Abstract
Free full text

Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior.
Abstract
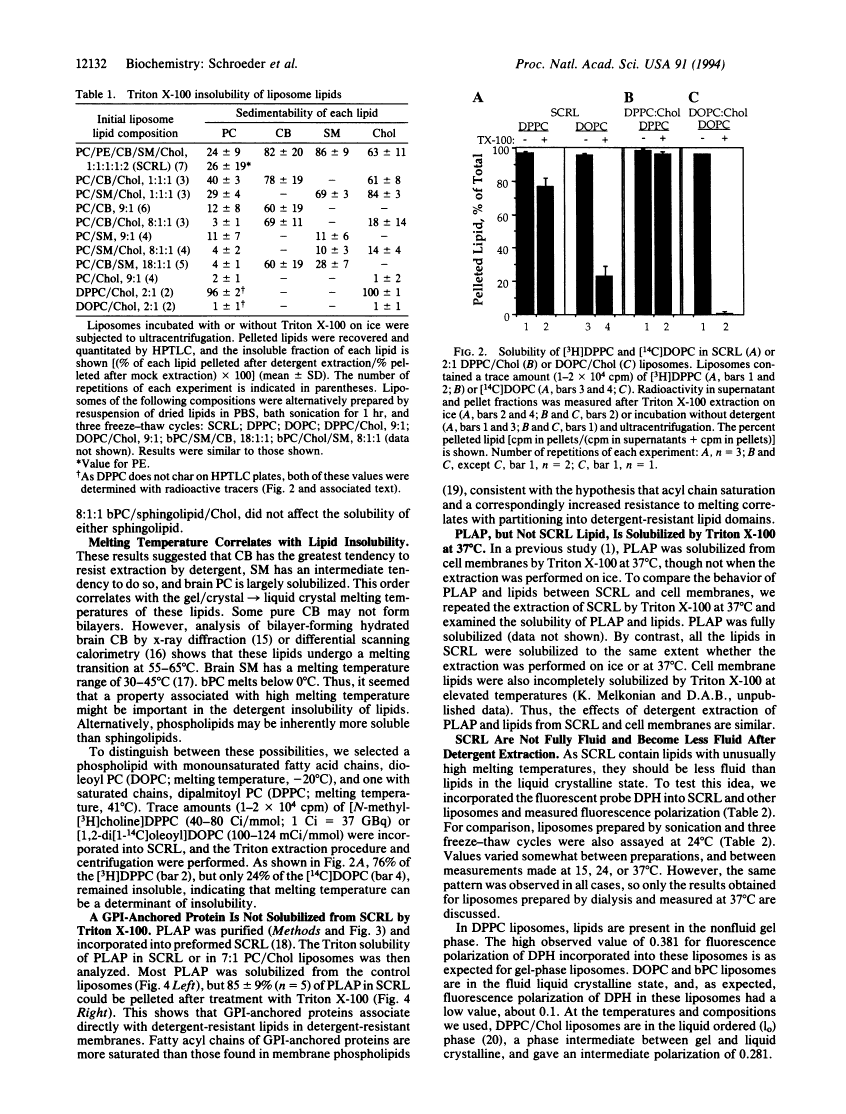
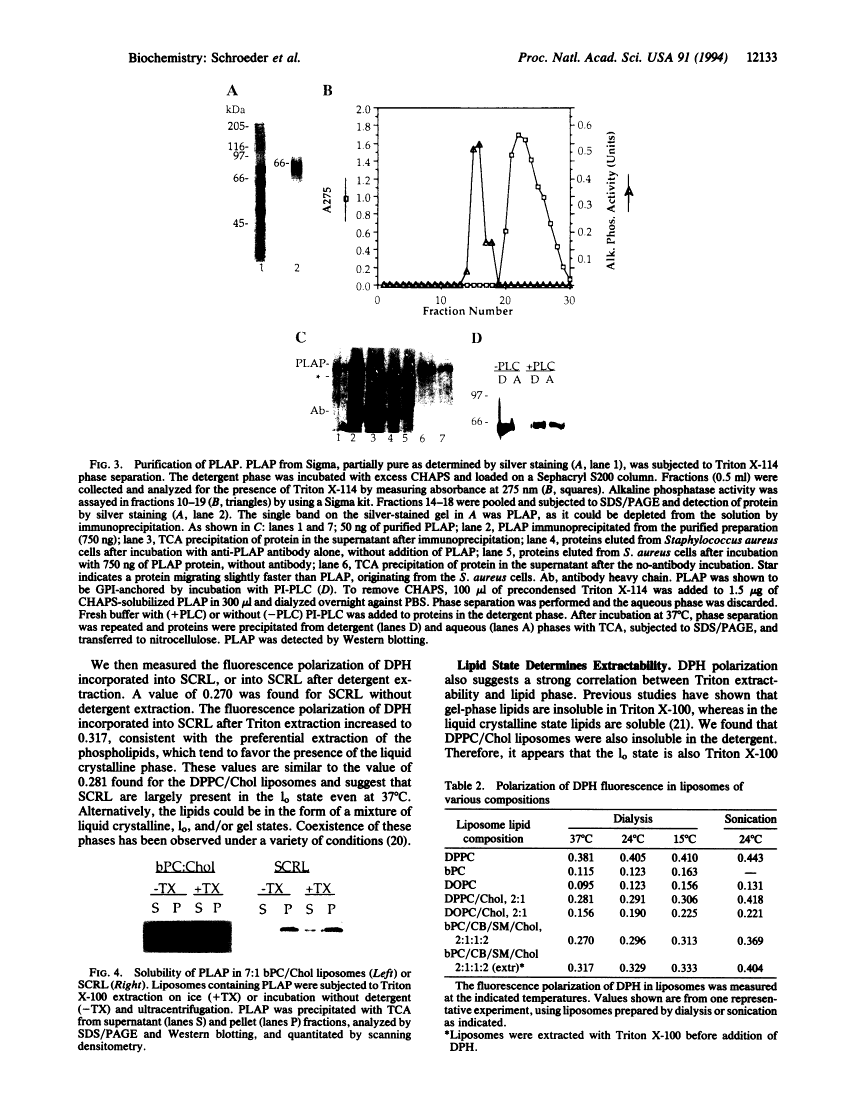
Proteins anchored by GPI are poorly solubilized from cell membranes by cold nonionic detergents because they associate with detergent-resistant membranes rich in cholesterol and sphingolipids. In this study, we demonstrated that cholesterol and sphingolipid-rich liposomes were incompletely solubilized by Triton X-100. GPI-anchored placental alkaline phosphatase incorporated in these liposomes was also not solubilized by cold Triton X-100. As sphingolipids have much higher melting temperatures (Tm) than cellular phospholipids, a property correlated with Tm might cause detergent inextractability. In support of this idea, we found that the low-Tm lipid dioleoyl phosphatidylcholine (DOPC) was efficiently extracted from detergent-resistant liposomes by Triton X-100, whereas the high-Tm lipid dipalmitoyl phosphatidylcholine (DPPC) was not. The fluorescence polarization of liposome-incorporated diphenylhexatriene was measured to determine the "fluidity" of the detergent-resistant liposomes. We found that these liposomes were about as fluid as DPPC/cholesterol liposomes, which were present in the liquid-ordered phase, and much less fluid than DOPC or DOPC/cholesterol liposomes. These findings may explain the behavior of GPI-anchored proteins, which often have saturated fatty acyl chains and should prefer a less-fluid membrane. Therefore, we propose that acyl chain interactions can influence the association of GPI-anchored proteins with detergent-resistant membrane lipids. The affinity of GPI-anchored proteins for a sphingolipid-rich membrane phase that is not in the liquid crystalline state may be important in determining their cellular localization.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. [Abstract] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. [Europe PMC free article] [Abstract] [Google Scholar]
- Cinek T, Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [Abstract] [Google Scholar]
- Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992 Sep;118(5):1003–1014. [Europe PMC free article] [Abstract] [Google Scholar]
- Arreaza G, Melkonian KA, LaFevre-Bernt M, Brown DA. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem. 1994 Jul 22;269(29):19123–19127. [Abstract] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. [Abstract] [Google Scholar]
