Abstract
Free full text

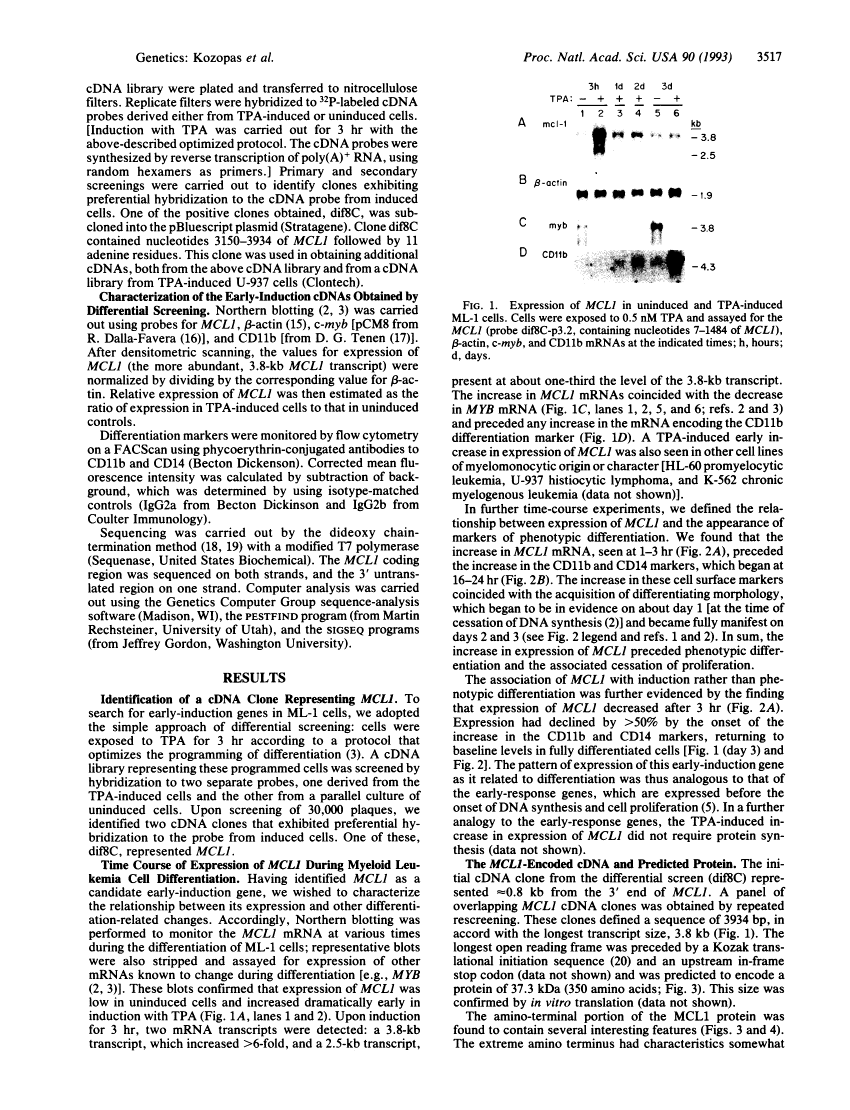
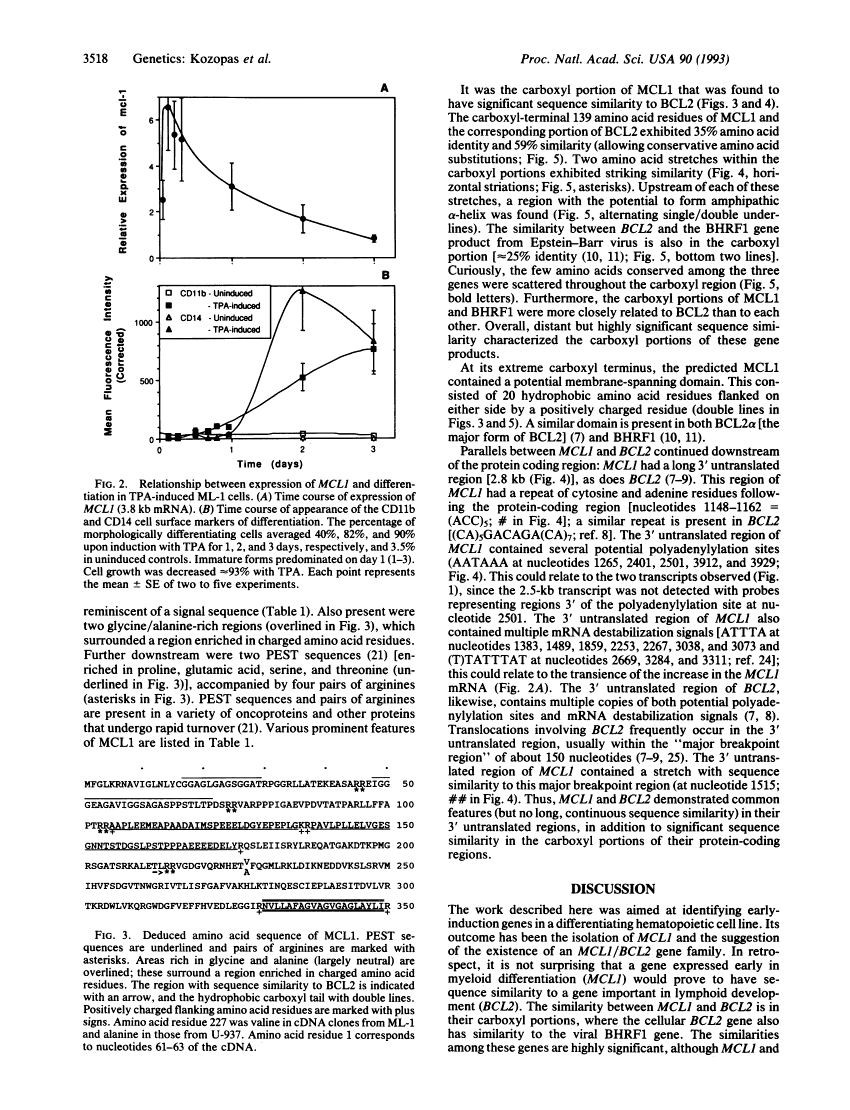
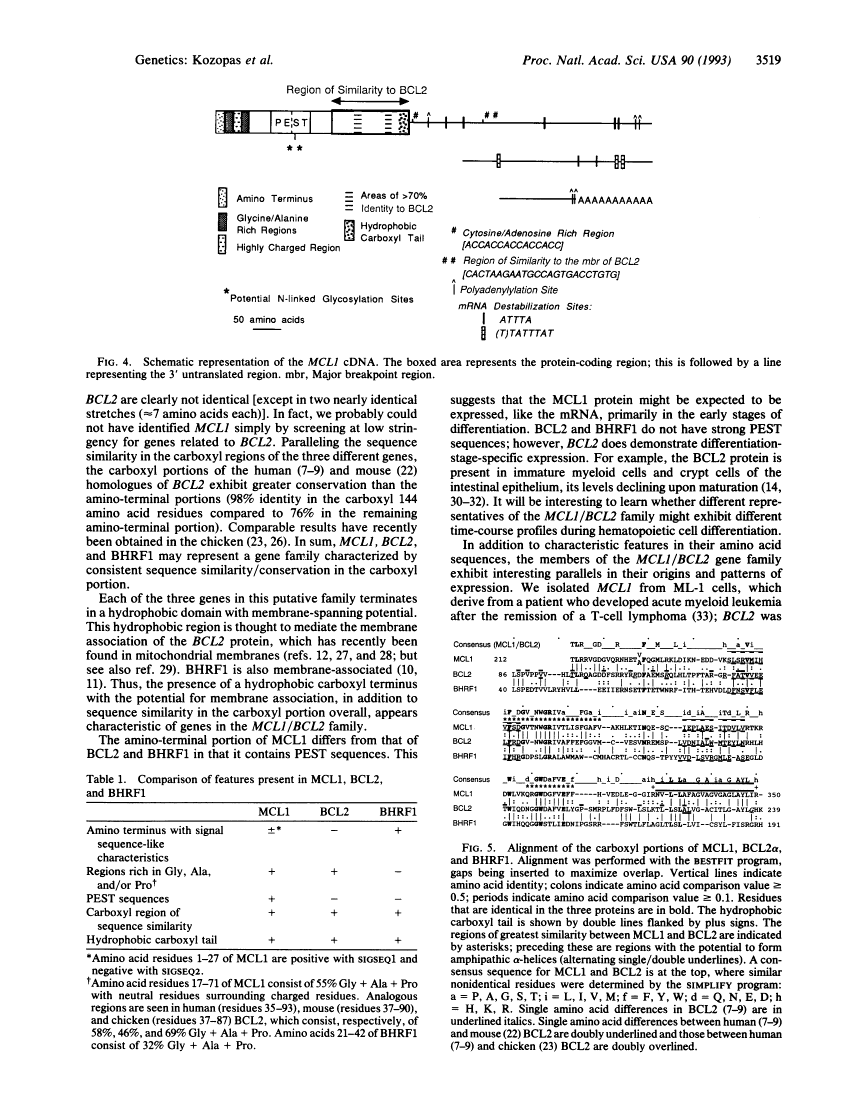
MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2.
Abstract
During their lifespan, immature cells normally pass through sequential transitions to a differentiated state and eventually undergo cell death. This progression is aberrant in cancer, although the transition to differentiation can be reestablished in inducible leukemia cell lines. This report describes a gene, MCL1, that we isolated from the ML-1 human myeloid leukemia cell line during phorbol ester-induced differentiation along the monocyte/macrophage pathway. Our results demonstrate that expression of MCL1 increases early in the induction, or "programming," of differentiation in ML-1 (at 1-3 hr), before the appearance of differentiation markers and mature morphology (at 1-3 days). They further show that MCL1 has sequence similarity to BCL2, a gene involved in normal lymphoid development and in lymphomas with the t(14;18) chromosome translocation. MCL1 and BCL2 do not fall into previously known gene families. BCL2 differs from many oncogenes in that it inhibits programmed cell death, promoting viability rather than proliferation; this parallels the association of MCL1 with the programming of differentiation and concomitant maintenance of viability but not proliferation. Thus, in contrast to proliferation-associated genes, expression of MCL1 and BCL2 relates to the programming of differentiation and cell viability/death. The discovery of MCL1 broadens our perspective on an emerging MCL1/BCL2 gene family and will allow further comparison with oncogene families.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig RW, Frankfurt OS, Sakagami H, Takeda K, Bloch A. Macromolecular and cell cycle effects of different classes of agents inducing the maturation of human myeloblastic leukemia (ML-1) cells. Cancer Res. 1984 Jun;44(6):2421–2429. [Abstract] [Google Scholar]
- Craig RW, Bloch A. Early decline in c-myb oncogene expression in the differentiation of human myeloblastic leukemia (ML-1) cells induced with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 Feb;44(2):442–446. [Abstract] [Google Scholar]
