Abstract
Free full text

Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery
Abstract
The use of nanoparticulate pharmaceutical drug delivery systems (NDDSs) to enhance the in vivo effectiveness of drugs is now well established. The development of multifunctional and stimulus-sensitive NDDSs is an active area of current research. Such NDDSs can have long circulation times, target the site of the disease and enhance the intracellular delivery of a drug. This type of NDDS can also respond to local stimuli that are characteristic of the pathological site by, for example, releasing an entrapped drug or shedding a protective coating, thus facilitating the interaction between drug-loaded nanocarriers and target cells or tissues. In addition, imaging contrast moieties can be attached to these carriers to track their real-time biodistribution and accumulation in target cells or tissues. Here, I highlight recent developments with multifunctional and stimuli-sensitive NDDSs and their therapeutic potential for diseases including cancer, cardiovascular diseases and infectious diseases.
Nanoparticulate pharmaceutical drug delivery systems (NDDSs) are widely used in pharmaceutical research and in clinical settings to enhance the effectiveness of diagnostic agents and drugs, including anticancer, antimicrobial and antiviral drugs1,2. The types of nano-carriers that exist are diverse and include the following: liposomes; polymeric nanoparticles; polymeric micelles; silica, gold, silver and other metal nanoparticles; carbon nanotubes; solid lipid nanoparticles; niosomes; and dendrimers. The use of NDDSs can overcome several problems that are associated with traditional drugs, such as poor aqueous solubility, low bioavailability and nonspecific distribution in the body.
The first generation of NDDSs mainly aimed to address single challenges, such as the need to increase drug stability in vivo and the circulation time in the blood, or the need to target a drug to a specific tissue or pathology. Now, research has led to the development of NDDSs that can perform two or more functions (either simultaneously or sequentially) to overcome multiple physiological barriers to optimize delivery and deliver their loads (which can be single or multiple) to the required target sites (such as organs, tissues, cells) or specific pathologies in the body3 (FIG. 1). The properties of multifunctional NDDSs include the ability to bear a sufficient load of a drug or DNA-related material, have increased circulation times (through the use of soluble polymers) and target the intended site of action both nonspecifically (for example, via the enhanced permea-bility and retention (EPR) effect) and specifically (via the attachment of target-specific ligands). In addition, multifunctional NDDSs can respond to several stimuli that are characteristic of the pathological site, which is achieved through the inclusion of components that react to abnormal pH, temperature and redox conditions, and to the overexpression of certain biological molecules. Multifunctional NDDSs can also respond to stimuli from outside the body, such as magnetic or ultrasound fields, and can be supplemented with an imaging contrast moiety to enable their biodistribution, target accumulation or the efficacy of the therapy to be monitored.
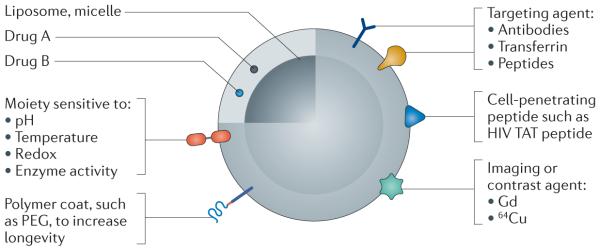
Drugs (Drug A and Drug B) can be loaded into a pharmaceutical nanocarrier, such as a liposome or polymeric micelle. Depending on the purpose of the nanoparticulate pharmaceutical drug delivery system (NDDS), various agents can be added to the nanoparticle to target the NDDS to a particular tissue, to increase cell penetration, to enable imaging or to release the drugs in response to a given stimulus.
PEG, poly(ethylene glycol).
Although as yet there is no broadly recognized and accepted single classification system for multifunctional NDDSs, they can generally be divided into three groups. The first group consists of drug-loaded NDDSs that com-bine at least two different functions, such as longevity, targetability, stimuli-sensitivity or cell penetration. The second group of NDDSs, in addition to the previously described properties, are loaded with more than one drug and/or gene therapy-related material, such as antisense oligonucleotides or small interfering RNAs (siRNAs). The third group consists of so-called theranostic NDDSs, which have an additional diagnostic label for use with current clinical imaging modalities.
Research in the area of multifunctional NDDSs4,5 is very active, but substantial work remains to make them a clinical reality. Here, I highlight recent developments relating to multifunctional NDDSs. The majority of the currently available data relate to cancer, although there are some examples with other diseases.
NDDS longevity and targeting
One of the most common uses of NDDSs is to combine prolonged circulation times with targetabilty. Such NDDSs are particularly useful for tumour targeting because tumours (as well as other inflammation zones) usually have increased vascular permeability as well as poor lymphatic drainage6,7. This enables long-circulating NDDSs to accumulate in tumours through the EPR effect, which forms the basis for passive targeting8 . Nevertheless, EPR-based drug delivery strategies face several challenges. First, tumours — especially large, solid tumours — are pathophysiologically heterogeneous. Some parts of such tumours are not vascularized, do not exhibit the EPR effect, may have sizeable necrotic areas9,10 and have varied microvascular permeability10. In addition, the increased interstitial pressure that exists within tumours may limit the EPR-mediated accumulation of NDDSs even if the vasculature is leaky11.
NDDSs that are used for passive targeting and/or spontaneous accumulation must have long circulation times to ensure that sufficient drug is delivered to the target tissue. The usual approach to obtain long-circulating NDDSs is to coat them with hydrophilic and flexible polymers, such as poly(ethylene glycol) (PEG), as was first suggested for liposomes12. The pegylation of NDDSs prevents their interaction with opsonins and impedes their capture and clearance by the mononuclear phagocyte system. However, pegylated NDDSs can induce the production of antibodies that can accelerate the blood clearance of nanoparticles (as was demonstrated for clinically used pegylated liposomes), particularly after repetitive administration; this is the so-called accelerated blood clearance phenomenon13,14.
Although PEG is still the gold-standard polymer that is used to prepare long-circulating NDDSs, other hydrophilic polymers that are used include poly[N-(2-hydroxypropyl) methacrylamide]15, poly(acryloyl morpholine), poly-N-vinylpyrrolidones16 and polyvinyl alcohol17. In general, the concept of pegylation, as well as the use of alternative polymers for longevity, is well established and reviewed; thus, in the next section only some of the key developments in this area are briefly mentioned. Importantly, the shape of NDDSs can also influence their pharmacokinetics and biodistribution18,19.
Liposomal long-circulating NDDSs are the most frequently studied type of NDDS; however, synthetic amphiphilic polymers have also been used to sterically stabilize several other types of NDDS to alter their bio-distribution. For example, pegylation of gold nanoparticles reduced their uptake by the mononuclear phagocyte system and their subsequent clearance from the body20. One potential application of pegylated gold nanoparticles is their use in photothermal tumour therapy (known as ablation) following their accumulation in the tumour21. Pegylated methotrexate-conjugated poly-l-lysine dendrimers also accumulated efficiently in solid tumours in rats and mice via the EPR affect22, and pegylation reduced the toxicity of positive charge-bearing dendrimers in cell-culture experiments23. Quantum dots can also be modified by pegylation. In mice, pegylated quantum dots had prolonged circulation times, which were attrib-utable to their decreased uptake in organs (such as the spleen and liver) in which the mononuclear phagocyte system is active24.