- Lisanti MP, Tang ZL, Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J Cell Biol. 1993 Nov;123(3):595–604. [Europe PMC free article] [Abstract] [Google Scholar]
- Chang WJ, Ying YS, Rothberg KG, Hooper NM, Turner AJ, Gambliel HA, De Gunzburg J, Mumby SM, Gilman AG, Anderson RG. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994 Jul;126(1):127–138. [Europe PMC free article] [Abstract] [Google Scholar]
- Thompson TE, Tillack TW. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. [Abstract] [Google Scholar]
- Skibbens JE, Roth MG, Matlin KS. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989 Mar;108(3):821–832. [Europe PMC free article] [Abstract] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. [Abstract] [Google Scholar]
- Macala LJ, Yu RK, Ando S. Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res. 1983 Sep;24(9):1243–1250. [Abstract] [Google Scholar]
- Micanovic R, Bailey CA, Brink L, Gerber L, Pan YC, Hulmes JD, Udenfriend S. Aspartic acid-484 of nascent placental alkaline phosphatase condenses with a phosphatidylinositol glycan to become the carboxyl terminus of the mature enzyme. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1398–1402. [Europe PMC free article] [Abstract] [Google Scholar]
- Koke JA, Yang M, Henner DJ, Volwerk JJ, Griffith OH. High-level expression in Escherichia coli and rapid purification of phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thuringiensis. Protein Expr Purif. 1991 Feb;2(1):51–58. [Abstract] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. [Abstract] [Google Scholar]
- Clowes AW, Cherry RJ, Chapman D. Physical properties of lecithin-cerebroside bilayers. Biochim Biophys Acta. 1971 Oct 12;249(1):301–317. [Abstract] [Google Scholar]
- Barenholz Y, Suurkuusk J, Mountcastle D, Thompson TE, Biltonen RL. A calorimetric study of the thermotropic behavior of aqueous dispersions of natural and synthetic sphingomyelins. Biochemistry. 1976 Jun 1;15(11):2441–2447. [Abstract] [Google Scholar]
- Schreier H, Moran P, Caras IW. Targeting of liposomes to cells expressing CD4 using glycosylphosphatidylinositol-anchored gp120. Influence of liposome composition on intracellular trafficking. J Biol Chem. 1994 Mar 25;269(12):9090–9098. [Abstract] [Google Scholar]
