SF, Vorojeikina DP, Notides AC. Phosphorylation of tyrosine 537 on the human estrogen receptor is required for binding to an estrogen response element. The Journal of Biological Chemistry. 1995;270:30205–30212. [Abstract] [Google Scholar]
- Bagatell R, Khan O, Paine-Murrieta G, Taylor CW, Akinaga S, Whitesell L. Destabilization of steroid receptors by heat shock protein 90-binding drugs: a ligand-independent approach to hormonal therapy of breast cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2001;7:2076–2084. [Abstract] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Molecular and Cellular Biology. 1999;19:4535–4545. [Europe PMC free article] [Abstract] [Google Scholar]
- Bastien J, Adam-Stitah S, Riedl T, Egly JM, Chambon P, Rochette-Egly C. TFIIH interacts with the retinoic acid receptor gamma and phosphorylates its AF-1-activating domain through cdk7. The Journal of Biological Chemistry. 2000;275:21896–21904. 10.1074/jbc.M001985200. [Abstract] [CrossRef] [Google Scholar]
- Vom Baur E, Zechel C, Heery D, Heine MJ, Garnier JM, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. The EMBO Journal. 1996;15:110–124. [Europe PMC free article] [Abstract] [Google Scholar]
- Beitel LK, Elhaji YA, Lumbroso R, Wing SS, Panet-Raymond V, Gottlieb B, Pinsky L, Trifiro MA. Cloning and characterization of an androgen receptor N-terminal-interacting protein with ubiquitin-protein ligase activity. Journal of Molecular Endocrinology. 2002;29:41–60. [Abstract] [Google Scholar]
- Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell. 2008;14:10–21. 10.1016/j.ccr.2008.06.001. [Abstract] [CrossRef] [Google Scholar]
- Berndsen CE, Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nature Structural & Molecular Biology. 2014;21:301–307. 10.1038/nsmb.2780. [Abstract] [CrossRef] [Google Scholar]
- Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS. Phosphorylation by p38 Mitogen-Activated Protein Kinase Promotes Estrogen Receptor α Turnover and Functional Activity via the SCFSkp2 Proteasomal Complex. Molecular and Cellular Biology. 2012;32:1928–1943. 10.1128/MCB.06561-11. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Seminars in Cancer Biology. 2003;13:41–47. [Abstract] [Google Scholar]
- Bremm A, Komander D. Emerging roles for Lys11-linked polyubiquitin in cellular regulation. Trends in Biochemical Sciences. 2011;36:355–363. 10.1016/j.tibs.2011.04.004. [Abstract] [CrossRef] [Google Scholar]
- Bresnick EH, Dalman FC, Sanchez ER, Pratt WB. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. The Journal of Biological Chemistry. 1989;264:4992–4997. [Abstract] [Google Scholar]
- Brown JS, Jackson SP. Ubiquitylation, neddylation and the DNA damage response. Open Biology. 2015;5 10.1098/rsob.150018. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. The EMBO Journal. 1996;15:2174–2183. [Europe PMC free article] [Abstract] [Google Scholar]
- Byun B, Jung Y. Repression of transcriptional activity of estrogen receptor alpha by a Cullin3/SPOP ubiquitin E3 ligase complex. Molecules and Cells. 2008;25:289–293. [Abstract] [Google Scholar]
- Caboni L, Lloyd DG. Beyond the Ligand-Binding Pocket: Targeting Alternate Sites in Nuclear Receptors. Medicinal Research Reviews. 2013;33:1081–1118. 10.1002/med.21275. [Abstract] [CrossRef] [Google Scholar]
- Calligé M, Kieffer I, Richard-Foy H. CSN5/Jab1 is involved in ligand-dependent degradation of estrogen receptor {alpha} by the proteasome. Molecular and Cellular Biology. 2005;25:4349–4358. 10.1128/MCB.25.11.4349-4358.2005. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Calvo V, Beato M. BRCA1 Counteracts Progesterone Action by Ubiquitination Leading to Progesterone Receptor Degradation and Epigenetic Silencing of Target Promoters. Cancer Research. 2011;71:3422–3431. 10.1158/0008-5472.CAN-10-3670. [Abstract] [CrossRef] [Google Scholar]
- Cardozo CP, Michaud C, Ost MC, Fliss AE, Yang E, Patterson C, Hall SJ, Caplan AJ. C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Archives of Biochemistry and Biophysics. 2003;410:134–140. 10.1016/S0003-9861(02)00680-X. [Abstract] [CrossRef] [Google Scholar]
- Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, Rychak E, Corral LG, Ren YJ, Wang M, et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nature Structural & Molecular Biology. 2014;21:803–809. 10.1038/nsmb.2874. [Abstract] [CrossRef] [Google Scholar]
- Chastagner P, Israël A, Brou C. Itch/AIP4 mediates Deltex degradation through the formation of K29-linked polyubiquitin chains. EMBO Reports. 2006;7:1147–1153. 10.1038/sj.embor.7400822. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science (New York, NY ) 1989;243:1576–1583. [Abstract] [Google Scholar]
- Chen J, Chen ZJ. Regulation of NF-κB by ubiquitination. Current Opinion in Immunology. 2013;25:4–12. 10.1016/j.coi.2012.12.005. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chen D, Pace PE, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Molecular and Cellular Biology. 1999;19:1002–1015. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen D, Riedl T, Washbrook E, Pace PE, Coombes RC, Egly JM, Ali S. Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Molecular Cell. 2000;6:127–137. [Abstract] [Google Scholar]
- Chymkowitch P, May NL, Charneau P, Compe E, Egly J-M. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. The EMBO Journal. 2011;30:468–479. 10.1038/emboj.2010.337. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cirit M, Grant KG, Haugh JM. Systemic Perturbation of the ERK Signaling Pathway by the Proteasome Inhibitor, MG132. PLoS ONE. 2012;7:e50975. 10.1371/journal.pone.0050975. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Clark DE, Poteet-Smith CE, Smith JA, Lannigan DA. Rsk2 allosterically activates estrogen receptor alpha by docking to the hormone-binding domain. The EMBO Journal. 2001;20:3484–3494. 10.1093/emboj/20.13.3484. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Höhfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nature Cell Biology. 2001;3:93–96. 10.1038/35050618. [Abstract] [CrossRef] [Google Scholar]