Active targeting of NDDSs can be achieved by attaching targeting ligands, such as monoclonal antibodies, transferrin, various peptides, folate, aptamers (single-stranded oligonucleotides) or certain sugar moieties, onto their surface25,26. To prevent steric hindrance between the targeting moiety and the protective polymer (such as PEG), the targeting ligand is usually attached to the chemically activated distal end of the NDDS-grafted polymeric chain27. An example of such active targeting of NDDSs is pegylated doxorubicin-loaded liposomes that have human epidermal receptor 2 (HER2; also known as ERBB2)-specific antibodies attached; these were suc-cessfully used to target HER2-overexpressing SK-BR3 cells in mice28. A nucleosome-specific monoclonal antibody (mAb 2C5) that recognized multiple types of tumour cells via tumour cell-surface-bound nucleosomes improved the targeting of doxorubicin-loaded liposomes to tumour cells and increased cancer cell cytotoxicity in in vitro and in vivo models29 , including nude mice xeno-grafted with the U-87 cell line, which was derived from a human glioblastoma30. In another example of active targeting, pegylated gold nanoparticles conjugated to monoclonal F19 antibodies were used as targeted label-ling agents for human pancreatic carcinoma tissues31. Lactoferrin-conjugated PEG–polylactic-acid nanoparticles improved the delivery of the conjugated particles to the brain in an experimental murine model32.
An interesting approach to increase targeting centres on self-assembling polyalkylcyanoacrylate-based nanoparticles. These types of nanoparticles could serve as pharmaceutical carriers for various drugs and could be produced in pegylated and ‘activated’ forms, enabling various ligands to be easily attached33. Polyisohexyl-cyanoacrylate nanoparticles loaded with doxorubicin produced by BioAlliance Pharma as Livatag (doxorubicin Transdrug) are currently in a Phase III trial in Europe and in the United States as a second-line treatment of hepatocellular carcinoma after sorafenib, at a stage with no available approved treatment (Livatag press release; see Further information).
Aptamers that impart affinity and specificity by electrostatic, hydrogen or hydrophobic bonding, but not via the base pairing, have also been used as stable and efficient targeting moieties for NDDSs. For example, liposomal NDDSs modified with specific aptamers have been used to target leukaemia cells34.
Targetability can also be applied to stimuli-sensitive NDDSs. Gemcitabine-loaded pegylated pH-sensitive liposomes modified with an epidermal growth factor receptor (EGFR)-specific antibody efficiently inhibited tumour growth in mice35. Micellar NDDSs that were composed of amphiphilic conjugates of PEG and a co-polymer of poly(ε-caprolactone) and poly(malic acid) loaded with doxorubicin conjugated to poly(malic acid) via a pH-sensitive bond were modified with folate for enhanced cellular uptake36. This preparation provided an enhanced release of doxorubicin at lowered pH values inside cancer cells36. NDDSs with similar properties based on pH-sensitive polymethacrylate (PMA)-grafted poly(amidoamine) nanocarriers and additionally pegylated and modified with a folate moiety efficiently delivered paclitaxel to tumours and inhibited tumour growth in mice37.
Clearly, the separation of long-circulating NDDSs into those that achieve active targeting and passive targeting is conditional. That is, both phenomena are closely connected because NDDSs will accumulate in the target area via the EPR effect (passive targeting) before ligand-mediated interaction with target cells (active targeting) occurs.
Another interesting issue is associated with the use of targeting ligands that are also naturally present (in their free form) in the circulation, such as folate, transferrin or certain peptides. Although one may expect competition between the ligands attached to NDDSs and the native ligands for the binding sites, the success of folate-and transferrin-targeted NDDSs (see below) demonstrates that this potential problem can be overcome. This is probably achieved through multipoint interactions of ligand-modified NDDSs with the target and/or because of the rapid recirculation of the cognate receptors, which provides sufficient opportunities for an interaction of the NDDS with the receptor.
Disease applications for NDDS-based therapies
Cardiovascular diseases
NDDSs hold promise as therapeutic, diagnostic and theranostic agents for cardio-vascular diseases38, particularly atherosclerosis39 (FIG. 2). Multifunctional micellar NDDSs that combined a targeting pentapeptide, a fluorophore and a drug targeting atherosclerotic plaques by specifically binding to clotted plasma proteins were used to visualize atherosclerotic lesions in a mouse model. This preparation was shown to increase the amount of the anticoagulant agent biva-lirudin that was delivered to the lesion40. Because the increased uptake of low-density lipoproteins (LDLs) by resident macrophages in plaques is associated with the progression of atherosclerosis, targeting plaque-associated macrophages could be a method of inhibiting LDL uptake41. Indeed, targeted polymeric NDDSs loaded with pravastatin specifically and dramatically inhibited the phagocytic activity of macrophages without affecting non-target cells42.
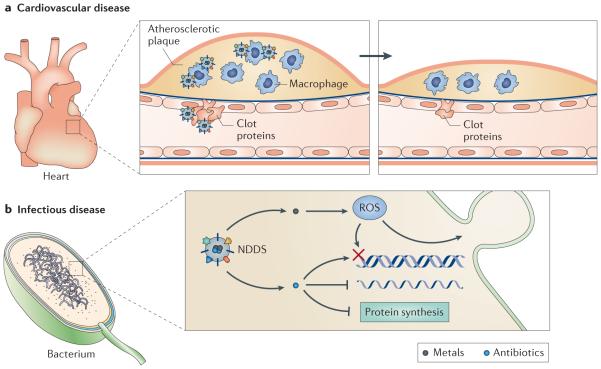
a | Nanoparticulate pharmaceutical drug delivery systems (NDDSs) can be engineered to target activated macrophages in atherosclerotic plaques or proteins present in a clot. These nanoparticles can thereby reduce plaque size through these two distinct mechanisms. b | NDDSs can be used to deliver metals to bacteria, where they can generate reactive oxygen species (ROS) to induce membrane blebbing and DNA damage. Antibiotics can also be delivered intracellularly using NDDSs.
Multimodal diagnostic micellar NDDSs that were fluorescent and paramagnetic and targeted to macrophage scavenger receptors using specific antibodies have been used to visualize lesions in the abdominal aortas of mice with atherosclerosis43. Furthermore, polymeric NDDSs made of polyketals that were used to deliver small-molecule drugs, such as the p38 mitogen-activated protein kinase (MAPK) inhibitor SB239063, and anti-oxidant proteins, such as superoxide dismutase, to the area of the myocardial infarction in a rat model significantly improved cardiac function44.
Liposome-based systems have also been frequently used to study the potential use of NDDSs in cardiovascular diseases45. For example, liposomes loaded with ATP or co-enzyme Q accumulated well in the infarcted areas of the myocardium and improved cardiac parameters in rat and rabbit models of myocardial infarct46,47. In a rat model of heart transplant, organ rejection was simultaneously imaged and treated using multifunctional polymeric NDDSs co-loaded with superparamagnetic iron oxide nanoparticles (SPIONs) and plasmid DNA48. The SPION acted as a magnetic resonance imaging (MRI) contrast agent, whereas the plasmid DNA suppressed the local immune response48. NDDSs for targeted thrombolytic therapy were developed by conjugating a thrombolytic agent (recombinant tissue plasminogen activator) to dextran-coated iron oxide nanoparticles, which were additionally modified with a thrombus-targeted peptide that was sensitive to activated factor XIII49. These NDDSs had increased binding to the margins of intravascular thrombi and good thrombolytic activity in a mouse model of pulmonary embolism.
