Abstract
Free full text

Plasmodium vivax Transmission in Africa
Abstract
Malaria in sub-Saharan Africa has historically been almost exclusively attributed to Plasmodium falciparum (Pf). Current diagnostic and surveillance systems in much of sub-Saharan Africa are not designed to identify or report non-Pf human malaria infections accurately, resulting in a dearth of routine epidemiological data about their significance. The high prevalence of Duffy negativity provided a rationale for excluding the possibility of Plasmodium vivax (Pv) transmission. However, review of varied evidence sources including traveller infections, community prevalence surveys, local clinical case reports, entomological and serological studies contradicts this viewpoint. Here, these data reports are weighted in a unified framework to reflect the strength of evidence of indigenous Pv transmission in terms of diagnostic specificity, size of individual reports and corroboration between evidence sources. Direct evidence was reported from 21 of the 47 malaria-endemic countries studied, while 42 countries were attributed with infections of visiting travellers. Overall, moderate to conclusive evidence of transmission was available from 18 countries, distributed across all parts of the continent. Approximately 86.6 million Duffy positive hosts were at risk of infection in Africa in 2015. Analysis of the mechanisms sustaining Pv transmission across this continent of low frequency of susceptible hosts found that reports of Pv prevalence were consistent with transmission being potentially limited to Duffy positive populations. Finally, reports of apparent Duffy-independent transmission are discussed. While Pv is evidently not a major malaria parasite across most of sub-Saharan Africa, the evidence presented here highlights its widespread low-level endemicity. An increased awareness of Pv as a potential malaria parasite, coupled with policy shifts towards species-specific diagnostics and reporting, will allow a robust assessment of the public health significance of Pv, as well as the other neglected non-Pf parasites, which are currently invisible to most public health authorities in Africa, but which can cause severe clinical illness and require specific control interventions.
Author Summary
Plasmodium vivax (Pv) is the most widely distributed malaria parasite globally, but conspicuously “absent” from Africa. The majority of African populations do not express the Duffy blood group antigen, which is the only known receptor for Pv infection. Since this discovery in the 1970s, the low clinical incidence of Pv in Africa has resulted in a perception of Pv being completely absent and any apparent cases being misdiagnoses, and no public health allowances are made for this parasite in terms of diagnosis, treatment or surveillance reporting. As more sensitive diagnostics become available, Pv infection in Africa is increasingly reported from a variety of different survey types: entomological, serological, community prevalence surveys, as well as clinical infection data from local residents and travellers returning to malaria-free countries. A literature review was conducted to assemble these reports and assess the current status of evidence about Pv transmission in Africa. Moderate to conclusive evidence of transmission was available from 18 of the 47 malaria-endemic countries examined, distributed across all parts of the continent. Mechanisms explaining this reported transmission are evaluated, as well as alternative explanations for the observations. Combinations of complementary explanations are likely, varying according to regional ecology and population characteristics. The public health implications of these observations and recommendations for increased awareness of Pv transmission on this continent are discussed.
Introduction
Malaria in sub-Saharan Africa has historically been almost exclusively attributed to Plasmodium falciparum (Pf). The identification of the Duffy antigen as the obligate trans-membrane receptor for P. vivax (Pv) infection of red blood cells by Miller et al. during the 1970s [1,2] stalled research into the epidemiology of Pv in Africa as indigenous populations on this continent were known to rarely express the Duffy antigen (and therefore be resistant to infection) and the dogma of “Pv absence from Africa” became entrenched [3,4]. However, against a backdrop of increasing appreciation for the clinical severity of Pv infection [5–7], multiple sources of evidence suggest that Pv may be more prevalent on this continent than commonly perceived [3,8–10]. The absence of any thorough effort to unify these sporadic reports, however, precludes appraisal of their significance, and assessment of the public health significance of Pv in sub-Saharan Africa.
Plasmodium vivax has certain biological and epidemiological characteristics distinguishing it from Pf. Lower peripheral parasitaemia of blood-stage infections and a perception of lower risk of clinical complications during the era of malariotherapy of neuro-syphilis patients [5,11] contributed to the classification of Pv as “benign”, despite the parasite causing the same spectrum of clinical symptoms as Pf [5,7,12,13]. A key difference is the Pv parasite’s ability to form dormant liver-stages (“hypnozoites”) which evade the human immune system and blood-stage therapy, and can trigger relapses of clinical episodes weeks to months following the initial infective mosquito bite [14,15]. Without adequate treatment, hypnozoites therefore impose on the host a cumulative burden of blood-stage infection, contributing to the disease’s severe malaria phenotype, notably as severe anaemia [16]. These (and other) life-stage features unique to Pv mean that several aspects of the control interventions designed against Pf, including preventive measures related to vector biting behaviour [17,18], diagnosis and treatment [15,19,20] are not effective against Pv, therefore making this parasite a greater challenge than Pf in achieving malaria elimination [21,22]. Current WHO diagnosis and treatment guidelines for the WHO African Region (AFR) do not allow for the peculiarities of Pv infections, and are exclusively focused on Pf (see Box 1). Untreated, however, chronic relapses of Pv can cause severe morbidity and mortality [16].
