Abstract
Free full text

Hepatitis C virus-induced hepatocellular carcinoma
Abstract
Hepatitis C virus (HCV) is a leading etiology of hepatocellular carcinoma (HCC). The interaction of HCV with its human host is complex and multilayered; stemming in part from the fact that HCV is a RNA virus with no ability to integrate in the host's genome. Direct and indirect mechanisms of HCV-induced HCC include activation of multiple host pathways such as liver fibrogenic pathways, cellular and survival pathways, interaction with the immune and metabolic systems. Host factors also play a major role in HCV-induced HCC as evidenced by genomic studies identifying polymorphisms in immune, metabolic, and growth signaling systems associated with increased risk of HCC. Despite highly effective direct-acting antiviral agents, the morbidity and incidence of liver-related complications of HCV, including HCC, is likely to persist in the near future. Clinical markers to selectively identify HCV subjects at higher risk of developing HCC have been reported however they require further validation, especially in subjects who have experienced sustained virological response. Molecular biomarkers allowing further refinement of HCC risk are starting to be implemented in clinical platforms, allowing objective stratification of risk and leading to individualized therapy and surveillance for HCV individuals. Another role for molecular biomarker-based stratification could be enrichment of HCC chemoprevention clinical trials leading to smaller sample size, shorter trial duration, and reduced costs.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a growing clinical problem, being the second leading cause of cancer deaths worldwide (GLOBOCAN, http://globocan.iarc.fr) and one of the only increasing causes of cancer-related mortality in the U.S.1 In contrast to developing countries in the Asia-Pacific regions and sub-Saharan Africa, where hepatitis B virus (HBV) is the major risk factor for HCC, chronic infection with hepatitis C virus (HCV) has been a leading cause of HCC in developed countries2 and is the first indication for liver transplantation for patients with HCC in the U.S.3 Worldwide, the World Health Organization (WHO) estimates that 3% of the world's population has been infected with HCV and that more than 170 million people are currently chronic carriers of HCV (WHO, www.who.int). In the U.S., more than 75% of HCV-infected adults were born between 1945 and 1965, so-called "baby boomers" and more than 1 million of the baby-boomer population is predicted to develop HCV-related cirrhosis, hepatic decompensation, or HCC by 2020 with current hospital inpatient management costs for this population over $15 billion annually.4,5
The risk of HCC, in chronic HCV infection, is associated with fibrosis stage. In cirrhotic subjects, the annual incidence of HCC is extremely high (1-7% per year), although HCC rarely develops in livers with less fibrosis.6,7 Although the emergence of highly effective direct-acting antivirals (DAAs) for HCV is expected to reduce the incidence of HCV-related HCC,8 the achievement of a sustained virological response (SVR) does not eliminate the risk of HCC, especially when the patients have already developed advanced liver fibrosis.9,10 Although molecular mechanisms of HCV-induced HCC development have not been fully elucidated, these epidemiological observations suggest that the major role of HCV in carcinogenesis is to create a cirrhotic tissue microenvironment that serves as a carcinogenic milieu. In addition, direct carcinogenic effects of HCV proteins have been suggested in a variety of experimental models as additional drivers of HCV-induced HCC development.11
In this review, we overview the current molecular understanding of HCV-induced hepatocarcinogenesis and discuss potential strategies that may allow to stratify subjects with HCV infection at different risks of HCC occurrence based on clinical or, more refined, molecular markers.