The translation of these NDDSs and others identified in similar studies into clinical practice remains the primary challenge to advancing the field and will be addressed in the closing section of this article.
Infectious diseases
NDDSs have a growing role in combatting infectious diseases50. Of particular interest here are multicomponent NDDSs that contain metals, which can facilitate the formation of reactive oxygen species to eliminate pathogenic bacteria (FIG. 2). For example, NDDSs that combined iodinated chitosan and silver nanoparticles bound to and killed bacteria better than either component alone51. Hydrogel and glass-based nanoparticles capable of releasing nitric oxide facilitated the cleaning and healing of open wounds in mice52. NDDSs can be used to deliver antibiotics inside cells; murine salmonellosis was successfully treated (with a greater than tenfold decrease in the level of pathogen in the liver and spleen of experimental mice) using gentamicin in silica xerogel-based NDDSs53. The bacteria-mediated degradation of a polyphosphoester core of antibiotic-loaded NDDSs by bacterial enzymes54 provided an interesting example of a targeted, responsive NDDS. Nevertheless, this area is in early stages of development and more animal-based studies will be required to reveal its full potential.
Cancer
The majority of preclinical research with NDDSs relates to cancer. Based on one of the most popular pharmaceutical nanocarriers — liposomes — several anticancer NDDSs are available at different stages of development (see TABLE 1 for a selected list).
Table 1
Liposome-based drugs for cancer therapy
| Drug formulation | Indication | Status* | Refs |
|---|---|---|---|
| Liposomal doxorubicin (Doxil, also known as Caelyx; Janssen) | AIDS-related Kaposi’s sarcoma, recurrent ovarian cancer, metastatic breast cancer and multiple myeloma | Approved | 165 |
|
| |||
| Non-pegylated liposomal doxorubicin (Myocet; Sopherion Therapeutics) | Metastatic breast cancer | Approved | 168,169 |
|
| |||
| Liposomal doxorubicin (LipoDox; Sun Pharma Global FZE) | Kaposi’s sarcoma, breast and ovarian cancer | Approved | 170,171 |
|
| |||
| Thermally sensitive liposomal doxorubicin (Thermodox; Celsion ) | Hepatocellular carcinoma | Phase III, active: NCT00617981 | 182 – 184 |
|
| |||
| Breast cancer | Phase I/II, recruiting: NCT00826085 | ||
|
| |||
| Hepatocellular carcinoma | Phase III, recruiting: NCT02112656 | ||
|
| |||
| Liposomal paclitaxel (Lipusu; Luye Pharma) | Solid tumours | Approved in China | 166 |
|
| |||
| Liposomal paclitaxel (EndoTAG-1; Medigene) | Breast cancer | Phase II, completed: NCT01537536, NCT00448305 | 175,176 |
|
| |||
| Pancreatic cancer | Phase II, completed: NCT00377936 | ||
|
| |||
| Liver cancer | Phase II, completed: NCT00542048 | ||
|
| |||
| Liposomal paclitaxel (LEP-ETU; NeoPharm) | Breast cancer | Phase II, completed: NCT01190982 | 202 |
|
| |||
| Liposomal daunorubicin (DaunoXome; Galen) | Kaposi’s sarcoma | Approved | 167 |
|
| |||
| Liposomal vincristine (Marqibo; Talon Therapeutics) | Acute lymphoblastic leukaemia | Approved | 172 – 174 |
|
| |||
| Liposomal cisplatin (LiPlaCis; LiPlasome Pharma) | Advanced or refractory solid tumours | Phase I, recruiting: NCT01861496 | 203 |
|
| |||
| Liposomal cisplatin (Lipoplatin; Regulon) | Pancreatic cancer, non-small-cell lung cancer, head and neck cancer and breast cancers | Phase I/II/III | 175, 177–179 |
|
| |||
| Liposomal cisplatin (SPI-077; Azla Corporation) | Ovarian cancer | Phase II, completed: NCT00004083 | 175,180, 181 |
|
| |||
| Liposomal formulation of SN-38, the active metabolite of irinotecan (LE-SN38; NeoPharm) | Colorectal carcinoma | Phase II, completed: NCT00311610 | 175,185 |
|
| |||
| Liposomal irinotecan HCl: floxuridine mixture (CPX-1; Celator Pharmaceuticals) | Colorectal cancer | Phase II, completed: NCT00361842 | 182 |
|
| |||
| Liposomal irinotecan (MM-398; Merrimack Pharmaceuticals) | Metastatic pancreatic cancer | Phase III, active: NCT01494506 | 204 |
|
| |||
| Liposomal L-annamycin | Acute lymphocytic leukaemia, doxorubicin-resistant blood cancer | Phase I/II | 186,187 |
|
| |||
| Liposomal vinorelbine, INX-0125 (AlocrestTM; Hana Biosciences) | Hodgkin’s disease, non-Hodgkin’s lymphoma | Phase I, active: NCT00364676 | 188 |
The next step in the development of anticancer NDDSs is to target them to tumours. Cancer cells contain several targets discussed below that can be used by NDDSs. Each of these targets could be specific for a certain type or types of cancer. Although it is possible that there will be overlapping expression of a target in cancer cells and normal cells, the level of expression is usually much higher in cancer cells; this characteristic is a prerequisite for an NDDS-targeting ligand.
Folate has been used as a targeting ligand in preclinical studies of NDDSs, owing to its easy conjugation to nanocarriers, high affinity for folate receptors and the lower expression of folate receptors in normal cells then in cancer cells and activated macrophages that are typical of inflammatory diseases55. Numerous folate-targeted liposomal systems for cancer have been described (reviewed in REFS 56,57). For example, liposomes modified with folate via a PEG spacer efficiently delivered their cargo intracellularly through receptor-mediated endocytosis (which could bypass multidrug resistance), and doxorubicin-mediated cytotoxicity against cancer cells expressing folate receptors was 85-fold higher with drug-loaded targeted liposomes than with unmodified liposomes58. In addition, when glutathione-conjugated folate was coupled to a PEG–distearoylphosphatidyl-ethanolamine (PEG–DSPE) polymer and incorporated into the liposomal membrane, there was increased activity of the liposomal vincristine and favourable pharmaco-kinetics in mice59. Folate-based modified NDDSs loaded with paclitaxel60 or carboplatin61 had high antitumour activity in mouse models. Liposome targeting to folate receptors has also been used to improve gene delivery of the herpes simplex virus type 1 thymidine kinase suicide gene; the liposome preparation inhibited tumour growth in a mouse model of oral cancer62.