- Di Croce L, Okret S, Kersten S, Gustafsson JA, Parker M, Wahli W, Beato M. Steroid and nuclear receptors. The EMBO Journal. 1999;18:6201–6210. 10.1093/emboj/18.22.6201. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dace A, Zhao L, Park KS, Furuno T, Takamura N, Nakanishi M, West BL, Hanover JA, Cheng S. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proceedings of the National Academy of Sciences. 2000;97:8985–8990. 10.1073/pnas.160257997. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Davidovich S, Ben-Izhak O, Shapira M, Futerman B, Hershko DD. Over-expression of Skp2 is associated with resistance to preoperative doxorubicin-based chemotherapy in primary breast cancer. Breast Cancer Research: BCR. 2008;10:R63. 10.1186/bcr2122. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deng C-X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Research. 2006;34:1416–1426. 10.1093/nar/gkl010. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deshaies RJ, Joazeiro CAP. RING Domain E3 Ubiquitin Ligases. Annual Review of Biochemistry. 2009;78:399–434. 10.1146/annurev.biochem.78.101807.093809. [Abstract] [CrossRef] [Google Scholar]
- Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains - from structures to functions. Nature Reviews. Molecular Cell Biology. 2009;10:659–671. 10.1038/nrm2767. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dizin E, Irminger-Finger I. Negative feedback loop of BRCA1–BARD1 ubiquitin ligase on estrogen receptor alpha stability and activity antagonized by cancer-associated isoform of BARD1. The International Journal of Biochemistry & Cell Biology. 2010;42:693–700. 10.1016/j.biocel.2009.12.025. [Abstract] [CrossRef] [Google Scholar]
- Duong V, Boulle N, Daujat S, Chauvet J, Bonnet S, Neel H, Cavaillès V. Differential Regulation of Estrogen Receptor α Turnover and Transactivation by Mdm2 and Stress-Inducing Agents. Cancer Research. 2007;67:5513–5521. 10.1158/0008-5472.CAN-07-0967. [Abstract] [CrossRef] [Google Scholar]
- Durcan TM, Kontogiannea M, Thorarinsdottir T, Fallon L, Williams AJ, Djarmati A, Fantaneanu T, Paulson HL, Fon EA. The Machado-Joseph disease-associated mutant form of ataxin-3 regulates parkin ubiquitination and stability. Human Molecular Genetics. 2011;20:141–154. 10.1093/hmg/ddq452. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, Helgason E, Fairbrother WJ, Deshayes K, Kirkpatrick DS, et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. The EMBO Journal. 2010;29:4198–4209. 10.1038/emboj.2010.300. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Eakin CM, MacCoss MJ, Finney GL, Klevit RE. Estrogen receptor α is a putative substrate for the BRCA1 ubiquitin ligase. Proceedings of the National Academy of Sciences. 2007;104:5794–5799. 10.1073/pnas.0610887104. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB. Molecular chaperones function as steroid receptor nuclear mobility factors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2876–2881. 10.1073/pnas.0400116101. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- El Khissiin A, Leclercq G. Implication of proteasome in estrogen receptor degradation. FEBS Letters. 1999;448:160–166. 10.1016/S0014-5793(99)00343-9. [Abstract] [CrossRef] [Google Scholar]
- Emanuele S, Calvaruso G, Lauricella M, Giuliano M, Bellavia G, D’Anneo A, Vento R, Tesoriere G. Apoptosis induced in hepatoblastoma HepG2 cells by the proteasome inhibitor MG132 is associated with hydrogen peroxide production, expression of Bcl-XS and activation of caspase-3. International Journal of Oncology. 2002 10.3892/ijo.21.4.857. [Abstract] [CrossRef] [Google Scholar]
- Emmerich CH, Ordureau A, Strickson S, Arthur JSC, Pedrioli PGA, Komander D, Cohen P. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15247–15252. 10.1073/pnas.1314715110. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157:255–266. 10.1016/j.cell.2014.03.012. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fan S, Wang J-A, Yuan R, Ma Y, Meng Q, Erdos MR, Pestell RG, Yuan F, Auborn KJ, Goldberg ID, et al. BRCA1 Inhibition of Estrogen Receptor Signaling in Transfected Cells. Science. 1999;284:1354–1356. 10.1126/science.284.5418.1354. [Abstract] [CrossRef] [Google Scholar]
- Fan M, Park A, Nephew KP. CHIP (Carboxyl Terminus of Hsc70-Interacting Protein) Promotes Basal and Geldanamycin-Induced Degradation of Estrogen Receptor-α Molecular Endocrinology. 2005;19:2901–2914. 10.1210/me.2005-0111. [Abstract] [CrossRef] [Google Scholar]
- Faresse N, Ruffieux-Daidie D, Salamin M, Gomez-Sanchez CE, Staub O. Mineralocorticoid receptor degradation is promoted by Hsp90 inhibition and the ubiquitin-protein ligase CHIP. American Journal of Physiology - Renal Physiology. 2010;299:F1462–F1472. 10.1152/ajprenal.00285.2010. [Abstract] [CrossRef] [Google Scholar]
- Fischer ES, Böhm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512:49–53. 10.1038/nature13527. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Freudenthal BD, Gakhar L, Ramaswamy S, Washington MT. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nature Structural & Molecular Biology. 2010;17:479–484. 10.1038/nsmb.1776. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z. Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology. 2005;146:1707–1712. 10.1210/en.2004-1198. [Abstract] [CrossRef] [Google Scholar]
- Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biology. 2010;12:119–131. 10.1038/ncb2012. [Abstract] [CrossRef] [Google Scholar]
- Glauser L, Sonnay S, Stafa K, Moore DJ. Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. Journal of Neurochemistry. 2011;118:636–645. 10.1111/j.1471-4159.2011.07318.x. [Abstract] [CrossRef] [Google Scholar]
- Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. Journal of Biological Chemistry. 1994;269:4458–4466. [Abstract] [Google Scholar]
- Goto E, Yamanaka Y, Ishikawa A, Aoki-Kawasumi M, Mito-Yoshida M, Ohmura-Hoshino M, Matsuki Y, Kajikawa M, Hirano H, Ishido S. Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization. The Journal of Biological Chemistry. 2010;285:35311–35319. 10.1074/jbc.M110.112763. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gronemeyer H, Gustafsson J-Å, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nature Reviews Drug Discovery. 2004;3:950–964. 10.1038/nrd1551. [Abstract] [CrossRef] [Google Scholar]
- Gunther JR, Parent AA, Katzenellenbogen JA. Alternative inhibition of androgen receptor signaling: peptidomimetic pyrimidines as direct androgen receptor/coactivator disruptors. ACS Chemical Biology. 2009;4:435–440. 10.1021/cb900043e. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gustafsson N, Zhao C, Gustafsson J-Å, Dahlman-Wright K. RBCK1 Drives Breast Cancer Cell Proliferation by Promoting Transcription of Estrogen Receptor α and Cyclin B1. Cancer Research. 2010;70:1265–1274. 10.1158/0008-5472.CAN-09-2674. [Abstract] [CrossRef] [Google Scholar]