Pathogenesis of HCV-induced HCC
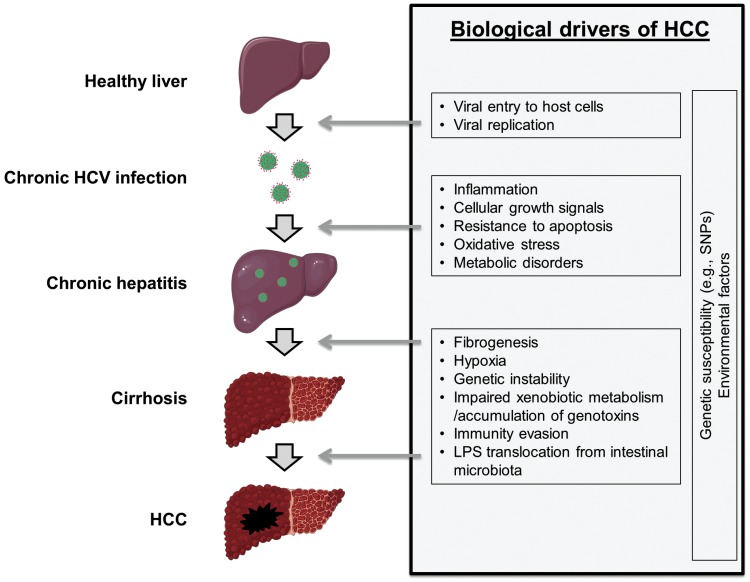
HCV-induced HCC development is a multi-step process that may progress over 20-40 years and involves a number of steps: establishment of chronic HCV infection, chronic hepatic inflammation, progressive liver fibrosis, initiation of neoplastic clones accompanied by irreversible somatic genetic/epigenetic alterations, and progression of the malignant clones in a carcinogenic tissue microenvironment (Fig. 1). Each step of HCV-induced hepatocarcinogenesis is a potential target for therapeutic intervention or chemoprevention.12 Unlike HBV which can integrate into the host genome leading to potential direct carcinogenic activity, HCV is a RNA virus with limited integration of its genetic material into the host's genome. Therefore, the carcinogenic potential of HCV is generally assumed to be linked to indirect mechanisms, although the lack of a convenient in vitro model system to study biology is a major obstacle for the understanding of the mechanisms linking HCV infection, inflammation and carcinogenesis.13,14
Interaction of HCV with cellular pathways
The "field effect" or "field cancerization" refers to the cirrhotic milieu/microenvironment that allows initiation and promotion of neoplastic clones by facilitating genetic aberrations and cellular transformation.15 Chronic damage to hepatocytes, such as HCV infection, leads to a release of inflammatory and fibrotic mediators such as reactive oxygen species, cell death signals, hedgehog ligands and nucleotides.16 A complex series of mechanisms centering on the hepatic stellate cell, mediated through intracellular inflammasome activation, the nuclear receptor family, (farsenoid-X-receptor, peroxisome proliferator-activated receptors and others) and other transcriptional events contribute to stellate cell activation. The activated hepatic stellate cell promotes liver scarring through proliferation, contractility, fibrogenesis, matrix degradation and inflammatory signaling. HCV broadly infects hepatocytes, monocytes, lymphocytes and other secretory cells, and contributes to stellate cell activation. HCV core and nonstructural proteins stimulate profibrogenic mediators such as TGF-beta.17 HCV infection induces TGFB1 through reactive oxygen species (ROS) production, p38 MAPK, JNK, ERK, and NF-kappaB pathways.18 Platelet-derived growth factor (PDGF) is the most potent mitogenic signal, inducing expression of beta PDGF receptor expression in stellate cells together with other cell surface receptors of growth signaling such as integrins.19 Transgenic mice expressing PDGF-C develop liver fibrosis and HCC,20 and an acyclic retinoid, peretinoin, represses fibrosis and HCC development in the model.21 Severity of liver fibrosis is tightly correlated with increasing risk of HCC in patients with chronic HCV infection, suggesting that cirrhosis-driven carcinogenesis is the major mechanism in the development of HCV-related HCC.6,22
Clinical data suggest the role of HCV viral factors in disease progression and HCC development. For example, genotype 3 HCV has a worse response to DAAs, increased steatosis, and increased fibrosis progression rate,23,24 whilst, although the evidence is conflicting, HCC risk seems to be dependent on viral genotype and potentially increased in subjects with HCV genotype 3.25,26,27 Experimental data also seem to suggest that HCV proteins interact with cellular proliferation and survival pathways leading to an increased risk of HCC. Over-expression of HCV proteins, promotes cellular proliferation, transformation, and/or tumor formation in mice, suggesting the direct contribution of viral proteins to activating oncogenic molecular pathways.28,29,30,31 The viral core protein inhibits tumor suppressor genes TP53, TP73, and RB1 as well as negative regulator of cell cycle such as CDKN1A.32,33,34,35 NS3 and NS5A also inhibit TP53,36,37 and NS5B inhibited RB1.38 HCV