Transferrin, an 80 kDa serum glycoprotein, binds to the transferrin receptor and is taken into cells via receptor-mediated endocytosis. Compared with normal cells, the expression of transferrin receptor in certain cancer cells is much higher because of their increased demand for iron. Consequently, transferrin-receptor-targeted NDDSs could be used for anticancer therapy. Indeed, this was demonstrated with doxorubicin-loaded long-circulating transferrin-modified liposomes in a rat model of liver cancer63. Other examples of the anticancer effects of transferrin-targeted liposomes in mouse models include those loaded with boron compounds64,65 and with ceramide66.
EGFRs are frequently overexpressed in solid tumours67,68 and are therefore popular targets for NDDSs. HER2, which is overexpressed in approximately 20% of breast cancers68 and some other cancers, is also a popular target for NDDSs. For example, there was greater accumulation of HER2-targeted pegylated liposomes loaded with doxorubicin than of non-targeted liposomes in HER2-overexpressing breast cancer cells69 and in ascitic lymphoma cells70.
The tumour vasculature is also an attractive target; destruction of the vasculature inhibits tumour growth and metastasis, it is not tumour type-specific and, importantly, NDDSs do not need to diffuse into the tumour mass to reach this target71. Targets that have been the focus of most studies include vascular endothelial growth factor (VEGF; especially for anti-angiogenic therapy), vascular cell adhesion molecule (VCAM), matrix metalloproteinases (MMPs) and integrins72,73. For example, peptides that bind specifically to the tumour vasculature coupled to liposomes loaded with doxorubicin improved drug efficacy against several human cancers in severe combined immunodeficient mice74. Pegylated VCAM-targeted immunoliposomes bound selectively to activated endothelial cells in vitro and accumulated in tumour vessels in vivo75. MMPs are overexpressed on the newly formed vessels and tumour tissues, and are involved in tissue remodelling, tumour invasiveness, resistance to apoptosis and metastasis. An antigen-binding fragment of the antibody against MMP that was conjugated to doxorubicin-loaded liposomes via a PEG spacer showed enhanced tumoural uptake and inhibited tumour growth in a mouse model76.
Considerations for NDDS receptor targeting
The success of receptor-mediated targeting depends strongly on the target affinity and the density of the receptors that are present on the cell surface. In addition, with pegylated NDDSs, the surface-attached PEG could prevent or hinder the interaction between the targeting ligand and its receptor. In a sense, this limits the opportunities of targeting because not all potential targets are expressed at sufficient levels. It is also important to consider, on a case by case basis, whether spontaneous targeting (passive accumula-tion; based on the EPR effect) or ligand-mediated specific targeting (which clearly requires more synthetic effort) is preferable. In general, if good accumulation of a drug in the pathological tissue is the primary goal, then EPR-mediated accumulation may suffice. However, if the presence of the drug inside the cell is needed then ligands that can be internalized may be required.
Stimuli-sensitive NDDSs
The use of NDDSs that respond to different types of stimuli to control the properties and behaviour of the NDDS is an important and growing area of research77 (FIG. 3; TABLE 2). The stimuli can be internal and intrinsic to the target tissue (such as changes in pH, temperature, redox condition or the activity of certain enzymes) or external and artificially applied (such as a magnetic field, ultrasound and various types of irradiation) (FIG. 4).
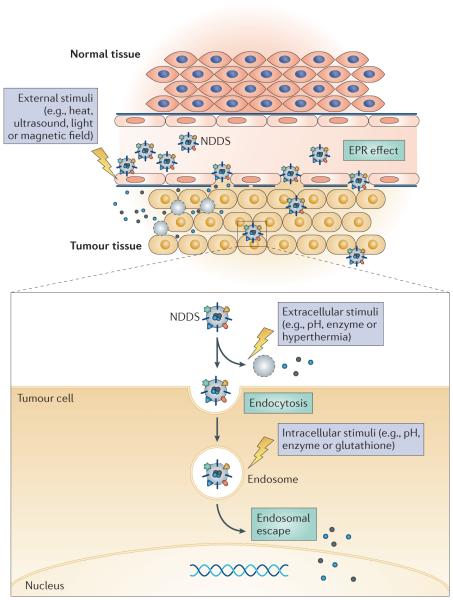
Moieties attached to nanoparticulate pharmaceutical drug delivery systems (NDDSs) can enhance their penetration into the target tissue or cell. Externally applied stimuli (such as heat) or intrinsic stimuli (such as a change in pH in the target tissue) can cause the NDDS to release its cargo in the vicinity of the target tissue (such as a tumour). Intracellular proteins specific to the target tissue can be used to ensure cargo release in the correct cell type. EPR, enhanced permeability and retention.
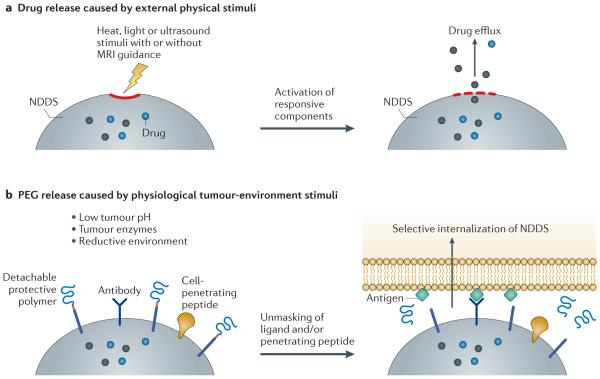
Various external stimuli can be applied to facilitate intracellular delivery (part a), and various local stimuli, such as enzymes present in the tumour environment, can be used to eliminate the protective poly(ethylene glycol) (PEG) layer from a nanoparticulate pharmaceutical drug delivery system (NDDS) to facilitate its interaction with target cells (part b). MRI, magnetic resonance imaging.