- Haglund K, Dikic I. Ubiquitylation and cell signaling. The EMBO Journal. 2005;24:3353–3359. 10.1038/sj.emboj.7600808. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Al-Hakim AK, Zagorska A, Chapman L, Deak M, Peggie M, Alessi DR. Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. The Biochemical Journal. 2008;411:249–260. 10.1042/BJ20080067. [Abstract] [CrossRef] [Google Scholar]
- Al-Hakim A, Escribano-Diaz C, Landry M-C, O’Donnell L, Panier S, Szilard RK, Durocher D. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair. 2010;9:1229–1240. 10.1016/j.dnarep.2010.09.011. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–4495. 10.1210/en.2011-1470. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- He Y, Xu Y, Zhang C, Gao X, Dykema KJ, Martin KR, Ke J, Hudson EA, Khoo SK, Resau JH, et al. Identification of a lysosomal pathway that modulates glucocorticoid signaling and the inflammatory response. Science Signaling. 2011;4:ra44. 10.1126/scisignal.2001450. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Held JM, Britton DJ, Scott GK, Lee EL, Schilling B, Baldwin MA, Gibson BW, Benz CC. Ligand binding promotes CDK-dependent phosphorylation of ER-alpha on hinge serine 294 but inhibits ligand-independent phosphorylation of serine 305. Molecular Cancer Research: MCR. 2012;10:1120–1132. 10.1158/1541-7786.MCR-12-0099. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Helenius K, Yang Y, Alasaari J, Mäkelä TP. Mat1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipocyte differentiation. Molecular and Cellular Biology. 2009;29:315–323. 10.1128/MCB.00347-08. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Henrich LM, Smith JA, Kitt D, Errington TM, Nguyen B, Traish AM, Lannigan DA. Extracellular signal-regulated kinase 7, a regulator of hormone-dependent estrogen receptor destruction. Molecular and Cellular Biology. 2003;23:5979–5988. [Europe PMC free article] [Abstract] [Google Scholar]
- Hershko A, Ciechanover A. The Ubiquitin System. Annual Review of Biochemistry. 1998;67:425–479. 10.1146/annurev.biochem.67.1.425. [Abstract] [CrossRef] [Google Scholar]
- Hilmi K, Hussein N, Mendoza-Sanchez R, El-Ezzy M, Ismail H, Durette C, Bail M, Rozendaal MJ, Bouvier M, Thibault P, et al. Role of SUMOylation in full antiestrogenicity. Molecular and Cellular Biology. 2012;32:3823–3837. 10.1128/MCB.00290-12. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hoege C, Pfander B, Moldovan G-L, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. 10.1038/nature00991. [Abstract] [CrossRef] [Google Scholar]
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang K-T, Pavlides SC, Lecanda J, Blank SV, Mittal KR, Gold LI. Estrogen and progesterone regulate p27kip1 levels via the ubiquitin-proteasome system: pathogenic and therapeutic implications for endometrial cancer. PloS One. 2012;7:e46072. 10.1371/journal.pone.0046072. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Husnjak K, Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annual Review of Biochemistry. 2012;81:291–322. 10.1146/annurev-biochem-051810-094654. [Abstract] [CrossRef] [Google Scholar]
- Hwang C-S, Shemorry A, Auerbach D, Varshavsky A. The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nature Cell Biology. 2010;12:1177–1185. 10.1038/ncb2121. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJL, Goswami P, Nagy V, Terzic J, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471:637–641. 10.1038/nature09814. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Imhof MO, McDonnell DP. Yeast RSP5 and its human homolog hRPF1 potentiate hormone-dependent activation of transcription by human progesterone and glucocorticoid receptors. Molecular and Cellular Biology. 1996;16:2594–2605. [Europe PMC free article] [Abstract] [Google Scholar]
- Inoue S, Orimo A, Hosoi T, Kondo S, Toyoshima H, Kondo T, Ikegami A, Ouchi Y, Orimo H, Muramatsu M. Genomic binding-site cloning reveals an estrogen-responsive gene that encodes a RING finger protein. Proceedings of the National Academy of Sciences. 1993;90:11117–11121. [Europe PMC free article] [Abstract] [Google Scholar]
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. 10.1126/science.1177319. [Abstract] [CrossRef] [Google Scholar]
- Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Molecular Cell. 2013;49:795–807. 10.1016/j.molcel.2013.01.017. [Abstract] [CrossRef] [Google Scholar]
- Jensen EV, Desombre ER, Kawashima T, Suzuki T, Kyser K, Jungblut PW. Estrogen-binding substances of target tissues. Science. 1967;158:529–530. 10.1126/science.158.3800.529-c. [Abstract] [CrossRef] [Google Scholar]
- Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. 10.1016/j.cell.2008.04.012. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jing X, Infante J, Nachtman RG, Jurecic R. E3 ligase FLRF (Rnf41) regulates differentiation of hematopoietic progenitors by governing steady-state levels of cytokine and retinoic acid receptors. Experimental Hematology. 2008;36:1110–1120. 10.1016/j.exphem.2008.04.001. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Johnsen SA, Güngör C, Prenzel T, Riethdorf S, Riethdorf L, Taniguchi-Ishigaki N, Rau T, Tursun B, Furlow JD, Sauter G, et al. Regulation of estrogen-dependent transcription by the LIM cofactors CLIM and RLIM in breast cancer. Cancer Research. 2009;69:128–136. 10.1158/0008-5472.CAN-08-1630. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jung SY, Malovannaya A, Wei J, O’Malley BW, Qin J. Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Molecular Endocrinology (Baltimore, Md) 2005;19:2451–2465. 10.1210/me.2004-0476. [Abstract] [CrossRef] [Google Scholar]
- Jung Y-S, Lee S-J, Yoon M-H, Ha NC, Park B-J. Estrogen receptor α is a novel target of the Von Hippel-Lindau protein and is responsible for the proliferation of VHL-deficient cells under hypoxic conditions. Cell Cycle (Georgetown, Tex) 2012;11:4462–4473. 10.4161/cc.22794. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kamata Y, Watanabe J, Nishimura Y, Arai T, Kawaguchi M, Hattori M, Obokata A, Kuramoto H. High expression of skp2 correlates with poor prognosis in endometrial endometrioid adenocarcinoma. Journal of Cancer Research and Clinical Oncology. 2005;131:591–596. 10.1007/s00432-005-0671-2. [Abstract] [CrossRef] [Google Scholar]
- Kar G, Keskin O, Nussinov R, Gursoy A. Human proteome-scale structural modeling of E2–E3 interactions exploiting interface motifs. Journal of Proteome Research. 2012;11:1196–1207. 10.1021/pr2009143. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Karp SE, Tonin PN, Bégin LR, Martinez JJ, Zhang JC, Pollak MN, Foulkes WD. Influence of BRCA1 mutations on nuclear grade and estrogen receptor status of breast carcinoma in Ashkenazi Jewish women. Cancer. 1997;80:435–441. [Abstract] [Google Scholar]