Community surveys in Liberia published in 1949 reported 2.0% of all malaria infections being due to Pv [23]. However, the acceptance of P. ovale (Po) as a separate species [24,25], first as a single species and later two [26], and the demonstration of the dependency of Pv on the Duffy antigen meant that the idea of Pv absence from Africa became entrenched, and during the latter part of the twentieth century apparent diagnoses of Pv were routinely reclassified as Po [3], a morphologically very similar parasite [24]. During this period of near-total reliance on microscopy diagnosis, Pv largely disappeared from sub-Saharan African epidemiological records. The rise of the molecular diagnostics era, however, provided the capacity to confidently differentiate human Plasmodium infections [27], and with it came the first substantive evidence bringing into doubt both the universality of Miller’s observations and the absence of Pv from populations across sub-Saharan Africa. The other endemic Plasmodium species, Po and P. malariae (Pm) have lower parasitaemia than Pv [28], so are also historically neglected by microscopy-based diagnostics, and therefore also rarely recorded without molecular diagnosis.
While Pv is evidently not a major co-endemic parasite in sub-Saharan Africa, Mendis et al in 2001 nevertheless estimated an annual burden of 6–15 million cases across Africa [9]. Reports of Pv transmission from almost all countries across the continent [8] further justify closer investigation. A shifting attitude is discernible in the language of the 2014 World Malaria Report, which suggests that Pv can occur throughout Africa, but with a low risk of infection due to high prevalence of Duffy negativity [29]; and the 2015 WHO Treatment Guidelines [30] indicate that cases are rare outside the Horn of Africa (with the exception of Mauritania and Mali) [30]. Current WHO guidelines for diagnosis and treatment in AFR, however, do not reflect this risk, and for example, recommend rapid diagnostic tests that do not detect non-Pf species. Moreover, most sub-Saharan African countries have no country-specific guidelines for Pv treatment (see Box 1).
The epidemiology of Pv in Africa remains poorly understood, despite being key to evaluating the need for adapting current control interventions, to estimating the burden of Pv disease globally, and to anticipating any changes that may result from reductions in Pf transmission [22]. Here we assess the existing evidence base of Pv transmission in sub-Saharan Africa and evaluate the hypothesised mechanisms which may be sustaining this.
Methods
The broad aims of this study were to evaluate what, if any, evidence existed of Pv transmission in Africa, and to explore whether the small subset of known susceptible individuals (i.e. Duffy positives) could be sustaining the observed prevalence levels reported from community surveys. Here, details are given about (i) the evidence base assembly, (ii) the unified framework weighting the evidence, (iii) quantification of the Duffy positive population at risk of infection (PvPAR), (iv) the prevalence data analysis in relation to PvPAR, and (v) reports of Pv infections in Duffy negative hosts.
Assembling an evidence base of P. vivax transmission in sub-Saharan Africa
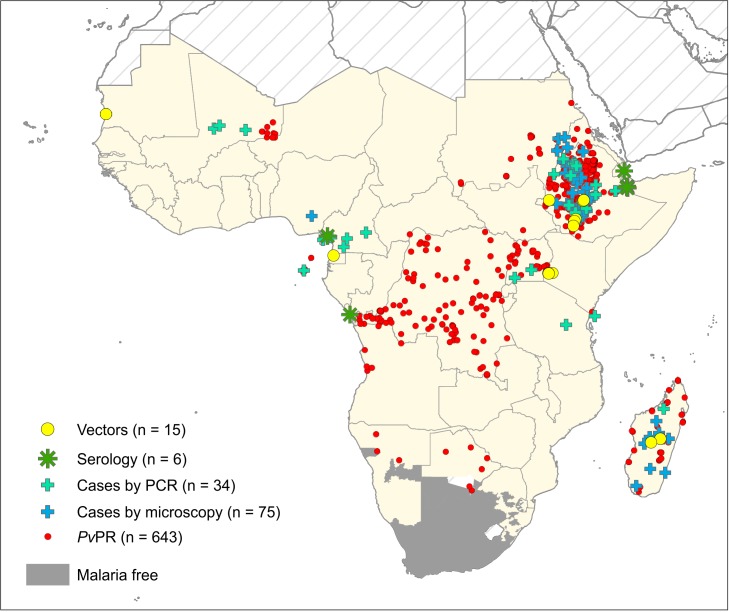
A PubMed literature search starting 01/01/1985 was run using keywords “vivax” AND “[African country names] or [Africa]” (last updated 19/12/2014). Following abstract review for relevance, several categories of data emerged: locally-diagnosed clinical case reports, serological surveys, observations of infected vectors, cross-sectional prevalence surveys, and reports of imported malaria into non-endemic countries by infected travellers (Fig 1). The location, diagnostic details and numbers of Pv infections were recorded by data type. Pv-specific annual parasite incidence (API) data reported by health management information systems was also assembled (see Gething et al 2012 for details [31]).

The geographic extent of the analysis included the 47 malaria-endemic countries of sub-Saharan Africa: all WHO African Regional Office countries except Algeria (0 indigenous cases in 2013 [29]), but including Djibouti, the Republic of Sudan and Somalia. ECDC: European Centre for Disease Prevention and Control. *Prevalence survey analyses were limited to those with sample size ≥50.
A further literature search for references about African Anopheles vectors was run (15/12/2014), using keywords “vivax” AND “[vector species names]”, with the seven dominant vector species across the Africa region [32]: Anopheles arabiensis, An. funestus, An. gambiae, An. melas, An. merus, An. moucheti and An. nili; An. pharoensis was also included as a WHO-defined “major Anopheles species” in certain African countries [29]. The numbers of Pv sporozoite-positive mosquitoes found in each survey location were recorded.