- Kozopas KM, Buchan HL, Craig RW. Improved coupling between proliferation-arrest and differentiation-induction in ML-1 human myeloblastic leukemia cells. J Cell Physiol. 1990 Dec;145(3):575–586. [Abstract] [Google Scholar]
- Kastan MB, Radin AI, Kuerbitz SJ, Onyekwere O, Wolkow CA, Civin CI, Stone KD, Woo T, Ravindranath Y, Craig RW. Levels of p53 protein increase with maturation in human hematopoietic cells. Cancer Res. 1991 Aug 15;51(16):4279–4286. [Abstract] [Google Scholar]
- Nathans D, Lau LF, Christy B, Hartzell S, Nakabeppu Y, Ryder K. Genomic response to growth factors. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):893–900. [Abstract] [Google Scholar]
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. [Abstract] [Google Scholar]
- Tsujimoto Y, Croce CM. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. [Europe PMC free article] [Abstract] [Google Scholar]
- Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. [Abstract] [Google Scholar]
- Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, Korsmeyer SJ. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearson GR, Luka J, Petti L, Sample J, Birkenbach M, Braun D, Kieff E. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology. 1987 Sep;160(1):151–161. [Abstract] [Google Scholar]
- Kocache MM, Pearson GR. Protein kinase activity associated with a cell cycle regulated, membrane-bound Epstein-Barr virus induced early antigen. Intervirology. 1990;31(1):1–13. [Abstract] [Google Scholar]
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. [Abstract] [Google Scholar]
- Liu YJ, Mason DY, Johnson GD, Abbot S, Gregory CD, Hardie DL, Gordon J, MacLennan IC. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991 Aug;21(8):1905–1910. [Abstract] [Google Scholar]
- Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. [Europe PMC free article] [Abstract] [Google Scholar]
- Hanukoglu I, Tanese N, Fuchs E. Complementary DNA sequence of a human cytoplasmic actin. Interspecies divergence of 3' non-coding regions. J Mol Biol. 1983 Feb 5;163(4):673–678. [Abstract] [Google Scholar]