- Kawai H, Li H, Chun P, Avraham S, Avraham HK. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730–7739. 10.1038/sj.onc.1205971. [Abstract] [CrossRef] [Google Scholar]
- Kilroy G, Kirk-Ballard H, Carter LE, Floyd ZE. The Ubiquitin Ligase Siah2 Regulates PPARγ Activity in Adipocytes. Endocrinology. 2012;153:1206–1218. 10.1210/en.2011-1725. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim MY, Woo EM, Chong YTE, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Molecular Endocrinology (Baltimore, Md) 2006;20:1479–1493. 10.1210/me.2005-0531. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Molecular Cell. 2011;44:325–340. 10.1016/j.molcel.2011.08.025. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim J-H, Park KW, Lee E-W, Jang W-S, Seo J, Shin S, Hwang K-A, Song J. Suppression of PPARγ through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death and Differentiation. 2014;21:594–603. 10.1038/cdd.2013.181. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A ubiquitin ligase complex assembles linear polyubiquitin chains. The EMBO Journal. 2006;25:4877–4887. 10.1038/sj.emboj.7601360. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kirkpatrick DS, Denison C, Gygi SP. Weighing in on ubiquitin: the expanding role of mass-spectrometry-based proteomics. Nature Cell Biology. 2005;7:750–757. 10.1038/ncb0805-750. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Komander D. The emerging complexity of protein ubiquitination. Biochemical Society Transactions. 2009;37:937. 10.1042/BST0370937. [Abstract] [CrossRef] [Google Scholar]
- Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nature Reviews. Molecular Cell Biology. 2009;10:550–563. 10.1038/nrm2731. [Abstract] [CrossRef] [Google Scholar]
- Kopf E, Plassat J-L, Vivat V, de Thé H, Chambon P, Rochette-Egly C. Dimerization with Retinoid X Receptors and Phosphorylation Modulate the Retinoic Acid-induced Degradation of Retinoic Acid Receptors α and γ through the Ubiquitin-Proteasome Pathway. Journal of Biological Chemistry. 2000;275:33280–33288. 10.1074/jbc.M002840200. [Abstract] [CrossRef] [Google Scholar]
- Kulathu Y, Komander D. Atypical ubiquitylation — the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nature Reviews Molecular Cell Biology. 2012;13:508–523. 10.1038/nrm3394. [Abstract] [CrossRef] [Google Scholar]
- Lecanda J, Parekh TV, Gama P, Lin K, Liarski V, Uretsky S, Mittal K, Gold LI. Transforming growth factor-beta, estrogen, and progesterone converge on the regulation of p27Kip1 in the normal and malignant endometrium. Cancer Research. 2007;67:1007–1018. 10.1158/0008-5472.CAN-06-0235. [Abstract] [CrossRef] [Google Scholar]
- Lee I, Schindelin H. Structural insights into E1-catalyzed ubiquitin activation and transfer to conjugating enzymes. Cell. 2008;134:268–278. 10.1016/j.cell.2008.05.046. [Abstract] [CrossRef] [Google Scholar]
- Lee MO, Kim EO, Kwon HJ, Kim YM, Kang HJ, Kang H, Lee JE. Radicicol represses the transcriptional function of the estrogen receptor by suppressing the stabilization of the receptor by heat shock protein 90. Molecular and Cellular Endocrinology. 2002;188:47–54. [Abstract] [Google Scholar]
- Li L, Li Z, Howley PM, Sacks DB. E6AP and Calmodulin Reciprocally Regulate Estrogen Receptor Stability. Journal of Biological Chemistry. 2006;281:1978–1985. 10.1074/jbc.M508545200. [Abstract] [CrossRef] [Google Scholar]
- Li H, Xu LL, Masuda K, Raymundo E, McLeod DG, Dobi A, Srivastava S. A feedback loop between the androgen receptor and a NEDD4-binding protein, PMEPA1, in prostate cancer cells. The Journal of Biological Chemistry. 2008;283:28988–28995. 10.1074/jbc.M710528200. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li B, Lu W, Yang Q, Yu X, Matusik RJ, Chen Z. Skp2 regulates androgen receptor through ubiquitin-mediated degradation independent of Akt/mTOR pathways in prostate cancer. The Prostate. 2014;74:421–432. 10.1002/pros.22763. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Licchesi JDF, Mieszczanek J, Mevissen TET, Rutherford TJ, Akutsu M, Virdee S, El Oualid F, Chin JW, Ovaa H, Bienz M, et al. An ankyrin-repeat ubiquitin-binding domain determines TRABID’s specificity for atypical ubiquitin chains. Nature Structural & Molecular Biology. 2012;19:62–71. 10.1038/nsmb.2169. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-Specific Phosphorylation of the Estrogen Receptor Changes Receptor Interactions with Ligand, Deoxyribonucleic Acid, and Coregulators Associated with Alterations in Estrogen and Tamoxifen Activity. Molecular Endocrinology. 2006;20:3120–3132. 10.1210/me.2006-0068. [Abstract] [CrossRef] [Google Scholar]
- Lin H-K, Wang L, Hu Y-C, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. The EMBO Journal. 2002;21:4037–4048. 10.1093/emboj/cdf406. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Loman N, Johannsson O, Bendahl PO, Borg A, Fernö M, Olsson H. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer. 1998;83:310–319. [Abstract] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S Proteasome Is Required for Estrogen Receptor-α and Coactivator Turnover and for Efficient Estrogen Receptor-α Transactivation. Molecular Cell. 2000;5:939–948. 10.1016/S1097-2765(00)80259-2. [Abstract] [CrossRef] [Google Scholar]
- Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong K-K, Bradner JE, Kaelin WG. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. 10.1126/science.1244917. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ma Y, Fan S, Hu C, Meng Q, Fuqua SA, Pestell RG, Tomita YA, Rosen EM. BRCA1 Regulates Acetylation and Ubiquitination of Estrogen Receptor-α Molecular Endocrinology. 2010;24:76–90. 10.1210/me.2009-0218. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Malovannaya A, Lanz RB, Jung SY, Bulynko Y, Le NT, Chan DW, Ding C, Shi Y, Yucer N, Krenciute G, et al. Analysis of the human endogenous coregulator complexome. Cell. 2011;145:787–799. 10.1016/j.cell.2011.05.006. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Malyukova A, Brown S, Papa R, O’Brien R, Giles J, Trahair TN, Dalla Pozza L, Sutton R, Liu T, Haber M, et al. FBXW7 regulates glucocorticoid response in T-cell acute lymphoblastic leukaemia by targeting the glucocorticoid receptor for degradation. Leukemia. 2013;27:1053–1062. 10.1038/leu.2012.361. [Abstract] [CrossRef] [Google Scholar]