The most commonly used epidemiological metric of malaria endemicity is the parasite rate (PR), assessed by cross-sectional surveys of infection prevalence [33]. The Malaria Atlas Project (www.map.ox.ac.uk) PR surveys database, the product of nearly ten years of archiving [33], was reviewed for evidence of Pv infections reported since 1985 (last updated 17/2/15).
A dataset of imported malaria infections into malaria-free countries by travellers returning from sub-Saharan African countries was assembled from national and regional (notably the European Centre for Disease Prevention and Control, ECDC) surveillance programme reports (search window November 2014 –January 2015). A selection of malaria-free countries with robust surveillance systems were contacted to obtain summary surveillance data since 2000 on imported malaria cases into their country. Given that individuals from these non-endemic countries would have similar prior exposure (i.e. be predominantly immunologically naïve), their country of origin was not relevant to the analysis, although varied countries were approached to increase the coverage of countries of infection (for instance, countries from several continents [Europe, Americas, Asia, Oceania] and linguistic groups [Dutch, English, French, German, Portuguese, Spanish, etc]). Contributing countries and the reporting time intervals which could be assembled are listed in Fig 1. Total numbers of infections were aggregated by probable country of infection.
A weighted framework of the strength of reported evidence of transmission
A framework was developed to assess the overall evidence of Pv transmission at the first sub-national administrative level (Admin1). First, the data available for each data type from each Admin1 were classified as being of category 1 (strongest), 2, or 3 (weakest) evidence (Fig 2), and second, the scores of the seven surfaces representing each evidence type were summed to provide an overall assessment of the relative strength of evidence of Pv transmission occurring in each Admin1. The varied nature of the available data restricted this to being a qualitative analysis, without aiming at any quantitative estimate of endemicity/incidence or quantitative comparison with other Plasmodium parasites. The absolute number of positive reports was used as the metric of strength of evidence, rather than any proportional metric.
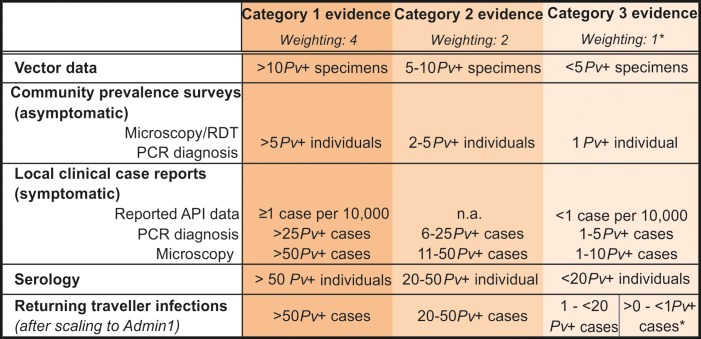
Ranks refer to the relative strength of the evidence as an indicator of ongoing Pv transmission in an area. *The total number of returning traveller infections from each country was divided between the number of Admin1 regions per country. If there was less than 1 reported infection per Admin1, the weighting score was 0.5. Note that because the traveller infections data were retrospective and not sub-nationally specific, it was not possible to distinguish the Republic of Sudan from South Sudan, thus results were considered for Sudan pre-separation and the same score allocated to both countries based on the overall data.
The criteria for each evidence category varied according to the time window of exposure represented by the data type (and thus the probability of local infection), the denominator size, and the diagnostic method sensitivity/specificity. For example, due to their limited range of movement, infected vector specimens were considered relatively strong evidence of local transmission. In contrast, the longer window of exposure detected by serological surveys meant sero-positivity could result from a transmission event in a different location prior to relocation to the study site. To reflect the reduced temporal specificity of the serological evidence, a relatively higher number of reports of sero-positive individuals was therefore necessary to provide the same strength of evidence of transmission as infections observed in real-time. The single time-point snapshot investigated during a community prevalence survey contrasts with a much larger time window and population denominator for clinical case reporting across a region. Therefore, evidence of parasitaemia from prevalence surveys was considered stronger evidence of ongoing transmission than routine reporting of symptomatic cases, and a larger number of positive symptomatic individuals was required to equate to the strength of evidence of asymptomatic infections from prevalence surveys. Annual Parasite Incidence data (API) indicating stable transmission (≥1 case/10,000) equated to the strongest category 1, while unstable transmission (<1 case/10,000) was category 3.
Clinical cases diagnosed by molecular diagnostic tools based on nucleic acid amplification techniques (notably PCR) were considered stronger evidence of transmission than by light microscopy or rapid diagnostic tests (RDT) due to the increased species-specific diagnostic accuracy. In the case of prevalence surveys, however, where infections were more likely to be of low parasitaemia, RDT and microscopy were expected to diagnose a smaller proportion of total infections than PCR-based diagnostics due to their differing limits of the detection [28]. It has been estimated that molecular diagnostics identify at least double the number of infections compared to conventional methods [34]. Therefore the numbers of infections diagnosed by the two methods correspond to differing proportions of the true parasite prevalence, with RDT and microscopy-based underestimating true prevalence. On the other hand, RDT and microscopy diagnostics were less specific. These two aspects therefore balanced out and it was considered that prevalence data from both diagnostics would correspond to similar strength of evidence of transmission.
Reports of Pv infection among travellers returning from African countries could not be attributed the same strength of evidence as observations of infection among local residents. The relapsing nature of Pv parasites means that infections could have been acquired from travel prior to the reported journey. Furthermore, the nature of the imported infections dataset assembled here is highly opportunistic and incomplete. This data type was therefore down-weighted relative to reports of local infections.