Table 2
Stimuli that can control the behaviour and/or properties of NDDSs
| Stimulus | Mechanistic basis | Key studies | Refs |
|---|---|---|---|
| pH | Low pH occurs in pathological areas such as tumours, infarcts and sites of inflammation, because of hypoxia (owing to increased glycolysis) and massive cell death; low pH also occurs in endosomes and the cytoplasm | pH-responsive complexes were achieved by engineering prefunctionalized polymers; these complexes successfully co-delivered doxorubicin and siRNA into cancer cells | 189 |
|
| |||
| Redox conditions | There is an increased concentration of glutathione inside many pathological cells | Bioreducible compounds containing disulphide bonds, such as poly(disulphide amine) and disulphide-based poly(amidoamine) polymers and dendrimers, increased the efficiency of intracellular gene delivery | 190 – 192 |
|
| |||
| An NDDS comprised of an siRNA–lipid conjugate (composed of a GFP-targeted siRNA conjugated to lipids via a disulphide bond) incorporated into polymeric micelles (made of a PEG2000–PE conjugate) protected siRNA from degradation; siRNA was released in reducing glutathione-rich surroundings, and downregulated the GFP-encoding gene 30-fold more effectively than did free siRNA | 193 | ||
|
| |||
| Enzyme activity | Certain enzymes are upregulated in pathological areas; for example, MMPs are upregulated in tumours | 100 nm nanoparticles composed of a gelatin (an MMP substrate) core and amino-PEG quantum dots conjugated to the core surface were degraded by MMPs in a mouse tumour model; the MMP degradation caused release of the quantum dots, which penetrated further into the tumour mass | 194 |
|
| |||
| Multifunctional liposomes containing an MMP2-responsive moiety (maleimide– PEG-cleavable peptide–DOPE) increased tumour targeting and internalization of the nanocarrier by tumour cells | 105 | ||
|
| |||
| Temperature | Inflammation-induced hyperthermia | The temperature difference between normal and diseased tissues is not usually high enough for most thermosensitive NDDSs, so an external heating source (such as a water bath, electromagnet, laser or high-intensity focused ultrasound to control the local temperature) is required | 103,195, 196 |
|
| |||
| Grafting a co-polymer of vinylpyrrolidinone and N-isopropylacryamide onto PE enhanced the accumulation of the now-thermosensitive conjugate in hyperthermia (42 °C)-treated tumours in a mouse model; there was a tenfold increase in DNA deposition in heated tumours compared with non-heated tumours | 89 | ||
|
| |||
| Magnetic nanoparticles (15 nm) introduced directly into a tumour in a mouse model and then heated using an alternating high frequency magnetic field were able to irreparably damage tumour cells or sensitize the tumour cells for additional chemo- or radiotherapy | 98 | ||
|
| |||
| Ultrasound | Local ultrasound increases temperature and microconvection in target tissues, resulting in a temporary or permanent destruction of cell membranes | Locally applied ultrasound led to the tumour-specific release of ~70% of loaded cisplatin from liposomes (compared with <3% release without ultrasound); this inhibited the growth of lymphoma and colon tumours in mouse models | 197 |
|
| |||
| Liposomes co-loaded with inert gases or perfluorated hydrocarbon and drugs could be used as contrast agents or for local drug release; this approach may work better on superficial tumours | 198 | ||
|
| |||
| Magnetic field | A magnetic field can concentrate magnetically sensitive NDDSs in specific areas | Conjugation of paclitaxel to PEG–SPION via a pH-sensitive ester bond resulted in NDDSs with high anticancer activity in vitro and in a mouse H22 tumour model | 97,199 |
|
| |||
| Methotrexate- and glutamic acid-complexed iron oxide co-loaded into liposomes caused a fivefold increase in drug accumulation in the target tissue of experimental mice when an external magnetic field was applied compared with a magnetically insensitive preparation | 200 | ||
|
| |||
| Light | Light-sensitive components can be incorporated into NDDSs; light induces photo-triggered structural changes in the NDDSs, leading to their direct interaction with the target cells. This interaction occurs via membrane fusion, photoisomerism, photofragmentation or photopolymerization, and/or drug leakage from NDDSs | A photosensitive, amphiphilic, dendritic conjugate based on the near-infrared- sensitive diazonaphthoquinone assembled into a photosensitive micellular nanocarrier loaded with doxorubicin enabled rapid drug release in vitro | 201 |
DOPE, dioleoylphosphatidylethanolamine; GFP, green fluorescent protein; MMP, matrix metalloproteinase; NDDS, nanoparticulate pharmaceutical drug delivery system; PE, phosphatidyl ethanolamine; PEG, poly(ethylene glycol); siRNA, small interfering RNA; SPION, superparamagnetic iron oxide nanoparticles.
pH-triggered drug delivery
The acidic environment of tumours78,79 and endosomes can be harnessed in the design of stimuli-sensitive NDDSs. For example, an NDDS can contain pH-sensitive components that protonate at lowered pH, resulting in the destabilization of the carrier and release of the drug. Pegylated poly l-histidine can form fairly stable polymeric micelles, but at pH values of approximately 6.5 and lower, histidine residues in the polyhistidine block become protonated, which results in an increase in the critical micelle concentration and micelle destabilization. As a result, the micelle-incorporated drug is released80; the high resultant drug concentration in the tumour interstitium or in the cytoplasm of the cancer cell may overcome drug efflux and other mechanisms of drug resistance81,82.
To avoid the premature degradation of drugs in lysosomes, pH-sensitive components (such as dioleoylphosphatidylethanolamine (DOPE)) that are capable of destabilizing the endosomal membrane or rupturing endosomes are used to modify NDDSs (such as liposomal NDDSs). This facilitates the fusion of the NDDS with the endovacuolar membrane after endocytosis and the subsequent release of the contents of the NDDS into the cytoplasm in response to the lowered endosomal pH83. Lipid conjugates of polyethyleneimine, such as DOPE-modified low-molecular-mass polyethyl-eneimine (PEI; 1,800 Da), were used to prepare nanoparticles to improve the PEI-mediated intracellular delivery of DNA84 and siRNA85 for various therapeutic purposes. Such conjugates have the combined advantages of positive charge, buffering capacity, fusogenicity and lowered toxicity.
The stimulus sensitivity of PEG coatings can enable the preparation of NDDSs with functions that are unveiled and activated only after the PEG molecules are detached. The use of ester and hydrazone moieties between the PEG moieties and NDDSs (which are stable at a neutral pH, such as in the blood, but hydrolyse at a pH of 6 or below) eliminates the protective effect of PEG during internalization by the target cell but also enables prolonged circulation in the blood. PEG is then removed once the NDDS is inside the acidified targets. PEG was detached from pH-sensitive PEG–hydrazone– phosphatidylethanolamine-based micelles and liposomes at lowered pH values, resulting in a cellular uptake that was similar to that of PEG-free NDDSs86.
Temperature-triggered drug delivery
Many pathological areas, such as tumours and inflamed areas, are hyperthermic68, which could be used as a possible trigger for stimuli-sensitive NDDSs. Alternatively, the tumour site could be heated from the outside to increase the size of pores within the microvasculature and increase blood flow, which results in an increased extravasation of drug-loaded NDDSs. Lipids with a suitable gel-to-liquid phase-transition temperature are usually used to prepare thermosensitive liposomes, such as dipalmitoylphosphatidylcholine or lysolipids. Indeed, temperature-sensitive lysolipid doxorubicin-loaded liposomes (named ThermoDox) have improved efficacy for liver cancer-targeted drug delivery in mice87,88 and have also been tested in human patients. Celsion, which developed Thermodox, recently reported a 57% improvement in overall survival when heat-activated liposomal doxorubicin in combination with radio-frequency ablation (RFA) was used in Phase III clinical trials to treat primary liver cancer (Celsion press release; see Further information). Temperature-sensitive NDDSs can also be prepared by incorporating polymers that have a lower physiological critical solution temperature (such as poly(N-isopropylacrylamide)) into the NDDS. In mice, it was shown that at temperatures above the low critical solution temperature, the polymer precipitates and causes disruption of the NDDSs to release the drug89.
Redox-triggered drug delivery
The difference in redox potential between normal and tumour tissues, and between the intracellular and extracellular environment, can also be exploited for drug delivery in cancer. For example, the concentration of glutathione in cancer cells is 100-to 1,000-fold higher than in the blood, and in a tumour mass the glutathione concentration is also markedly (100-fold) higher than the extracellular level of glutathione in normal tissue5. Reduction-sensitive liposomes that have two forms of disulphide-conjugated multifunctional lipids on their surface have been tested in a breast cancer cell line90. The cleavage of the disulphide bond resulted in the removal of the hydrophilic head group of the conjugate, membrane re-organization and the release of encapsulated calcein90. The disulphide bond has been widely used as the cleavable and/or reversible linker in NDDSs to confer redox sensitivity.