- Mao C, Patterson NM, Cherian MT, Aninye IO, Zhang C, Montoya JB, Cheng J, Putt KS, Hergenrother PJ, Wilson EM, et al. A new small molecule inhibitor of estrogen receptor alpha binding to estrogen response elements blocks estrogen-dependent growth of cancer cells. The Journal of Biological Chemistry. 2008;283:12819–12830. 10.1074/jbc.M709936200. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Markson G, Kiel C, Hyde R, Brown S, Charalabous P, Bremm A, Semple J, Woodsmith J, Duley S, Salehi-Ashtiani K, et al. Analysis of the human E2 ubiquitin conjugating enzyme protein interaction network. Genome Research. 2009;19:1905–1911. 10.1101/gr.093963.109. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Martiniani R, Di Loreto V, Di Sano C, Lombardo A, Liberati AM. Biological activity of lenalidomide and its underlying therapeutic effects in multiple myeloma. Advances in Hematology. 2012;2012:842945. 10.1155/2012/842945. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Matsumoto ML, Dong KC, Yu C, Phu L, Gao X, Hannoush RN, Hymowitz SG, Kirkpatrick DS, Dixit VM, Kelley RF. Engineering and structural characterization of a linear polyubiquitin-specific antibody. Journal of Molecular Biology. 2012;418:134–144. 10.1016/j.jmb.2011.12.053. [Abstract] [CrossRef] [Google Scholar]
- McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Current Opinion in Pharmacology. 2010;10:620–628. 10.1016/j.coph.2010.09.007. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. [Abstract] [Google Scholar]
- Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, Floore A, Velds A, van’t Veer L, Neefjes J. Tamoxifen resistance by a conformational arrest of the estrogen receptor α after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. 10.1016/j.ccr.2004.05.016. [Abstract] [CrossRef] [Google Scholar]
- Miesfeld R, Okret S, Wikström AC, Wrange O, Gustafsson JA, Yamamoto KR. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984;312:779–781. [Abstract] [Google Scholar]
- Mitsiades CS, Davies FE, Laubach JP, Joshua D, San Miguel J, Anderson KC, Richardson PG. Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29:1916–1923. 10.1200/JCO.2010.34.0760. [Abstract] [CrossRef] [Google Scholar]
- Moilanen AM, Poukka H, Karvonen U, Häkli M, Jänne OA, Palvimo JJ. Identification of a novel RING finger protein as a coregulator in steroid receptor-mediated gene transcription. Molecular and Cellular Biology. 1998;18:5128–5139. [Europe PMC free article] [Abstract] [Google Scholar]
- Morris JR, Solomon E. BRCA1: BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Human Molecular Genetics. 2004;13:807–817. 10.1093/hmg/ddh095. [Abstract] [CrossRef] [Google Scholar]
- Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science (New York, NY) 2007;315:201–205. 10.1126/science.1127085. [Abstract] [CrossRef] [Google Scholar]
- Mund T, Lewis MJ, Maslen S, Pelham HR. Peptide and small molecule inhibitors of HECT-type ubiquitin ligases. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16736–16741. 10.1073/pnas.1412152111. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Nagaich AK, Walker DA, Wolford R, Hager GL. Rapid periodic binding and displacement of the glucocorticoid receptor during chromatin remodeling. Molecular Cell. 2004;14:163–174. [Abstract] [Google Scholar]
- Nakajima A, Maruyama S, Bohgaki M, Miyajima N, Tsukiyama T, Sakuragi N, Hatakeyama S. Ligand-dependent transcription of estrogen receptor α is mediated by the ubiquitin ligase EFP. Biochemical and Biophysical Research Communications. 2007;357:245–251. 10.1016/j.bbrc.2007.03.134. [Abstract] [CrossRef] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proceedings of the National Academy of Sciences. 1999a;96:1858–1862. 10.1073/pnas.96.5.1858. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai M-J, O’Malley BW. The Angelman Syndrome-Associated Protein, E6-AP, Is a Coactivator for the Nuclear Hormone Receptor Superfamily. Molecular and Cellular Biology. 1999b;19:1182–1189. [Europe PMC free article] [Abstract] [Google Scholar]
- Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. 10.1016/j.cell.2008.07.039. [Abstract] [CrossRef] [Google Scholar]
- Nishikawa H, Ooka S, Sato K, Arima K, Okamoto J, Klevit RE, Fukuda M, Ohta T. Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. The Journal of Biological Chemistry. 2004;279:3916–3924. 10.1074/jbc.M308540200. [Abstract] [CrossRef] [Google Scholar]
- Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP. Peptide antagonists of the human estrogen receptor. Science. 1999;285:744–746. [Abstract] [Google Scholar]
- Pan X, Zhou T, Tai Y-H, Wang C, Zhao J, Cao Y, Chen Y, Zhang P-J, Yu M, Zhen C, et al. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nature Medicine. 2011;17:708–714. 10.1038/nm.2369. [Abstract] [CrossRef] [Google Scholar]
- Parent AA, Gunther JR, Katzenellenbogen JA. Blocking estrogen signaling after the hormone: pyrimidine-core inhibitors of estrogen receptor-coactivator binding. Journal of Medicinal Chemistry. 2008;51:6512–6530. 10.1021/jm800698b. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pavlides SC, Huang K-T, Reid DA, Wu L, Blank SV, Mittal K, Guo L, Rothenberg E, Rueda B, Cardozo T, et al. Inhibitors of SCF-Skp2/Cks1 E3 Ligase Block Estrogen-Induced Growth Stimulation and Degradation of Nuclear p27kip1: Therapeutic Potential for Endometrial Cancer. Endocrinology. 2013;154:4030–4045. 10.1210/en.2013-1757. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nature Biotechnology. 2003;21:921–926. 10.1038/nbt849. [Abstract] [CrossRef] [Google Scholar]
- Peng D-J, Zeng M, Muromoto R, Matsuda T, Shimoda K, Subramaniam M, Spelsberg TC, Wei W-Z, Venuprasad K. Noncanonical K27-linked polyubiquitination of TIEG1 regulates Foxp3 expression and tumor growth. Journal of Immunology (Baltimore, Md: 1950) 2011;186:5638–5647. 10.4049/jimmunol.1003801. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pesiri V, Rosa PL, Stano P, Acconcia F. Identification of an estrogen receptor α non covalent ubiquitin-binding surface: role in 17β-estradiol-induced transcriptional activity. Journal of Cell Science. 2013;126:2577–2582. 10.1242/jcs.123307. [Abstract] [CrossRef] [Google Scholar]
- Phu L, Izrael-Tomasevic A, Matsumoto ML, Bustos D, Dynek JN, Fedorova AV, Bakalarski CE, Arnott D, Deshayes K, Dixit VM, et al. Improved quantitative mass spectrometry methods for characterizing complex ubiquitin signals. Molecular & Cellular Proteomics: MCP. 2011;10:M110.003756. 10.1074/mcp.M110.003756. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Picard N, Charbonneau C, Sanchez M, Licznar A, Busson M, Lazennec G, Tremblay A. Phosphorylation of Activation Function-1 Regulates Proteasome-Dependent Nuclear Mobility and E6-Associated Protein Ubiquitin Ligase Recruitment to the Estrogen Receptor β Molecular Endocrinology. 2008;22:317–330. 10.1210/me.2007-0281. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pickart CM. Mechanisms Underlying Ubiquitination. Annual Review of Biochemistry. 2001;70:503–533. 10.1146/annurev.biochem.70.1.503. [Abstract] [CrossRef] [Google Scholar]