- McConville MJ, Ferguson MA. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. [Europe PMC free article] [Abstract] [Google Scholar]
- Sankaram MB, Thompson TE. Cholesterol-induced fluid-phase immiscibility in membranes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8686–8690. [Europe PMC free article] [Abstract] [Google Scholar]
- Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. [Abstract] [Google Scholar]
- Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994 Feb;42(2):155–166. [Abstract] [Google Scholar]
- Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994 Jun 24;264(5167):1948–1951. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.91.25.12130
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc45390?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.91.25.12130
Article citations
Biomimetic Lipid Raft: Domain Stability and Interaction with Physiologically Active Molecules.
Adv Exp Med Biol, 1461:15-32, 01 Jan 2024
Cited by: 0 articles | PMID: 39289271
Review
Critical Role of Molecular Packing in Lo Phase Membrane Solubilization.
Membranes (Basel), 13(7):652, 07 Jul 2023
Cited by: 1 article | PMID: 37505018 | PMCID: PMC10385406
Glycosphingolipid and Glycosylphosphatidylinositol Affect Each Other in and on the Cell.
Chembiochem, 24(13):e202200761, 01 Jun 2023
Cited by: 2 articles | PMID: 36935354 | PMCID: PMC10330729
Review Free full text in Europe PMC
A compendium of single extracellular vesicle flow cytometry.
J Extracell Vesicles, 12(2):e12299, 01 Feb 2023
Cited by: 46 articles | PMID: 36759917 | PMCID: PMC9911638
Review Free full text in Europe PMC
Dysregulation of cellular membrane homeostasis as a crucial modulator of cancer risk.
FEBS J, 291(7):1299-1352, 07 Nov 2022
Cited by: 3 articles | PMID: 36282100
Review
Go to all (401) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains.
J Biol Chem, 273(2):1150-1157, 01 Jan 1998
Cited by: 239 articles | PMID: 9422781
Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes.
J Biol Chem, 270(11):6254-6260, 01 Mar 1995
Cited by: 120 articles | PMID: 7890763
Role of GPI-anchored enzyme in liposome detergent-resistance.
J Membr Biol, 191(3):215-221, 01 Feb 2003
Cited by: 5 articles | PMID: 12571756
Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes?
Biochem Biophys Res Commun, 240(1):1-7, 01 Nov 1997
Cited by: 298 articles | PMID: 9367871
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: GM48596
Grant ID: GM47897