core, E2, NS5A, and NS5B activate cellular proliferative RAF/MAPK/ERK kinase pathways and E2F1 pathway, which are associated with more aggressive biological phenotype of HCC tumors.34,38,39,40,41 HCV proteins such as core are known to induce generation of ROS and transactivate MAPK and AP1 pathways.42 Insulin-like growth factor signaling is activated via IGF1R in early stage HCV-related HCC.43 NS5A was found to be involved in activation of PI3K/AKT and β-catenin/WNT pathways, and evasion from apoptosis by caspase-3 inhibition.44 NS5A inhibits TGF-beta signaling by preventing nuclear translocation of Smad proteins, resulting in downregulation of the tumor suppressor CDKN1A.45
Interferon pathway activation is a well-known innate immune response to HCV infection, and recent studies have elucidated its role in anti-tumor immunity.46 The HCV core protein inhibits the NF-kappaB mediated immune responses47 whereas the JNK pathway, activated in non-parenchymal liver cells by proinflammatory signals such as ROS, generates an inflammatory hepatic microenvironment that supports HCC development.48 Viral proteins also appear to subvert innate immune pathways via the suppression of innate immunity, inhibition of natural killer cells and impairment of antigen-presenting cells by viral proteins.49,50,51
Clinically, the interaction of HCV, steatosis and the metabolic syndrome has suggested a modulation of metabolic pathways by chronic HCV infection.52,53 In addition, HCV-related HCC is often accompanied by histological steatosis within the tumor and nontumoral liver.54 Expression of core protein in transgenic mice induces steatosis, insulin resistance and HCC, whereas HCV core protein co-localizes with apolipoprotein A2 on the surface of triglycerides, suggesting its association with lipid metabolism.31,55 HCV core protein suppresses microsomal triglyceride transfer protein activity and interferes with hepatic assembly and secretion of triglyceride-rich very low density lipoproteins, further contributing to steatosis.56 HCV core protein interacts with RXR-alpha and peroxisome proliferator-activated receptor-alpha (PPAR-alpha), and modulates cell differentiation, proliferation and fatty acid transport and catabolism in mice.57 In the presence of HCV core induced mitochondrial dysfunction, PPAR-alpha exacerbates steatosis, induces oxidative stress, and increases cell growth signals.58
Activating somatic mutations in telomerase reverse-transcriptase promoter is a frequent early neoplastic event in HCC with mixed etiologies including HCV.59 Hepatocyte proliferation is generally decreased at the stage of cirrhosis after many rounds of regeneration accompanied by telomere shortening that triggers cellular senescence though ATM, TP53, and CDKN1A as a safeguard to prevent carcinogenesis.60 HCV core protein overcomes stressinduced hepatocyte senescence by down-regulating CDKN2A expression via DNA methylation.61
Host factors affecting susceptibility to HCV-related HCC
A multi-kinase inhibitor, sorafenib, is the only drug currently approved for advanced HCC.62 Sorafenib showed anti-HCC effect by blocking paracrine hepatocyte growth factor from stromal cells in response to vascular endothelial growth factor-A secreted from HCC cells.63 Interestingly it improved portal hypertension in cirrhotic patients, supposedly due to its anti-angiogenic activity.64 Epidermal growth factor (EGF) is a mitogen involved in cellular growth, proliferation, differentiation, and carcinogenesis. In rodent models of cirrhosis-driven HCC, pharmacological inhibition with a small molecule EGF receptor (EGFR) inhibitor, erlotinib, regressed fibrosis and inhibited HCC development65 despite no inhibition of EGF pathway in the tumors, suggesting that the HCC preventive effect was through action on the cirrhotic tissue microenvironment that supports initiation of neoplastic clones. In contrast, another small molecule EGFR inhibitor, gefitinib, suppressed growth of initiated HCC clones in rats.66 EGFR was recently identified as a co-factor for HCV cellular entry, and erlotinib inhibited HCV infection, suggesting its role as a potential anti-HCV drug.67,68