- Poukka H, Aarnisalo P, Santti H, Jänne OA, Palvimo JJ. Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms. The Journal of Biological Chemistry. 2000;275:571–579. [Abstract] [Google Scholar]
- Powers GL, Ellison-Zelski SJ, Casa AJ, Lee AV, Alarid ET. Proteasome inhibition represses ERalpha gene expression in ER+ cells: a new link between proteasome activity and estrogen signaling in breast cancer. Oncogene. 2010;29:1509–1518. 10.1038/onc.2009.434. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pratt WB. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annual Review of Pharmacology and Toxicology. 1997;37:297–326. 10.1146/annurev.pharmtox.37.1.297. [Abstract] [CrossRef] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrine Reviews. 1997;18:306–360. 10.1210/edrv.18.3.0303. [Abstract] [CrossRef] [Google Scholar]
- Prenzel T, Begus-Nahrmann Y, Kramer F, Hennion M, Hsu C, Gorsler T, Hintermair C, Eick D, Kremmer E, Simons M, et al. Estrogen-dependent gene transcription in human breast cancer cells relies upon proteasome-dependent monoubiquitination of histone H2B. Cancer Research. 2011;71:5739–5753. 10.1158/0008-5472.CAN-11-1896. [Abstract] [CrossRef] [Google Scholar]
- Qi J, Tripathi M, Mishra R, Sahgal N, Fazil L, Ettinger S, Placzek WJ, Claps G, Chung LWK, Bowtell D, et al. The E3 Ubiquitin Ligase Siah2 Contributes to Castration-Resistant Prostate Cancer by Regulation of Androgen Receptor Transcriptional Activity. Cancer Cell. 2013;23:332–346. 10.1016/j.ccr.2013.02.016. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136:1098–1109. 10.1016/j.cell.2009.03.007. [Abstract] [CrossRef] [Google Scholar]
- Rajbhandari P, Schalper KA, Solodin NM, Ellison-Zelski SJ, Ping Lu K, Rimm DL, Alarid ET. Pin1 modulates ERα levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene. 2014;33:1438–1447. 10.1038/onc.2013.78. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ramamoorthy S, Nawaz Z. E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nuclear Receptor Signaling. 2008;6:e006. 10.1621/nrs.06006. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, Proteasome-Mediated Turnover of Unliganded and Liganded ERα on Responsive Promoters Is an Integral Feature of Estrogen Signaling. Molecular Cell. 2003;11:695–707. 10.1016/S1097-2765(03)00090-X. [Abstract] [CrossRef] [Google Scholar]
- Ren Y, Jiang H, Ma D, Nakaso K, Feng J. Parkin degrades estrogen-related receptors to limit the expression of monoamine oxidases. Human Molecular Genetics. 2011;20:1074–1083. 10.1093/hmg/ddq550. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rochette-Egly C, Adam S, Rossignol M, Egly JM, Chambon P. Stimulation of RAR alpha activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90:97–107. [Abstract] [Google Scholar]
- Le Romancer M, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L. Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Molecular Cell. 2008;31:212–221. 10.1016/j.molcel.2008.05.025. [Abstract] [CrossRef] [Google Scholar]
- Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir J-M, Corbo L. Cracking the Estrogen Receptor’s Posttranslational Code in Breast Tumors. Endocrine Reviews. 2011;32:597–622. 10.1210/er.2010-0016. [Abstract] [CrossRef] [Google Scholar]
- La Rosa P, Pesiri V, Marino M, Acconcia F. 17β-Estradiol-induced cell proliferation requires estrogen receptor (ER) α monoubiquitination. Cellular Signalling. 2011a;23:1128–1135. 10.1016/j.cellsig.2011.02.006. [Abstract] [CrossRef] [Google Scholar]
- La Rosa P, Marino M, Acconcia F. 17β-estradiol regulates estrogen receptor α monoubiquitination. IUBMB Life. 2011b;63:49–53. 10.1002/iub.414. [Abstract] [CrossRef] [Google Scholar]
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nature Reviews. Cancer. 2012;12:68–78. 10.1038/nrc3181. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sanchez M, Picard N, Sauvé K, Tremblay A. Coordinate regulation of estrogen receptor β degradation by Mdm2 and CREB-binding protein in response to growth signals. Oncogene. 2013;32:117–126. 10.1038/onc.2012.19. [Abstract] [CrossRef] [Google Scholar]
- Saville B, Poukka H, Wormke M, Janne OA, Palvimo JJ, Stoner M, Samudio I, Safe S. Cooperative coactivation of estrogen receptor alpha in ZR-75 human breast cancer cells by SNURF and TATA-binding protein. The Journal of Biological Chemistry. 2002;277:2485–2497. 10.1074/jbc.M109021200. [Abstract] [CrossRef] [Google Scholar]
- Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nature Reviews. Molecular Cell Biology. 2009;10:319–331. 10.1038/nrm2673. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Searle MS, Garner TP, Strachan J, Long J, Adlington J, Cavey JR, Shaw B, Layfield R. Structural insights into specificity and diversity in mechanisms of ubiquitin recognition by ubiquitin-binding domains. Biochemical Society Transactions. 2012;40:404–408. 10.1042/BST20110729. [Abstract] [CrossRef] [Google Scholar]
- Sengupta S, Wasylyk B. Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes & Development. 2001;15:2367–2380. 10.1101/gad.202201. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sentis S, Le Romancer M, Bianchin C, Rostan M-C, Corbo L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Molecular Endocrinology (Baltimore, Md) 2005;19:2671–2684. 10.1210/me.2005-0042. [Abstract] [CrossRef] [Google Scholar]
- Sheeler CQ, Singleton DW, Khan SA. Mutation of serines 104, 106, and 118 inhibits dimerization of the human estrogen receptor in yeast. Endocrine Research. 2003;29:237–255. [Abstract] [Google Scholar]
- Shinohara K, Tomioka M, Nakano H, Toné S, Ito H, Kawashima S. Apoptosis induction resulting from proteasome inhibition. The Biochemical Journal. 1996;317 (Pt 2):385–388. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Molecular Endocrinology (Baltimore, Md) 1993;7:1418–1429. 10.1210/mend.7.11.7906860. [Abstract] [CrossRef] [Google Scholar]
- Smith DF, Toft DO. Steroid receptors and their associated proteins. Molecular Endocrinology. 1993;7:4–11. 10.1210/mend.7.1.8446107. [Abstract] [CrossRef] [Google Scholar]
- Sobhian B, Shao G, Lilli DR, Culhane AC, Moreau LA, Xia B, Livingston DM, Greenberg RA. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science (New York, NY) 2007;316:1198–1202. 10.1126/science.1139516. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 2000;102:67–76. [Abstract] [Google Scholar]