Enzyme-sensitive systems
The increased expression of certain local enzymes in cancer, such as MMPs, could be considered as a biomarker for disease diagnosis and prognosis, as well as a means for the delivery of pharmaceuticals via an enzyme-triggered mechanism91,92 (FIG. 2). Several substrates that are sensitive to MMPs have been designed for the delivery of drugs and as imaging agents. Cancer-associated proteases are another set of enzymes that are upregulated in tumours and can be used to develop enzyme-sensitive NDDSs. For instance, conjugates composed of cholesterol, peptide and poly-acrylic acid were cleaved by a cancer-associated protease (urokinase) and induced rapid drug release in an in vitro model93.
Magnetically sensitive systems
NDDSs that can be magnetized either by incorporating magnetite or prepared by direct modification of magnetized metal particles with biocompatible polymers could be used for specific targeting under an external magnetic field. Unlike ferromagnetic materials, magnetite or ferromagnetic particles that are less than 10 nm in diameter have a lower intensity of stray magnetic field, which reduces the potential health risks caused by magnetic fields94. These particles are referred to as SPIONs and can be surface-modified with various biocompatible compounds, such as dextran, to reduce their aggregation and increase their biocompatibility95.
SPIONs can be loaded into nanocarriers such as liposomes94, micelles96,97 or solid nanoparticles98,99 together with drugs and can increase drug localization in tumours under the exposure to a magnetic field. For example, liposomes co-loaded with methotrexate and SPION94 localized to tumours in mice. NDDSs containing SPIONs and a taxane drug could be additionally modified with PEG for longevity or with antibodies for tumour targeting96.
Control of NDDS functionalities with stimulus-sensitive moieties for enhanced targeting
Multifunctional NDDSs that combine stimulus-sensitive properties with another drug delivery approach could improve the therapeutic outcome in animal models and, hopefully, in future clinical applications86,94,100–103. The combined use of two different stimulus-sensitive approaches could be beneficial, as was shown for co-polymeric micelles that were sensitive to both pH and redox potential. For example, doxorubicin delivery to HepG2 tumour cells by these dual-sensitive NDDSs was more effective than with single-stimulus-sensitive control NDDSs104. Thermosensitive and magnetically sensitive liposomes loaded with methotrexate had more favourable drug pharmacokinetic properties (that is, better drug accumulation in skeletal muscle tissue in the tested animal model) than single-stimulus-sensitive delivery systems94. In addition, a multifunctional liposomal nanocarrier modified simultaneously with an antitumour monoclonal antibody (which actively targeted the tumour), a cell-penetrating peptide (which enabled intracellular delivery) and an MMP2-sensitive PEG–lipid conjugate (which facilitated MMP2-triggered PEG detachment from the lipid anchor) enhanced tumour targeting in vitro105.
Of all the stimuli considered above, pH and temperature (which can also be controlled by external factors) are the easiest to use among the intrinsic stimuli, whereas magnetic field and ultrasound — which are likely to be used clinically in the near future — are the external stimuli that are easiest to use. The use of redox condi-tions or local enzymatic activity still requires substantial synthetic efforts, whereas light-based approaches could be restricted primarily to superficial lesions unless fibre optics or near-infrared irradiation can be used.
Intracellular delivery and organelle targeting
After reaching the target, NDDSs may still need to cross the barrier of the cell membrane to deliver their drug load into specific organelles within the cytoplasm. Several strategies to prevent lysosomal degradation following the cellular internalization of NDDSs have been described106, including the use of targeting and cell-penetrating moieties as well as agents that can destabilize the lysosomal membrane, such as fusogenic lipids and pore-forming proteins107.
Cell-penetrating peptides are able to internalize nanosized particles, including liposomes, into cells108–110. Complexes of HIV TAT peptide (TATp)–liposomes and a plasmid encoding green fluorescence protein (pGFP) were used for successful in vitro transfection of several tumour and normal cells, as well as for the in vivo transfection of Lewis lung carcinoma tumour cells in mice111. Octa-arginine-modified, bleomycin- or doxorubicin-loaded liposomes demonstrated good intracellular penetration and strong inhibition of tumour growth in murine models112,113.
To provide intracellular drug delivery, the cell-penetrating function should be shielded — for example, under a PEG coating — until the NDDS is inside the target cell (where PEG is cleaved by local conditions)5,86. NDDSs such as targeted long-circulating pegylated liposomes and PEG–phosphatidylethanolamine-based micelles that possess several functionalities86,114 have been described. A pegylated TATp-modified pGFP-loaded liposomal preparation that had a PEG coating that detached at a lowered pH provided highly efficient transfection of tumour cells in vivo115. The potential therapeutic use of this approach was demonstrated in mice bearing ovarian cancer xenografts using a liposomal system that exposed a hidden cell-penetrating peptide (TATp) upon removal of PEG by the lower pH environment inside the tumour116,117.
Other micellar-based NDDSs were constructed using degradable ε-caprolactone-based co-polymers; the func-tional group on the co-polymer was used to incorporate siRNA against multidrug resistance protein 1 (MDR1; also known as ABCB1) or doxorubicin118. These micelles were also modified with the integrin-specific RGD4C peptide for cancer targeting and with the cell-penetrating TATp. Fluorescently labelled micelles were effectively taken up by cells and they improved drug delivery into cell nuclei, resulting in enhanced cytotoxicity in a mouse tumour model118.
However, delivering drugs or drug-loaded NDDSs inside cells may not be sufficient to achieve the maximum therapeutic response, and can even result in increased off-target effects. As such, the delivery of drug-loaded NDDSs directly to the organelle of interest could maximize the intended effect. This requires developing NDDSs that combine longevity, targetability and organelle recognition.
As mitochondrial dysfunction has been linked to many diseases, including cancer119, several strategies including the incorporation of the lipophilic cation rhodamine 123 to target NDDSs to mitochondria have been described120. Cell lysosomes have been targeted with NDDSs modified with octadecyl-rhodamine B121. Cytotoxic ceramides have been delivered to lysosomes of cancer cells using transferrin-modified liposomes, which resulted in reduced tumour growth in mice bearing an ovarian carcinoma xenograft66. Rhodamine B-modified fusogenic liposomes have also been used to deliver glucocerebrosidase into the mitochondria of Gaucher’s-type cells with low glucocerebrosidase activity122. Glucocerebrosidase is an enzyme that is deficient in patients with Gaucher’s disease, and so this type of NDDS is of particular interest for the treatment of lysosomal storage diseases122.
NDDSs for multimodal imaging and theranostics
Currently used medical imaging modalities include γ-scintigraphy, MRI, computed tomography and ultra-sonography, together with some modifications of these methods. Because each modality has its own advantages and limitations, it is highly desirable to have more than one type of reporter group associated with the same NDDS. Multifunctional NDDSs have been constructed for multimodal imaging, which could overcome various problems associated with individual imaging modalities, such as insufficient sensitivity or resolution123. With this in mind, several NDDSs that combine optical imaging with computed tomography or positron emission tomography (PET), or combine PET with computed tomography have been developed; these systems enable higher resolution and can be based on traditional NDDSs, such as liposomes or polymeric micelles, or they can use template nanoparticles with intrinsic contrast properties (such as gold or iron oxide nanoparticles, or quantum dots), which could be additionally modified with contrast moieties for other imaging modalities.