- Majello B, Kenyon LC, Dalla-Favera R. Human c-myb protooncogene: nucleotide sequence of cDNA and organization of the genomic locus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9636–9640. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosmarin AG, Weil SC, Rosner GL, Griffin JD, Arnaout MA, Tenen DG. Differential expression of CD11b/CD18 (Mo1) and myeloperoxidase genes during myeloid differentiation. Blood. 1989 Jan;73(1):131–136. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Schuurman R, Keulen W. Modified protocol for DNA sequence analysis using Sequenase 2.0. Biotechniques. 1991 Feb;10(2):185–185. [Abstract] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. [Abstract] [Google Scholar]
- Negrini M, Silini E, Kozak C, Tsujimoto Y, Croce CM. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. [Abstract] [Google Scholar]
- Eguchi Y, Ewert DL, Tsujimoto Y. Isolation and characterization of the chicken bcl-2 gene: expression in a variety of tissues including lymphoid and neuronal organs in adult and embryo. Nucleic Acids Res. 1992 Aug 25;20(16):4187–4192. [Europe PMC free article] [Abstract] [Google Scholar]
- Cleveland DW, Yen TJ. Multiple determinants of eukaryotic mRNA stability. New Biol. 1989 Nov;1(2):121–126. [Abstract] [Google Scholar]
- Wyatt RT, Rudders RA, Zelenetz A, Delellis RA, Krontiris TG. BCL2 oncogene translocation is mediated by a chi-like consensus. J Exp Med. 1992 Jun 1;175(6):1575–1588. [Europe PMC free article] [Abstract] [Google Scholar]
- Cazals-Hatem DL, Louie DC, Tanaka S, Reed JC. Molecular cloning and DNA sequence analysis of cDNA encoding chicken homologue of the Bcl-2 oncoprotein. Biochim Biophys Acta. 1992 Aug 17;1132(1):109–113. [Abstract] [Google Scholar]
- Chen-Levy Z, Nourse J, Cleary ML. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen-Levy Z, Cleary ML. Membrane topology of the Bcl-2 proto-oncogenic protein demonstrated in vitro. J Biol Chem. 1990 Mar 25;265(9):4929–4933. [Abstract] [Google Scholar]