Various host polymorphisms associated with the immune system have been associated with HCV-induced HCC. A Japanese genome-wide association study comparing HCV-related HCC patients with chronic hepatitis C patients identified a single nucleotide polymorphism (SNP) in MHC class I polypeptide-related sequence A (MICA - rs2596542), which is involved in response of dendritic cells to type-I interferon in chronic hepatitis C.69,70 Another SNP in the MICA promoter (rs2596538) was associated with increased serum soluble MICA protein.71 A subsequent study in Caucasian hepatitis C patients in Switzerland did not replicate the association with HCC for this locus, but for a nearby locus in HCP5 (rs2244546), suggesting that the MICA/HCP5 region contains a potential susceptibility locus.72 An IL28B variant (rs12979860), initially identified as an interferon response predictor, may be associated with increased risk of HCV-related HCC.73,74
SNPs in genes associated with metabolic functions have also been weakly associated with HCV-induced HCC. Although some teams reported an association between HFE gene mutations, in particular H63D, and increased risk of HCC,75 these findings were not replicated in other studies.76 In patients with chronic hepatitis C with advanced fibrosis, positive association between liver iron deposition and higher incidence of HCC and poor prognosis was nevertheless observed.77 A SNP in the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene (rs738409) associated with alcoholic and non-alcoholic steatohepatitis may have weak association with HCV-related HCC.78
Assessment of risk of HCC in HCV-infected individuals
Considering the high prevalence of HCV-induced cirrhosis, the population at risk of development of HCC, requiring surveillance according to clinical guidelines, is huge and likely unmanageable. For instance, despite guidelines recommending HCC surveillance in subjects with cirrhosis,79 most patients at risk of HCC in the US do not receive recommended regular surveillance. Only 12% of cirrhotic HCV patients had routine annual surveillance in one US Veterans Affairs series and only 2% of HCV patients who developed HCC had previous appropriate screening in another series.80,81 Additionally, in a population-based US study, less than 20% of patients with cirrhosis who developed HCC received regular surveillance.82 Growing numbers of early-stage, asymptomatic cirrhotics identified by non-invasive fibrosis detection methods such as elastography may also add to the HCC screening burden.83 Therefore prognostic indicators are being actively developed to stratify HCV subjects, as well as patients with other liver diseases, into clearly defined risk groups to enable effective clinical management of the patients.12
Clinical stratification of HCC risk in HCV
A number of non-invasive and invasive clinical markers and systems have been proposed to assess risk of HCC in subjects with HCV, in particular in patients with HCV-induced cirrhosis (Table 1). Scores identifying subjects at risk of developing HCC after SVR have also been reported84,85 although these scores require further validation. This is especially relevant in this era of highly effective DAAs where the burden of HCV-related complications might shift to subjects who have achieved SVR, but remain at high risk of liver-related complications. Overall, although clinical variable-based prediction models for HCC development have been explored, their performance is limited and none of them has been established in practice.86,87
Table 1
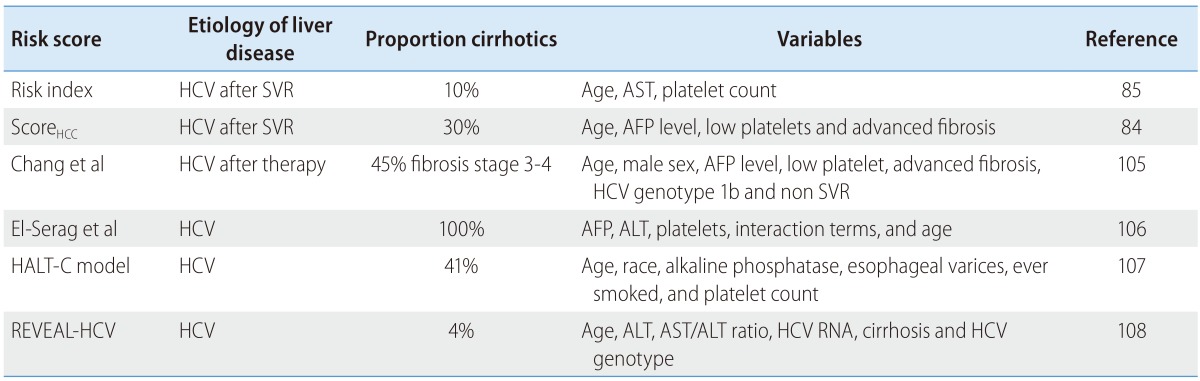
HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virological response; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; ALT, alanine aminotransferase.