Thus, iron-oxide-based nanoparticles have been modified using click chemistry with a near-infrared dye to yield a dual MRI–near-infrared contrast agent124. Pegylated liposomes co-loaded with iohexol (a computed tomography imaging agent) and gadoteridol (a Gd-containing agent for MRI) had a long circulation lifetime and enabled simultaneous enhancement of computed tomography and magnetic resonance signals in mice and rabbits125. Dual-mode computed tomography–MRI imaging in rats and mice was achieved using multifunctional Gd-loaded dendrimer-entrapped gold nanoparticles126. Simultaneous optical imaging, computed tomography imaging and MRI was made possible using liposomes labelled with a fluorophore tracer, 99mTc, 111In or 64Cu and Gd127,128 in mouse models. Moreover, the addition of a radionuclide129 enables the imaging system to become a therapeutic. The use of polychelating amphiphilic polymers, which enable the attachment of many reporter metal atoms and can be incorporated or absorbed onto the nanocarrier, enabled a marked increase in the number of bound reporter metal atoms per particle and image-signal intensity130. In mouse models, such preparations can be targeted to tumours with a cancer-specific mAb with rapid and specific tumour accumulation and could serve as effective contrast agents for MRI of tumours131.
A relatively novel development in the area of multi-functional NDDSs is the use of so-called theranostics; that is, the simultaneous use of pharmaceutical NDDSs for diagnostic and/or imaging purposes together with therapeutic use. Through the use of contrast reporter moieties, theranostics enable real-time monitoring of the biodistribution and target accumulation of NDDSs. Theranostics often use liposomes, polymeric micelles, dendrimers and iron oxide nanoparticles as the carrier component of NDDSs, and heavy metal atoms (such as 111 In, 99m Tc, Mn and Gd) as contrast moieties for γ-scintigraphy and MRI132,133. For example, liposomes co-loaded with quantum dots and the dopamine agonist apomorphine had increased uptake into the brains of mice (apomorphine accumulation in the brain was increased by 2.4-fold) and enabled fluorescent brain imaging134. Multifunctional receptor-targeted nano-complexes containing a neurotensin-targeting peptide, DNA, a fluorophore and Gd have been used for brain imaging combined with DNA delivery in rats135.
Multifunctional core-shell polymeric nanoparticles composed of a poly(lactic-co-glycolic acid) core and a positively charged glycol-chitosan shell and co-loaded with DNA and quantum dots enabled transdermal DNA delivery and transfection of epidermal Langerhans cells in mice, and allowed the migration of target cells to be monitored136. Long-circulating pegylated liposomes loaded with doxorubicin and additionally decorated with a tumour-specific antibody and contrast moieties137,138 had increased therapeutic activity in vivo, and their target accumulation could be easily followed by γ-scintigraphy or MRI.
Iron-oxide-based NDDSs, which combine magnetic drug targeting and MRI, have attracted attention in recent years. SPIONs (which are popular MRI imaging agents) are frequently combined with therapeutic agents139 and PEG for longevity. For example, chlorotoxin-conjugated iron oxide nanoparticles delivered therapeutic methotrexate and an MRI contrast agent to 9L glioma xenografts in mice140. Magnetic particles modified with a recombinant peptide containing the amino-terminal fragment of urokinase-type plasminogen activator (uPA) can target tumours overexpressing uPA receptors, which enabled enhanced imaging and delivery in mice141. A targeted theranostic NDDS was prepared by covalent attachment of a near-infrared imaging agent and a translocation peptide to magnetic nanoparticles co-loaded with siRNA and was used for multimodal tumour imaging and to monitor the silencing activity of siRNA in tumour models142,143. Furthermore, hydrophobic photosensitizers (such as protoporphyrin IX) were loaded into pH-sensitive PEG–poly(β-amino ester)block co-polymer micelles for simultaneous tumour imaging and photodynamic therapy in mice144. A pegylated, siRNA-containing, magnetic-resonance-contrast liposome-based NDDS that contained fluorescent labels for histology downregulated the expression of survivin and inhibited tumour growth in xenografted tumours in mice145. In addition, pegylated liposomes that were loaded with Gd(III) for MRI, siRNA against survivin for tumour therapy, and additionally labelled with rhodamine–PE (phosphatidylethanolamine) for fluorescent detection have been studied in mice145.
Targeting of theranostic NDDSs provides an additional advantage, as was shown for the co-delivery of Gd and doxorubicin in liposomes targeted with a peptide specific for a neural cell adhesion molecule, which resulted in higher drug concentration in tumour tissues, increased inhibition of tumour growth and enhanced MRI visualization of Kaposi’s tumours in mice146. Antibody-mediated targeting of liposomes co-loaded with iron oxide and doxorubicin to pancreatic tumours in mice enhanced both the antitumour activity and the tumour magnetic resonance signal compared with untargeted liposomes147. The combination of a chemotherapeutic drug and a contrast agent on the same NDDS facilitates the development of personalized nanotherapeutic approaches because the efficiency of nanocarrier accumulation in the tumour (which is determined by the leakiness of the blood vessels and traced by a technique that uses contrast, such as mammography) can assist in the determination of the correct dose of the therapeutic agent for the treatment of each individual tumour148,149.
NDDSs for combination therapy
Drug combinations that can be delivered by NDDSs may be useful to combat drug resistance in patients with cancer150. A targeted liposome-polycation-hyaluronic acid NDDS that co-delivered three different siRNAs and one miRNA (to downregulate proliferation pathways, angio-genesis and the cellular defence against apoptosis) into tumours resulted in an 80% decrease in tumour size in a mouse model of melanoma151. Pegylated liposomes modified with trilysinoyl oleyamide have been used to co-deliver myeloid cell leukaemia 1 (MCL1)-specific siRNA and the histone deacetylase inhibitor suberoxy-lanilide hydroxamic acid into tumours, which resulted in a greater inhibition of tumour growth in mice bearing KB (human cervical cancer) xenografts152.
Similarly, liposomes co-loaded with doxorubicin and DNA encoding a dominant mutant form of survivin and targeted to tumours had enhanced antitumour activity in a murine model of Lewis lung carcinoma153,154. Peptide-targeted liposomes loaded with doxorubicin or cisplatin together with oligonucleotides against the molecules involved in two main drug resistance mechanisms, B cell lymphoma 2 (BCL-2) and MDR1 (multidrug-resistance protein 1), had high efficacy in human ovarian cancer xenografts155. Moreover, a combination of the colorectal cancer drug irinotecan and 5-fluoroorotic acid in pegylated liposomes (at a 5:1 ratio) had synergistic effects and had higher therapeutic efficacy than did the free drug mixtures in animal models, including KB-tumour-bearing mice156,157. For example, in a Phase I clinical trial, liposomes loaded with irinotecan and floxuridine liposomes (in a 1:1 molar ratio) were well tolerated and had antitumour activity in patients with advanced colorectal cancer, whereby 13 out of 15 patients responded to therapy158. The drug is currently in Phase II (TABLE 1).