The data were mapped to the Admin1 level. Where multiple surveys within a data type were available from a single administrative region, the scores were based on the total number of reports. Returning traveller infections were only reported to the national level, so to allocate scores to Admin1 units, the overall number of reported Pv infections was divided by the total number of Admin1 units in the country. These infections were therefore strongly down-weighted relative to the other data.
Finally, once the component data types were categorised and mapped, these were summed together in ArcMap 10.1 [35]. The weighting system accounted both for the strength of evidence in each category and for corroboration between data types: the more data types reporting Pv transmission, the higher the overall strength of evidence. The highest level, “conclusive evidence”, was only attained if two or more category 1 sources of evidence were available. The lowest level, “very weak evidence” corresponded to Admin1 units from which no direct evidence was available, only a relatively small number of infected traveller reports (less than 1 case per number of Admin1 units, thus a low probability of local transmission).
Estimating the population at risk of infection
Using an approach similar to that previously published [8,31], the geographic limits of Pv transmission were overlaid onto a 2015 population surface to estimate the PvPAR. Medical intelligence data and biological exclusions were applied to define the limits of potential transmission. First, API and routinely reported incidence data were reviewed [31] to identify areas which could be excluded for being risk-free of malaria; this exclusion was not species-specific due to the previously discussed imperfect diagnostic capacity of most countries, but instead only areas defined as “malaria free” were excluded. Second, areas where temperatures could not support Pv sporogony at any time in an average year were excluded by a modelled temperature suitability mask [36]. Aridity was not used as exclusion given that man-made conditions can facilitate vector breeding even in areas of extreme aridity; previous PvPAR estimates have “downgraded” risk from stable to unstable transmission levels based on aridity, but the lack of distinction between these levels here means no aridity exclusion was applied. Third, urban areas, as identified based on the Global Rural Urban Mapping Project (GRUMP) urban extents layer [37], were excluded. While potentially overly-conservative, the exclusion of urban populations was consistent with previous PvPAR estimates [8,31], and is based on the significantly lower infection risk in urban areas [38,39].
A population surface for 2015 was compiled from the WorldPop Project projections (www.worldpop.org), supplemented by data from the Gridded Population of the World (GPW) v3 (http://sedac.ciesin.columbia.edu/data/set/gpw-v3-population-count-future-estimates) for the Comoros and São Tomé and Príncipe, which were not available from WorldPop. This was adjusted to the Duffy positive population using the previously published Duffy blood group frequency maps developed by geostatistical modelling of population surveys of blood group frequencies [4]. The median model prediction of the Duffy surface was used, together with the 25% and 75% quartiles (50% confidence interval) of the Duffy model outputs. National-level PvPAR estimates were derived for the three Duffy thresholds within the defined limits of transmission. All spatial manipulations were run at 5 x 5 km resolution in ArcMap and ArcScene 10.1 [35].
Investigation of reported PvPR in relation to the Duffy positive PvPAR
The PR database was used to investigate aspects of Pv transmission across sub-Saharan Africa and compare them to the PvPAR and to Pf. Estimates of the prevalence of Duffy negativity at the PR survey locations were extracted from the modelled Duffy group frequency maps [4]. For the comparisons with Pf, only surveys with the diagnostic capacity to identify both species were included, ensuring that paired estimates of PvPR and PfPR were matched in space, time, and population sample characteristics. The analysis was also restricted to surveys of ≥50 individuals. A trio of spatially-matched estimates were therefore collated for PvPR, PfPR and Duffy negativity prevalence.
This dataset was used to assess: (i) whether the observed PvPR values were consistent with infections being potentially limited exclusively to Duffy positive hosts, (ii) how the prevalence of infection and relative risk of infection differed between species among their specific subset of known susceptible hosts (based on the assumption that the PvPAR was limited to Duffy positive hosts: PvPRFy+ = PvPR/Proportion of Duffy positive hosts), (iii) the relationship between infection prevalence of the two species, and whether prevalence of one could inform predictions of the other. Full methodological details are available in Text S1.
Collating reports of P. vivax infections in Duffy negative hosts
Reports of Pv infections in Duffy negative hosts were compiled from the different data types reviewed. Only studies that used molecular confirmation of both Pv infection and Duffy genotype were included. The reports were geopositioned and presented in relation to the local prevalence of Duffy blood group phenotypes estimated by the modelled geostatistical maps [4].
Results
Evidence of transmission: The data
The literature search identified 1,479 publications, of which 529 were considered relevant to studies of Pv in Africa. All reports of Pv infection were collated and geopositioned (Fig 3). A total of 15 surveys reporting infected Anopheles vectors were identified (S1 Table), as well as six reports of Pv sero-positivity (S2 Table), 109 sites with local Pv case reports (S3 Table) and 643 Pv-positive community prevalence surveys (www.map.ox.ac.uk). Overall, reports of Pv were available from 21 of the 47 malaria-endemic countries examined. The maps in Fig 4 represent the aggregated scores mapped to the Admin1 level.