- Stancato LF, Silverstein AM, Gitler C, Groner B, Pratt WB. Use of the thiol-specific derivatizing agent N-iodoacetyl-3-[125I]iodotyrosine to demonstrate conformational differences between the unbound and hsp90-bound glucocorticoid receptor hormone binding domain. The Journal of Biological Chemistry. 1996;271:8831–8836. [Abstract] [Google Scholar]
- Stavreva DA, Müller WG, Hager GL, Smith CL, McNally JG. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Molecular and Cellular Biology. 2004;24:2682–2697. [Europe PMC free article] [Abstract] [Google Scholar]
- Subramanian K, Jia D, Kapoor-Vazirani P, Powell DR, Collins RE, Sharma D, Peng J, Cheng X, Vertino PM. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Molecular Cell. 2008;30:336–347. 10.1016/j.molcel.2008.03.022. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sultana R, Theodoraki MA, Caplan AJ. Specificity in the actions of the UBR1 ubiquitin ligase in the degradation of nuclear receptors. FEBS Open Bio. 2013;3:394–397. 10.1016/j.fob.2013.09.003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sun J, Zhou W, Kaliappan K, Nawaz Z, Slingerland JM. ERα phosphorylation at Y537 by Src triggers E6-AP-ERα binding, ERα ubiquitylation, promoter occupancy, and target gene expression. Molecular Endocrinology (Baltimore, Md) 2012;26:1567–1577. 10.1210/me.2012-1140. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Takano Y, Adachi S, Okuno M, Muto Y, Yoshioka T, Matsushima-Nishiwaki R, Tsurumi H, Ito K, Friedman SL, Moriwaki H, et al. The RING finger protein, RNF8, interacts with retinoid X receptor alpha and enhances its transcription-stimulating activity. The Journal of Biological Chemistry. 2004;279:18926–18934. 10.1074/jbc.M309148200. [Abstract] [CrossRef] [Google Scholar]
- Talukder AH, Mishra SK, Mandal M, Balasenthil S, Mehta S, Sahin AA, Barnes CJ, Kumar R. MTA1 interacts with MAT1, a cyclin-dependent kinase-activating kinase complex ring finger factor, and regulates estrogen receptor transactivation functions. The Journal of Biological Chemistry. 2003;278:11676–11685. 10.1074/jbc.M209570200. [Abstract] [CrossRef] [Google Scholar]
- Tateishi Y, Sonoo R, Sekiya Y, Sunahara N, Kawano M, Wayama M, Hirota R, Kawabe Y, Murayama A, Kato S, et al. Turning Off Estrogen Receptor β-Mediated Transcription Requires Estrogen-Dependent Receptor Proteolysis. Molecular and Cellular Biology. 2006;26:7966–7976. 10.1128/MCB.00713-06. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 1999;5:2638–2645. [Abstract] [Google Scholar]
- Terpos E, Kanellias N, Christoulas D, Kastritis E, Dimopoulos MA. Pomalidomide: a novel drug to treat relapsed and refractory multiple myeloma. OncoTargets and Therapy. 2013;6:531–538. 10.2147/OTT.S34498. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Teyssier C, Ou C-Y, Khetchoumian K, Losson R, Stallcup MR. Transcriptional intermediary factor 1alpha mediates physical interaction and functional synergy between the coactivator-associated arginine methyltransferase 1 and glucocorticoid receptor-interacting protein 1 nuclear receptor coactivators. Molecular Endocrinology (Baltimore, Md) 2006;20:1276–1286. 10.1210/me.2005-0393. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Thénot S, Henriquet C, Rochefort H, Cavaillès V. Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1. The Journal of Biological Chemistry. 1997;272:12062–12068. [Abstract] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. The EMBO Journal. 2000;19:94–102. 10.1093/emboj/19.1.94. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proceedings of the National Academy of Sciences of the United States of America. 1966;55:1574–1581. [Europe PMC free article] [Abstract] [Google Scholar]
- Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nature Cell Biology. 2009;11:123–132. 10.1038/ncb1821. [Abstract] [CrossRef] [Google Scholar]
- Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. 10.1038/nature09815. [Abstract] [CrossRef] [Google Scholar]
- Totta P, Pesiri V, Marino M, Acconcia F. Lysosomal function is involved in 17β-estradiol-induced estrogen receptor α degradation and cell proliferation. PloS One. 2014;9:e94880. 10.1371/journal.pone.0094880. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science (New York, NY) 2009;324:787–790. 10.1126/science.1168175. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tzeng DZ, Klinge CM. Phosphorylation of purified estradiol-liganded estrogen receptor by casein kinase II increases estrogen response element binding but does not alter ligand stability. Biochemical and Biophysical Research Communications. 1996;223:554–560. 10.1006/bbrc.1996.0933. [Abstract] [CrossRef] [Google Scholar]
- Valley CC, Métivier R, Solodin NM, Fowler AM, Mashek MT, Hill L, Alarid ET. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Molecular and Cellular Biology. 2005;25:5417–5428. 10.1128/MCB.25.13.5417-5428.2005. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vanaja DK, Mitchell SH, Toft DO, Young CYF. Effect of geldanamycin on androgen receptor function and stability. Cell Stress & Chaperones. 2002;7:55–64. [Europe PMC free article] [Abstract] [Google Scholar]
- Wallace AD, Cidlowski JA. Proteasome-mediated Glucocorticoid Receptor Degradation Restricts Transcriptional Signaling by Glucocorticoids. Journal of Biological Chemistry. 2001;276:42714–42721. 10.1074/jbc.M106033200. [Abstract] [CrossRef] [Google Scholar]
- Wang X, DeFranco DB. Alternative Effects of the Ubiquitin-Proteasome Pathway on Glucocorticoid Receptor Down-Regulation and Transactivation Are Mediated by CHIP, an E3 Ligase. Molecular Endocrinology. 2005;19:1474–1482. 10.1210/me.2004-0383. [Abstract] [CrossRef] [Google Scholar]
- Wang LH, Yang XY, Zhang X, An P, Kim H-J, Huang J, Clarke R, Osborne CK, Inman JK, Appella E, et al. Disruption of estrogen receptor DNA-binding domain and related intramolecular communication restores tamoxifen sensitivity in resistant breast cancer. Cancer Cell. 2006;10:487–499. 10.1016/j.ccr.2006.09.015. [Abstract] [CrossRef] [Google Scholar]
- Weis KE, Ekena K, Thomas JA, Lazennec G, Katzenellenbogen BS. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Molecular Endocrinology (Baltimore, Md ) 1996;10:1388–1398. 10.1210/mend.10.11.8923465. [Abstract] [CrossRef] [Google Scholar]
- Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Molecular Endocrinology (Baltimore, Md) 1996;10:705–712. 10.1210/mend.10.6.8776730. [Abstract] [CrossRef] [Google Scholar]