Molecular markers of HCC risk in HCV
Molecular biomarkers of HCC risk and/or poor prognosis may enable further enrichment of the high-risk population and may significantly contribute to improvement of early HCC detection. Numerous germline SNPs have been reported as HCC risk variants, although very few of them are replicated in independent patient series/cohorts.88 The EGF 61*G allele (rs4444903) was associated with HCC risk in a prospective cohort of patients with HCVrelated advanced fibrosis or cirrhosis (Table 2),89,90 while a SNP in an antioxidant enzymes, myeloperoxidase, (MPO-463*G, rs2333227) was associated with HCC risk in another prospective study.91 A 186-gene-expression signature was associated with HCC risk in prospectively followed patients with early-stage HCV-related cirrhosis (HR=2.65).92 The gene signature has proven prognostic not only for HCC recurrence but also for liver disease progression, HCC development and overall survival in subjects with early-stage HCV cirrhosis.92,93,94 The signature was present in the liver of rodent models of fibrosis/cirrhosis-driven HCC, and the poor prognosis pattern of the signature was reversed in association with the HCC chemopreventive effect of an FDA-approved EGFR inhibitor, erlotinib,65 which is now being tested in a phase 1 trial with the gene signature as a companion biomarker (ClinicalTrials.gov, NCT02273362). Circulating cells or biomolecules such as miRNAs may be alternative sources to obtain similar molecular information less invasively.95 In addition, molecular imaging of collagen could potentially be used to monitor fibrosis regression, which may correlate with decreased HCC risk.96
Table 2
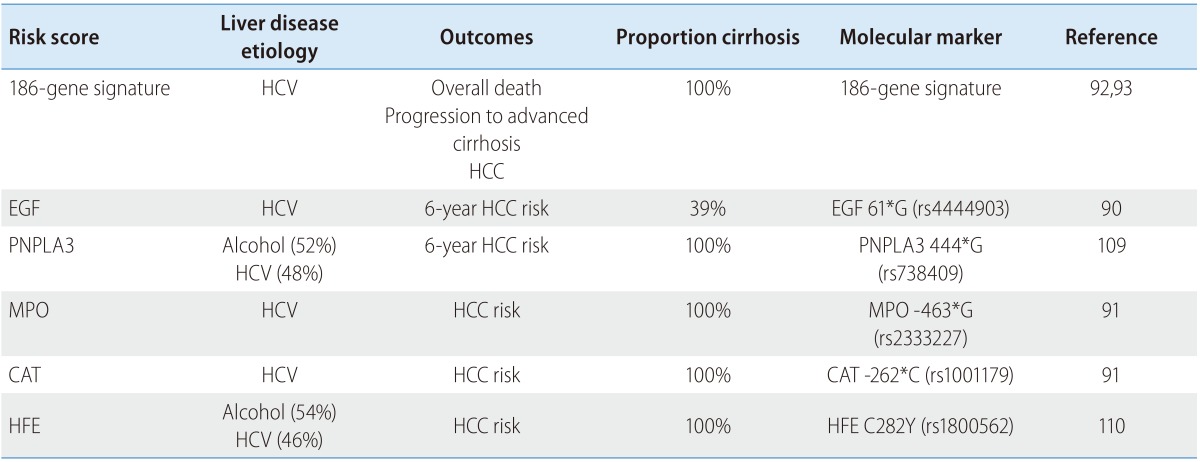
HCC, hepatocellular carcinoma; HCV, hepatitis C virus; EGF, epidermal growth factor; MPO, myeloperoxidase; CAT, catalase; HFE, hemochromatosis.
One possible role of molecular risk stratification of subjects with HCV-induced liver disease could be chemopreventive trial enrichment with subjects at high risk of HCC.12 Until now, several large phase 3 clinical trials have failed to show the usefulness of chemopreventive strategies in HCC despite the enrollment of thousands of patients and a follow-up time approaching decade(s).97,98,99 Enrichment of high-risk patients with the use of HCC risk biomarkers and/or prognostic indices is critical to increase HCC incidence and keep required sample size and study duration within practically feasible range. HCC risk biomarker-based clinical trial enrichment will drastically lower the bar to conduct cancer chemoprevention trials by substantially reducing required sample size and the duration of follow-up comparable to oncology trials enrolling advanced-stage cancer patients.100
CONCLUSIONS AND FUTURE PERSPECTIVES
Recent clinical trials of DAA-based interferon-free regimens have demonstrated SVR rates greater than 90% across a wide spectrum of HCV-induced disease101 hopefully leading to reduced rates of HCC development in subjects achieving SVR.9 However a number of challenges remain before HCV antiviral therapy will lead to eradication of HCV-induced HCC. The current cost of DAA-based therapy is prohibitive for most subjects infected with HCV outside developed countries suggesting that a reservoir of HCV-infected individuals is likely to remain for the foreseeable future. In addition, a significant proportion of individuals are not aware of their HCV-positive status; for instance the US Center for Disease Control estimates that 3% of baby boomers are HCV-positive, and has recently approved point-of-care testing and recommended universal one-time HCV screening for all adults born between 1945 and 1965.102 Although the number of HCV-infected people may stabilize or even decline in the next decades, most projections agree that the incidence of HCV-related complications will continue rising and peak around 2020-2030.103,104 Finally, and importantly, achievement of SVR leads to a reduction, but not an elimination of the risk of HCC9,10 suggesting that risk stratification for risk of HCC is still important even in this subgroup.