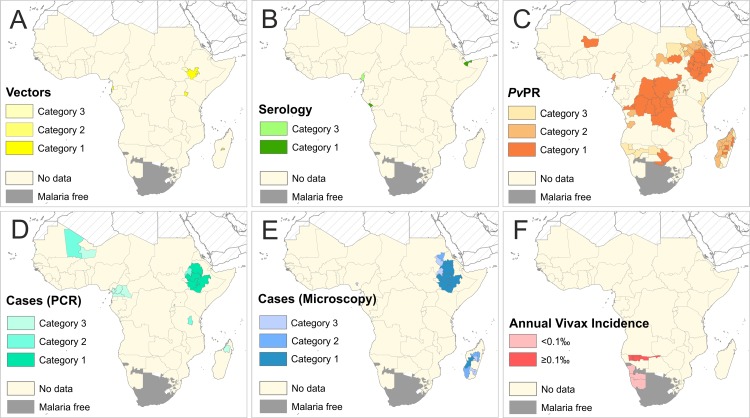
Evidence categories are defined in Fig 3. Panel A represents evidence of Pv from infected vectors, Panel B sero-positivity, Panel C Pv community prevalence surveys, Panel D molecularly-diagnosed clinical cases, Panel E microscopy-diagnosed clinical cases and Panel F annual Pv incidence data. See S1–S3 Tables for original data.
Records of Plasmodium infections in travellers were accessed for 31 malaria-free countries, corresponding to a period of 182 surveillance-years between 2000 and 2014 (Fig 1). Overall, 1,339 Pv infections were reported from 42 suspected countries of infection spread across sub-Saharan African countries, corresponding to all but five countries considered here. Fewer than 20 cases of Pv were reported from 24 countries, while from nine countries there were 20–50 cases, and nine countries were associated with infection of 50 of more travellers over the reporting period. The number of cases that our searches assembled and the proportion of Pv infections relative to Pf are shown in Fig 5, though these ratios were not included in the evidence-weighting framework which focussed exclusively on Pv with evidence strength classifications based on categorical classifications and not a quantitative analysis. Limitations to this dataset are discussed below.
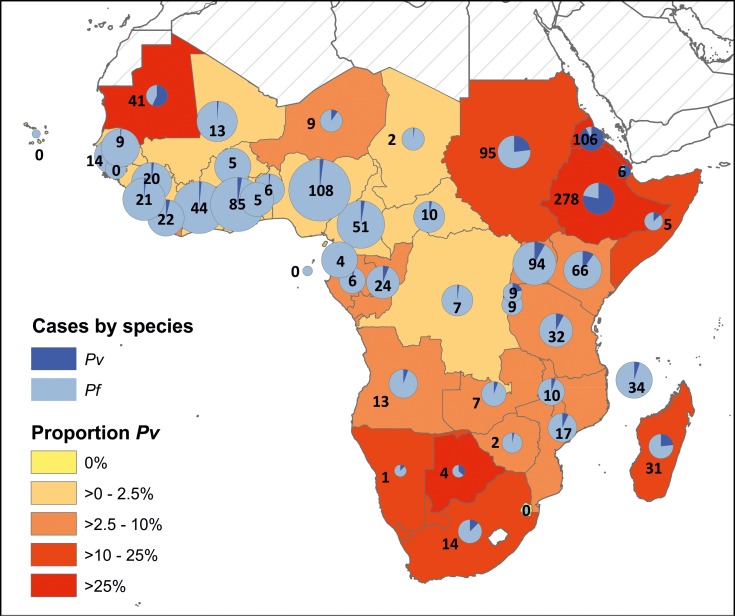
The relative contribution of Pf and Pv infections is reflected in each pie chart (with charts sized by overall number of infections, transformed on a square-root scale). Numbers indicate the number of exported Pv infections identified by this study (corresponding to the dark blue segment of the pie chart). Infections date from 2000 to 2014; data sources and countries of importation are listed in Fig 1. Where species-specific information was available for mixed infections, these were considered to be separate infections, so a single individual would have contributed two or more infections to the total count of malaria parasite infections. Returning patients for whom a single country of probable infection could not be determined were excluded.
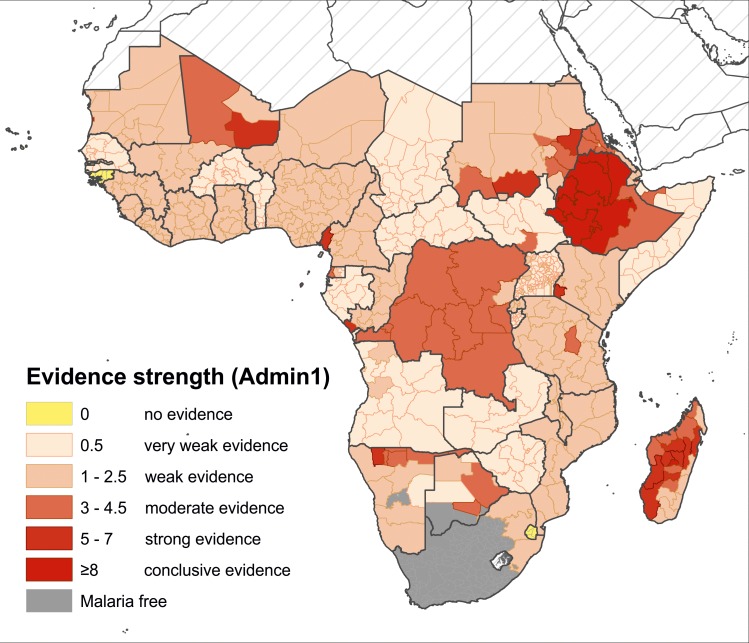
Evidence of transmission: The composite map
The composite map in Fig 6 reflects the strength of the overall available evidence in each Admin1 unit (summarised by country in S5 Table). Of the 629 Admin1 units across malaria-endemic Africa, there were 28 units for which no evidence of transmission was identified (island nations of Cape Verde and Mayotte, and the small states of Guinea-Bissau and Swaziland). In 488 Admin1 units, the only available evidence was from traveller infections, so evidence of transmission from these areas was considered weak (n = 217) or very weak (n = 271). Of the remaining Admin1 units from which direct evidence was identified (n = 113), eight had conclusive evidence (in Ethiopia, Madagascar and Mauritania), 20 had strong, 36 moderate and 49 weak evidence of local transmission. Overall, there was moderate to conclusive evidence of Pv from 18 countries, including four in West Africa, three in Central Africa, three in East Africa, two in Southern Africa, and all six countries of the “Horn of Africa+” (HoA+) region (which included Djibouti, Eritrea, Ethiopia, Somalia, Sudan and South Sudan). The weaker evidence categories represent areas of relatively higher uncertainty.