- Wickliffe KE, Williamson A, Meyer H-J, Kelly A, Rape M. K11-linked ubiquitin chains as novel regulators of cell division. Trends in Cell Biology. 2011;21:656–663. 10.1016/j.tcb.2011.08.008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wijayaratne AL, McDonnell DP. The Human Estrogen Receptor-α Is a Ubiquitinated Protein Whose Stability Is Affected Differentially by Agonists, Antagonists, and Selective Estrogen Receptor Modulators. Journal of Biological Chemistry. 2001;276:35684–35692. 10.1074/jbc.M101097200. [Abstract] [CrossRef] [Google Scholar]
- Van Wijk SJL, Timmers HTM. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2010;24:981–993. 10.1096/fj.09-136259. [Abstract] [CrossRef] [Google Scholar]
- Wu W, Nishikawa H, Hayami R, Sato K, Honda A, Aratani S, Nakajima T, Fukuda M, Ohta T. BRCA1 ubiquitinates RPB8 in response to DNA damage. Cancer Research. 2007;67:951–958. 10.1158/0008-5472.CAN-06-3187. [Abstract] [CrossRef] [Google Scholar]
- Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. The Journal of Biological Chemistry. 2003;278:34743–34746. 10.1074/jbc.C300249200. [Abstract] [CrossRef] [Google Scholar]
- Xu P, Peng J. Dissecting the ubiquitin pathway by mass spectrometry. Biochimica Et Biophysica Acta. 2006;1764:1940–1947. 10.1016/j.bbapap.2006.09.004. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative Proteomics Reveals the Function of Unconventional Ubiquitin Chains in Proteasomal Degradation. Cell. 2009a;137:133–145. 10.1016/j.cell.2009.01.041. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu K, Shimelis H, Linn DE, Jiang R, Yang X, Sun F, Guo Z, Chen H, Li W, Chen H, et al. Regulation of Androgen Receptor Transcriptional Activity and Specificity by RNF6-Induced Ubiquitination. Cancer Cell. 2009b;15:270–282. 10.1016/j.ccr.2009.02.021. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu M, Skaug B, Zeng W, Chen ZJ. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFalpha and IL-1beta. Molecular Cell. 2009c;36:302–314. 10.1016/j.molcel.2009.10.002. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Israël A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. [Abstract] [Google Scholar]
- Yang CH, Gonzalez-Angulo AM, Reuben JM, Booser DJ, Pusztai L, Krishnamurthy S, Esseltine D, Stec J, Broglio KR, Islam R, et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2006;17:813–817. 10.1093/annonc/mdj131. [Abstract] [CrossRef] [Google Scholar]
- Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nature Reviews. Molecular Cell Biology. 2009;10:755–764. 10.1038/nrm2780. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yokota K, Shibata H, Kobayashi S, Suda N, Murai A, Kurihara I, Saito I, Saruta T. Proteasome-mediated mineralocorticoid receptor degradation attenuates transcriptional response to aldosterone. Endocrine Research. 2004;30:611–616. [Abstract] [Google Scholar]
- Yudt MR, Vorojeikina D, Zhong L, Skafar DF, Sasson S, Gasiewicz TA, Notides AC. Function of Estrogen Receptor Tyrosine 537 in Hormone Binding, DNA Binding, and Transactivation† Biochemistry. 1999;38:14146–14156. 10.1021/bi9911132. [Abstract] [CrossRef] [Google Scholar]
- Zhang P-J, Zhao J, Li H-Y, Man J-H, He K, Zhou T, Pan X, Li A-L, Gong W-L, Jin B-F, et al. CUE domain containing 2 regulates degradation of progesterone receptor by ubiquitin–proteasome. The EMBO Journal. 2007;26:1831–1842. 10.1038/sj.emboj.7601602. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhao J, Zhang Z, Vucetic Z, Soprano KJ, Soprano DR. HACE1: A novel repressor of RAR transcriptional activity. Journal of Cellular Biochemistry. 2009;107:482–493. 10.1002/jcb.22146. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9587–9592. 10.1073/pnas.171174298. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhu J, Gianni M, Kopf E, Honoré N, Chelbi-Alix M, Koken M, Quignon F, Rochette-Egly C, de Thé H. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor α (RARα) and oncogenic RARα fusion proteins. Proceedings of the National Academy of Sciences. 1999;96:14807–14812. 10.1073/pnas.96.26.14807. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhu J, Zhao C, Kharman-Biz A, Zhuang T, Jonsson P, Liang N, Williams C, Lin C-Y, Qiao Y, Zendehdel K, et al. The atypical ubiquitin ligase RNF31 stabilizes estrogen receptor α and modulates estrogen-stimulated breast cancer cell proliferation. Oncogene. 2014;33:4340–4351. 10.1038/onc.2013.573. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1530/jme-14-0308
Read article for free, from open access legal sources, via Unpaywall:
https://jme.bioscientifica.com/downloadpdf/journals/jme/54/3/R151.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1530/jme-14-0308
Article citations
Ubiquitination and deubiquitination in the regulation of N6-methyladenosine functional molecules.
J Mol Med (Berl), 102(3):337-351, 30 Jan 2024
Cited by: 1 article | PMID: 38289385
Review
Regulation of PXR in drug metabolism: chemical and structural perspectives.
Expert Opin Drug Metab Toxicol, 20(1-2):9-23, 28 Jan 2024
Cited by: 1 article | PMID: 38251638
Review
Estrogen Receptor Signaling in Breast Cancer.
Cancers (Basel), 15(19):4689, 23 Sep 2023
Cited by: 10 articles | PMID: 37835383 | PMCID: PMC10572081
Review Free full text in Europe PMC
Human nuclear hormone receptor activity contributes to malaria parasite liver stage development.
Cell Chem Biol, 30(5):486-498.e7, 11 May 2023
Cited by: 2 articles | PMID: 37172592 | PMCID: PMC10878326
Mechanistic Studies and a Retrospective Cohort Study: The Interaction between PPAR Agonists and Immunomodulatory Agents in Multiple Myeloma.
Cancers (Basel), 14(21):5272, 27 Oct 2022
Cited by: 2 articles | PMID: 36358696 | PMCID: PMC9657746
Go to all (27) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A portrayal of E3 ubiquitin ligases and deubiquitylases in cancer.
Int J Cancer, 133(12):2759-2768, 25 Mar 2013
Cited by: 37 articles | PMID: 23436247
Review
Ubiquitination profiling identifies sensitivity factors for IAP antagonist treatment.
Biochem J, 466(1):45-54, 01 Feb 2015
Cited by: 5 articles | PMID: 25423073
Cullin 5-RING E3 ubiquitin ligases, new therapeutic targets?
Biochimie, 122:339-347, 04 Aug 2015
Cited by: 10 articles | PMID: 26253693
Review
Linking the ubiquitin-proteasome pathway to chromatin remodeling/modification by nuclear receptors.
J Mol Endocrinol, 34(2):281-297, 01 Apr 2005
Cited by: 65 articles | PMID: 15821097
Review
Funding
Funders who supported this work.
NCI NIH HHS (5)
Grant ID: CA159578
Grant ID: F31 CA186551
Grant ID: R01 CA159578
Grant ID: CA77474
Grant ID: R01 CA077474
NIGMS NIH HHS (2)
Grant ID: R01 GM083681
Grant ID: GM083681