- Alnemri ES, Robertson NM, Fernandes TF, Croce CM, Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. [Europe PMC free article] [Abstract] [Google Scholar]
- Gurfinkel N, Unger T, Givol D, Mushinski JF. Expression of the bcl-2 gene in mouse B lymphocytic cell lines is differentiation stage specific. Eur J Immunol. 1987 Apr;17(4):567–570. [Abstract] [Google Scholar]
- Graninger WB, Seto M, Boutain B, Goldman P, Korsmeyer SJ. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987 Nov;80(5):1512–1515. [Europe PMC free article] [Abstract] [Google Scholar]
- Delia D, Aiello A, Soligo D, Fontanella E, Melani C, Pezzella F, Pierotti MA, Della Porta G. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992 Mar 1;79(5):1291–1298. [Abstract] [Google Scholar]
- Ohyashiki K, Ohyashiki JH, Sandberg AA. Cytogenetic characterization of putative human myeloblastic leukemia cell lines (ML-1, -2, and -3): origin of the cells. Cancer Res. 1986 Jul;46(7):3642–3647. [Abstract] [Google Scholar]
- Reed JC, Tsujimoto Y, Alpers JD, Croce CM, Nowell PC. Regulation of bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science. 1987 Jun 5;236(4806):1295–1299. [Abstract] [Google Scholar]
- Marchini A, Tomkinson B, Cohen JI, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bc12, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991 Nov;65(11):5991–6000. [Europe PMC free article] [Abstract] [Google Scholar]
- McDonnell TJ, Nunez G, Platt FM, Hockenberry D, London L, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. [Europe PMC free article] [Abstract] [Google Scholar]
- Nuñez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [Abstract] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. [Abstract] [Google Scholar]
- Nuñez G, Hockenbery D, McDonnell TJ, Sorensen CM, Korsmeyer SJ. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. [Abstract] [Google Scholar]
- Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. [Abstract] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. [Abstract] [Google Scholar]
- McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991 Jan 17;349(6306):254–256. [Abstract] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. [Abstract] [Google Scholar]
- Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992 Apr 9;356(6369):494–499. [Abstract] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. [Abstract] [Google Scholar]
- Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.90.8.3516
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc46331?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Evaluation of MCL-1 as a prognostic factor in canine mammary gland tumors.
PLoS One, 19(7):e0306398, 16 Jul 2024
Cited by: 0 articles | PMID: 39012900 | PMCID: PMC11251587
Venetoclax Combination Treatment of Acute Myeloid Leukemia in Adolescents and Young Adult Patients.
J Clin Med, 13(7):2046, 01 Apr 2024
Cited by: 1 article | PMID: 38610812 | PMCID: PMC11012941
Review Free full text in Europe PMC
Personalized, Precision Medicine to Cure Alzheimer's Dementia: Approach #1.
Int J Mol Sci, 25(7):3909, 31 Mar 2024
Cited by: 1 article | PMID: 38612719 | PMCID: PMC11012190
Review Free full text in Europe PMC
Effective Targeting of Melanoma Cells by Combination of Mcl-1 and Bcl-2/Bcl-xL/Bcl-w Inhibitors.
Int J Mol Sci, 25(6):3453, 19 Mar 2024
Cited by: 1 article | PMID: 38542429 | PMCID: PMC10970841
PHA-665752's Antigrowth and Proapoptotic Effects on HSC-3 Human Oral Cancer Cells.
Int J Mol Sci, 25(5):2871, 01 Mar 2024
Cited by: 0 articles | PMID: 38474118 | PMCID: PMC10932316
Go to all (561) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation.
J Biol Chem, 274(3):1801-1813, 01 Jan 1999
Cited by: 85 articles | PMID: 9880563
Induction of BCL2 family member MCL1 as an early response to DNA damage.
Oncogene, 14(9):1031-1039, 01 Mar 1997
Cited by: 47 articles | PMID: 9070651
Expression of the antiapoptotic MCL1 gene product is regulated by a mitogen activated protein kinase-mediated pathway triggered through microtubule disruption and protein kinase C.
Oncogene, 17(10):1223-1234, 01 Sep 1998
Cited by: 56 articles | PMID: 9771965
Differentiation primary response genes and proto-oncogenes as positive and negative regulators of terminal hematopoietic cell differentiation.
Stem Cells, 12(4):352-369, 01 Jul 1994
Cited by: 34 articles | PMID: 7951003
Review