Population at risk of infection
An estimated 86.6 million individuals were at risk of Pv infection (PvPAR) across sub-Saharan Africa in 2015 (interquartile range, IQR: 43.9–156.7 million; S6 Table). These individuals were Duffy positive hosts living outside urban areas across malaria-endemic Africa where temperatures were suitable for sporogony at some point during an average year. The confidence interval is based only on the IQR of the Duffy positive frequency surface [4], without accounting for uncertainty in the population density dataset or the temperature suitability surface. The binary adjustment excluding risk of urban transmission results in a conservative PvPAR estimate which is also not represented in the confidence interval.
Overall, 28% of the PvPAR was outside the HoA+ region, with 10.0 million in East Africa (4.7 million excluding Madagascar), 7.2 million in West Africa, 4.1 million in Southern Africa and 3.3 million in Central Africa (S6 Table). In 12 countries distributed across all sub-regions, the national PvPAR was greater than one million; Ethiopia, Sudan and Madagascar carried the highest PvPARs. The spatial distribution of the PvPAR is illustrated in Fig 7, showing clustered areas of relatively increased PvPAR across the continent.
Investigation of PvPR in relation the Duffy positive PvPAR and to PfPR
Transmission of Pv in sub-Saharan Africa has been considered unlikely given the high prevalence of Duffy negativity across the continent. However, the evidence indicates that Pv is present, so here we investigated (i) whether the observed infection rates could be consistent with transmission exclusively limited to Duffy positive hosts, and (ii) how Pv and Pf infection rates were related to one another in sub-Saharan Africa. Full discussion of the results of these analyses and additional figures are available in the Supplementary Information (S1 Text and S1–S3 Figs).
The data (Fig 8A) were first examined for consistency with transmission within the Duffy positive population subset. The scatter of the data in Fig 8B being almost exclusively concentrated below the x = y dashed line (1,540 of 1,546 surveys) indicates that the proportion of individuals infected does not exceed the proportion of the population considered susceptible, thus fulfilling the necessary condition (but without proving) that Duffy negativity imposes an upper threshold on PvPR by infections being limited to Duffy positive hosts. Six PvPR values (1.7% of positive surveys) were outliers to this, all from coastal areas of Western/Central regions, including 4 from São Tomé and Príncipe. The survey with the highest discrepancy was from Cameroon, where 13 of 269 individuals were PCR-Pv positive [40]. Fig 8B also indicates PvPR rising as the frequency of susceptible hosts increases, as would be expected.

Only surveys of ≥50 individuals are included, and PvPR values are adjusted to the 1–99 age range. Panel A shows the PvPR values, their spatial distribution and the diagnostic method used for each survey. Panel B is a scatterplot of the relationship between the proportion of the population at risk of Pv infection (represented by the proportion of the population Fy+) and the proportion of individuals infected with Pv (PvPR).
Next, PvPR estimates were re-scaled to prevalence of infection among the subset of Duffy positive hosts (PvPRFy+ = PvPR/Proportion of Duffy positive hosts). S1 Fig plots PvPRFy+ against PfPR, revealing distributions of points across the graphs without any clear trends emerging between infection rates between species. Regional trends, however, were apparent, with higher relative risk of infection in regions of lower host availability. For instance, where PvPRFy+ was positive in the Western region (n = 13), all surveys showed higher likelihood of infection by Pv than Pf (among the respective susceptible population sub-groups) (S1 and S2 Figs). In areas where Duffy positive hosts were rare (across most of sub-Saharan Africa these are <5%), these individuals were at a higher risk of being infected by Pv than by Pf (S2 Fig). As Duffy positive hosts become more common, the relative risk of infection becomes more evenly distributed around 0.
Finally, a logistic regression model was developed to test for an association at the population level between the parasite rates of the two species: could PR data from one species predict PR of the other? No significant relationship could be identified from the paired PR dataset for surveys outside the HoA+, and the association was not significantly different from a flat line (S3 Fig). Within the HoA+, PfPR was a highly significant predictor of PvPRFy+ (p = 4.4x10-6) up to 15% PfPR, after which increases in PfPR did not result in further predicted increases in PvPRFy+; in contrast, PvPRFy+ within the HoA+ was a significant linear predictor of PfPR at all levels of endemicity. Substantial scatter and heterogeneity in the data, however, resulted in wide 95% confidence intervals around the predicted relationship even when a significant relationship was identified.