In conclusion, despite an improved understanding of direct and indirect mechanisms leading to HCV-induced HCC and despite the development of highly potent DAAs for HCV therapy HCV-related HCC will remain a major health challenge in the coming decades. Although prevention of HCV-induced HCC is not yet established, direct and indirect oncogenic roles of HCV and candidate target genes and molecular pathways have been suggested in experimental and clinical studies. Well validated clinical and molecular biomarkers will be key to target subjects at the higher end of the risk spectrum with more intensive interventions, surveillance and possibly chemoprevention trials.
Acknowledgements
This work was supported by the FLAGS foundation, the Nuovo-Soldati Cancer Research Foundation and an advanced training grant from Geneva University Hospital to NG and NIH/NIDDK R01 DK099558 and the Irma T. Hirschl Trust to YH.
Abbreviations
| DAA | direct-acting antiviral |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| SNP | single nucleotide polymorphysm |
| SVR | sustained virological response |
| WHO | World Health Organization |
| ROS | reactive oxygen species |
| PDGF | platelet-derived growth factor |
| PPAR | peroxisome proliferator-activated receptor |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
References
Articles from Clinical and Molecular Hepatology are provided here courtesy of Korean Association for the Study of the Liver
Citations & impact
Impact metrics
Citations of article over time
Article citations
Monocyte and Macrophage Functions in Oncogenic Viral Infections.
Viruses, 16(10):1612, 15 Oct 2024
Cited by: 0 articles | PMID: 39459945 | PMCID: PMC11512331
Review Free full text in Europe PMC
Intratumoral microbiota: an emerging force in diagnosing and treating hepatocellular carcinoma.
Med Oncol, 41(12):300, 25 Oct 2024
Cited by: 0 articles | PMID: 39453562
Review
Hepatitis B virus-related hepatocellular carcinoma exhibits distinct intratumoral microbiota and immune microenvironment signatures.
J Med Virol, 96(2):e29485, 01 Feb 2024
Cited by: 3 articles | PMID: 38377167
Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma.
Dig Dis Sci, 69(3):1055-1067, 01 Feb 2024
Cited by: 0 articles | PMID: 38300416
The potential role of the microbiota in prostate cancer pathogenesis and treatment.
Nat Rev Urol, 20(12):706-718, 25 Jul 2023
Cited by: 7 articles | PMID: 37491512
Review
Go to all (86) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02273362
SNPs (Showing 7 of 7)
- (1 citation) dbSNP - rs2596542
- (1 citation) dbSNP - rs12979860
- (1 citation) dbSNP - rs2333227
- (1 citation) dbSNP - rs4444903
- (1 citation) dbSNP - rs2244546
- (1 citation) dbSNP - rs2596538
- (1 citation) dbSNP - rs738409
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Complex Association of Virus- and Host-Related Factors with Hepatocellular Carcinoma Rate following Hepatitis C Virus Clearance.
J Clin Microbiol, 57(1):e01463-18, 02 Jan 2019
Cited by: 10 articles | PMID: 30381417 | PMCID: PMC6322466
Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma.
J Hepatol, 61(1 suppl):S79-90, 03 Nov 2014
Cited by: 114 articles | PMID: 25443348 | PMCID: PMC4435677
Review Free full text in Europe PMC
Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients.
J Hepatol, 71(2):265-273, 06 Apr 2019
Cited by: 76 articles | PMID: 30959157
Hepatitis C Virus-Induced Upregulation of MicroRNA miR-146a-5p in Hepatocytes Promotes Viral Infection and Deregulates Metabolic Pathways Associated with Liver Disease Pathogenesis.
J Virol, 90(14):6387-6400, 24 Jun 2016
Cited by: 59 articles | PMID: 27147737 | PMCID: PMC4936128
Funding
Funders who supported this work.
NIDDK NIH HHS (2)
Grant ID: R01 DK099558
Grant ID: R01DK099558

 1
1