Challenges in developing multifunctional NDDSs
When developing multifunctional and stimuli-sensitive NDDSs, one has to keep in mind that the different NDDS-attached functions may affect the biological activity of those NDDSs differently. Thus, although targeted ligands can result in improved cellular uptake, the surface properties, size and shape of the NDDSs will define their pharmacokinetics and biodistribution. Interestingly, some studies159,160 have demonstrated that the use of targeting ligands (that is, antibodies) does not necessarily increase the accumulation of NDDSs in the target area (with tumours as an example) when compared with EPR-mediated accumulation. Instead, the targeting ligand may enhance intracellular drug delivery in situations in which an internalizable ligand was used159,160, which could be beneficial for drugs that act intracellularly. It is also important to bear in mind that many surface-attached functions (most notably targeting moieties) could compromise the increased longevity of NDDSs that is imparted by pegylation; the surface density of such ligands should be closely monitored to provide a desirable targeting effect without overly compromising longevity.
I have mainly considered findings from early preclinical studies in this Review. The question then arises as to why, with so many interesting and impressive data, there still seems to be a long and arduous journey to bring these promising preclinical results into clinical practice? Potential answers to this question have been analysed in several recent publications161–164. Based on these discussions, one can first of all note that many data obtained in animal models cannot be easily translated into humans. This is particularly important in cancer research, in which subcutaneous tumour xenografts still represent the most frequently used model for targeted cancer therapy. Effective tumour targeting requires a sufficient number of productive contacts to be made between the targeted NDDSs and the target cells, which, in turn, requires effective preliminary accumulation of these NDDSs in the tumour via the EPR effect. In humans, this process is much more heterogeneous than in animal models because of a high heterogeneity in tumour blood flow. It is particularly problematic for animal models of subcutaneous tumours, in which the access of the blood to the tumour interstitium is greater than it is in solid tumours in patients. Unfortunately, the situation is even less clear with models of other non-cancer diseases.
In addition, there is ongoing discussion as to whether passive or active targeting will be more beneficial for various potential therapeutic indications. Nevertheless, the current thinking suggests that passive spontaneous accumulation of long-circulating NDDSs in tumours should be sufficient for drug delivery into the tumour mass, whereas intracellular drug delivery could be more effective if NDDSs are supplemented with specific targeting moieties that recognize cell-surface ligands to promote the internalization of drug-loaded NDDSs. Moreover, many experimental models of various human diseases are not uniformly standardized, which often makes the comparison of results difficult. From a technological point of view, many of the effective and experimentally elegant NDDSs are difficult to reproducibly scale up in the industrial setting to produce NDDSs on a commercial scale because of their complexity, which will also add substantially to the final price of the product.
Assuming the problems described above are eventu-ally resolved, the translation of the current knowledge into clinical applications will require the active involvement of pharmaceutical and biotechnology companies and an increased understanding of the benefits of NDDSs by the broad clinical community. Once more clinical data become available, multifunctional, stimuli-sensitive and theranostic NDDSs will continue to be developed and improved. To become a successful product, such NDDSs should be reasonably simple. That is, their preparation should not require complex multistep processes, should not include uncommon or expensive constituents, and they should preferably be made of components that are generally recognized as safe. In the future, the scalability of NDDSs should not represent a problem and be preferably based on known and accepted processes and protocols. The final product should also be sufficiently stable under normal storage conditions and easy to use; that is, it should not require complex administration protocols or regimens. If these requirements are met, multifunctional, stimuli-sensitive and theranostic NDDSs could become an important component of personalized therapy in the near future.
Acknowledgements
The author acknowledges US National Institutes of Health grant U54CA151881 and tremendous help by T. Levchenko.
Glossary
| Enhanced permeability and retention (EPR) effect | The property through which macromolecules (such as nanoparticles) accumulate in areas of inflammation including tumours, owing to the increased vascular permeability or abnormal blood vessel architecture. |
| Passive targeting | The mechanism through which nanoparticulate pharmaceutical drug delivery systems tend to accumulate in tumours, probably through the enhanced permeability and retention effect. |
| Quantum dots | Nanometre-scale particles of semiconductor materials that have quantum mechanical properties. |
| Active targeting | The mechanism through which specific moieties attached to nanoparticulate pharmaceutical drug delivery systems force them to interact with a specific type of cell or tissue. |
| HIV TAT peptide | An amino acid sequence within the HIV transactivator of transcription (TAT) protein. This peptide promotes cell entry as it is a key part of a protein transduction domain. |
| Theranostics | The simultaneous use of nanoparticulate pharmaceutical drug delivery systems for therapeutic as well as diagnostic and/or imaging purposes. |
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nrd4333
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4489143
Citations & impact
Impact metrics
Article citations
Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the targeted delivery of RNA therapeutics: a quality-by-design approach.
J Nanobiotechnology, 22(1):710, 14 Nov 2024
Cited by: 0 articles | PMID: 39543630 | PMCID: PMC11566655
Review Free full text in Europe PMC
Influence of lipid vesicle properties on the function of conjugation dependent membrane active peptides.
J Mater Chem B, 12(40):10320-10331, 17 Oct 2024
Cited by: 0 articles | PMID: 39291919 | PMCID: PMC11409839
Core-Shell Microspheres with Encapsulated Gold Nanoparticle Carriers for Controlled Release of Anti-Cancer Drugs.
J Funct Biomater, 15(10):277, 24 Sep 2024
Cited by: 0 articles | PMID: 39452576 | PMCID: PMC11509066
Nanomedicine marvels: crafting the future of cancer therapy with innovative statin nano-formulation strategies.
Nanoscale Adv, 18 Oct 2024
Cited by: 0 articles | PMID: 39478996 | PMCID: PMC11515941
Review Free full text in Europe PMC
Therapeutic targeting of senescent cells in the CNS.
Nat Rev Drug Discov, 23(11):817-837, 30 Sep 2024
Cited by: 0 articles | PMID: 39349637
Review
Go to all (488) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Fluorescent carbon dot-gated multifunctional mesoporous silica nanocarriers for redox/enzyme dual-responsive targeted and controlled drug delivery and real-time bioimaging.
Eur J Pharm Biopharm, 117:105-115, 28 Mar 2017
Cited by: 6 articles | PMID: 28363599
Recent developments in lipid-based pharmaceutical nanocarriers.
Front Biosci (Landmark Ed), 16(4):1388-1412, 01 Jan 2011
Cited by: 46 articles | PMID: 21196238
Review
Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers.
Adv Drug Deliv Rev, 60(4-5):548-558, 28 Nov 2007
Cited by: 198 articles | PMID: 18053612
Review
Multifunctional tumor-targeting nanocarriers based on hyaluronic acid-mediated and pH-sensitive properties for efficient delivery of docetaxel.
Pharm Res, 31(4):1032-1045, 24 Oct 2013
Cited by: 27 articles | PMID: 24154802
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: U54CA151881
Grant ID: U54 CA151881