Reports of P. vivax infection in Duffy negative hosts
The last few years have seen evidence emerging of Pv infections in Duffy negative hosts (Fy-Pv+ infections), providing an additional potential mechanism sustaining Pv transmission across sub-Saharan Africa. These observations are from diverse geographic, demographic and host genetic landscapes. The PubMed literature review identified 19 sites across 6 countries of sub-Saharan Africa where Fy-Pv+ infections in 54 individuals have been confirmed by molecular diagnosis, all published 2010–2015 (S4 Table and Fig 9). Observations of Fy-Pv+ infections were widely distributed across the continent, including in populations with heterogeneous Duffy phenotypes (with correspondingly higher PvPAR: Ethiopia and Madagascar) as well as in populations where Duffy negativity was at near fixation (where PvPAR was much lower: Angola, Cameroon, Equatorial Guinea and Mauritania). All Fy-Pv+ infections (except one survey from Yaoundé, Cameroon) were from areas classified as “rural” by GRUMP. The observations were from areas of varied population densities, ranging from <100 to >500,000 individuals in the surrounding 25 km2 area (WorldPop Project data). Both symptomatic and asymptomatic Fy-Pv+ infections were reported. The reported repartition of Pv infections among Duffy negative and positive hosts ranged widely between surveys (S4 Table), but the data did not permit any formal analysis of differential infection risk.

Yellow stars represent the number of Fy-Pv+ infections identified at each study location (including some as mixed infections with other Plasmodium species). Original citations and further details are given in S4 Table. Pie-charts summarise the predicted prevalence of the Duffy phenotypes in each country [41]. The background map is the predicted frequency of Duffy negativity [4]. Further Fy-Pv+ infections from western Kenya have been reported [42], but the diagnoses were inconclusive, so are not included in this map.
Discussion
It is evident that Pv is not absent from Africa. There is robust evidence from a variety of corroborating sources of Pv transmission across all sub-regions, with direct evidence from 21 of the 47 countries considered, and 42 countries are attributed with infection of visiting travellers. Overall, there was moderate to conclusive evidence of Pv transmission from within 18 countries. The remaining countries either had only weak evidence of transmission or no evidence could be found at all. These observations of widespread Pv infection should not be surprising: a suitable environment, competent vectors and susceptible human hosts make it unlikely that the parasite would not be present. The implications of the present Pv-specific study apply to all non-Pf species: without adequate diagnosis and reporting, their true public health burden cannot be determined. While Po and Pm are more widely acknowledged to be endemic to parts of Africa, Pf-specific diagnostics will not diagnose the low-level parasitaemia and non-specific symptoms that the non-Pf malarias present as, and radical cure is not available for either of the relapsing species, Pv or Po.
The opportunistic and highly varied nature of the available surveys restricted the sophistication of potential analyses, limiting the composite map to being a qualitative assessment of available evidence rather than providing any quantitative measures or comparisons with other parasite species. For instance, varied molecular diagnostic methodologies or inconsistent approaches for calculating sero-prevalence meant that standardization was not possible even within each data type, therefore favouring the categorical approach. The limited distribution of the data means that the resulting evidence map is both a map of sampling effort and of Pv presence in those sampled areas. Greater survey coverage would allow a distancing from the dependency on sampling effort. The traveller data are also biased according to popular travel destinations, and suffer further limitations: (i) the data assembled here are incomplete, formed from an opportunistic selection of countries and reporting years. The numbers of infections cannot be interpreted in any absolute sense; (ii) data are reported only to the national level, whereas the analysis was sub-national; (iii) suspected countries of infection may not be fully reliable given Pv’s ability to form latent infections. In recognition of these limitations, traveller data were therefore strongly down-weighted relative to other data types but nevertheless represented an important and complimentary component of the evidence of Pv transmission.
Although there were additional surveys reporting “zero Pv”, these were not incorporated into the evidence synthesis as they could not be interpreted as “absolute absences” [43], particularly given the lower sensitivity of conventional microscopy and RDT methods to Pv than Pf [27,29] which often underestimate the true prevalence of blood-stage Pv [44–48], especially in mixed infections [49]. The low anticipated prevalence of Pv means that very large sample sizes and diagnostic tools with higher sensitivity for detecting Pv would be needed to reliably give any confidence of “absence”.
While Pv infection levels appear plausible with transmission being restricted to the Duffy positive population at risk (estimated at 86.6 million), a number of other explanations discussed here have also been hypothesised to explain the observed infections.
Transmission among Duffy positive hosts
The PR data were found to be consistent with transmission being potentially sustained by Duffy positive hosts alone. However, an important limitation to the PR comparative analysis was the skewed distribution of positive surveys, with the majority coming from the HoA+ (73%), while the main area of interest was outside this region. The lack of any predictable association between PvPRFy+ and PfPR in regions outside the HoA+ suggests that change in the prevalence of one species is not reflected by the prevalence of the other species at the host population level. This outcome may indicate that the two infection rates are determined by different drivers of transmission linked to their differing biological characteristics, notably capacity to relapse. For instance in these areas of low Duffy positive host availability, the environmental drivers that are highly significant determinants of PfPR endemicity [50], may be secondary to the impact of the local host dynamics for predicting PvPRFy+. Alternatively, the noise from the heterogeneity of the small dataset (exacerbated by the PvPR adjustment to PvPRFy+) may be masking a relationship similar to that in the more data-rich HoA+ region which would be possible to detect through a larger dataset.
Bespoke transmission models accounting for relapse risks and the peculiarities of the predominantly Duffy negative landscape are required in order to estimate the critical community sizes and determine the transmission dynamics needed to sustain infection, to verify the plausibility of Pv in Africa being limited to Duffy positive individuals [51–56].
The quantitative PR data analysis presented here is heavily contingent on the Duffy negativity frequency map [4]. Of the 203 population surveys which informed that map, 41% were published pre-1990. The increase in large-scale population movement since this time [57,58] means that an influx of Duffy positive alleles to the gene pool may be increasing the frequency of susceptible hosts with a strongly spatially clustered distribution, making the 86.6 million PvPAR estimate a potentially significant underestimate. Furthermore, the historical waves of migration from Duffy positive British, French, Indian, Lebanese, Portuguese etc, populations may also be underrepresented. The wide confidence interval of the PvPAR (43.9–156.7 million) indicates the potential impact of changes to the Duffy maps. Furthermore, the GRUMP map which was used to identify urban populations for exclusion from the PvPAR, overestimate urban areas, so many high density rural populations may have been excluded, further contributing to a conservative PvPAR estimate.
Plasmodium ovale misdiagnoses
Plasmodium ovale represents an important potential confounder to the reliability of microscopic reports of Pv due to their morphological similarities [24] and the risk of Pv reports actually being misdiagnosed Po infections. Conversely, the opposite may also be true with Pv infections being misclassified as Po due to the belief of Pv absence [3]. Microscopy-based diagnoses were down-weighted relative to PCR-based data in the evidence framework to account for these uncertainties. There is also potential for cross-reactivity between Po- and Pv-specific antigens in serological screening. Little is known about the diversity of Po in particular, so there is a need for the development of additional species-specific reagents to reliably distinguish the two species.
The finding of Pv being a common cause of traveller infections is consistently reported from varied sources (Fig 5). For instance, of 618 cases of Pv imported into Europe between 1999 and 2003, 33.8% were reported to have been infected in Africa [59]. While molecular confirmation is not routine for returning traveller infections, this is increasingly common in China where thorough case investigations differentiate imported from indigenous cases [60]. Recent investigation of malaria infections among gold miners returning to China from Ghana after a median travel time of one year, found 42 of 874 infections were Pv and 1 was Po [61]. From a public health perspective, even if these several hundreds of infections are all misdiagnoses, given that the same treatment guidelines for radical cure apply to both species [30], the potential misdiagnosis is not significant in terms of treatment policy and requires the same increased diagnostic capacity away from the perception of Pf exclusivity.
Relapse as a confounder
A potential confounder to attributing all the assembled evidence of Pv to local transmission in Africa is the parasite’s ability to form dormant hypnozoites and present clinical symptoms only after a delayed period during which the patient may have travelled widely [14,62]. The evidence-weighting framework attempted to account for this possibility through the differing thresholds between categories. Again, though, even if a subset of the observed cases resulted from infection events elsewhere, the implications for diagnostic and treatment policy remain the same: appropriate capacity is required in the area of clinical presentation, irrespective of where transmission occurred.
Zoonotic transfer from an ape reservoir
Liu et al [63] have published conclusive evidence of the origin of human Pv being from a genetically diverse parasite population whose natural hosts are gorillas and chimpanzees in Africa. Although the human parasite clade is distinct from the more diverse parasite strains that indiscriminately infect various ape species, there is evidence (from one individual) of the plausibility of cross-infection of parasite strains between ape and human hosts with similar clinical presentation [63,64]. Liu et al therefore argue that Pv infections reported from regions of high Duffy negativity frequency are zoonotic infections spilling over from ape reservoirs. Entomological evidence suggests that An. moucheti and An. vinckei may be potential vector species bridging transmission between apes and humans [64,65], although the entomological evidence is very limited with only one reported infected specimen of each species and vector behaviour that is not conducive to frequent transmission events.
The natural ranges of these ape reservoir species are restricted to the forests of Central Africa (Fig 10). So, while this infection mechanism may explain transmission in specific contexts, this does not represent a universal explanation for the evidence of Pv across predominantly Duffy negative regions of Africa.
Duffy-independent transmission
The reports of Duffy negative infections from diverse epidemiological settings (54 molecularly-confirmed symptomatic and asymptomatic infections identified across 19 sites; S4 Table) represent a further potential mechanism sustaining Pv transmission across Africa. The public health burden of these infections is unclear. While the protective effect of Duffy negativity may not be as absolute as previously considered, this blood group’s high frequencies across Africa do nevertheless appear to represent a limiting force on potential Pv endemicity. In the absence of any limiting factor, and given the presence of competent vectors and a suitable climate, rates of Pv infection comparable to those in Asia or the Americas [31] would be expected. Fy-Pv+ infections demand further investigation, ideally across diverse epidemiological settings. The impact of reduced protection conferred by Duffy negativity (currently assumed to be 100%) would have important impacts on the resulting PvPAR (see estimates in Zimmerman et al [67]).
Complementary transmission mechanisms across a highly diverse continent
For the first time, this paper synthesises all available evidence of Pv transmission in Africa, and evaluates the varied mechanisms hypothesised to explain these observations across an area where transmission is largely unexpected. Here we argue that no single theory is sufficient to explain transmission across this varied continent, but instead, transmission pathways vary according to the complex ecology and epidemiology of each area and population.
Evidence relating to Pv transmission across Africa appears inconsistent. Pv is clearly widely present, causing a substantial proportion of returning traveller malaria infections, clinical illness and asymptomatic infection among local residents, infecting vectors and presenting a history of exposure by local communities to infected bites. However, extensive surveys using high-sensitivity molecular methods have repeatedly failed to diagnose Pv [68–70]. For instance, PCR-based screening of 1,402 blood samples in southern Cameroon found no trace of Pv [69], while a nearby PCR-based community survey of 269 individuals diagnosed a prevalence rate of 5% PvPR (representing 13.8% of all Plasmodium infections) [40]. The key difference between these neighbouring surveys in southern Cameroon was the demographic composition of the sampled populations: while no Pv was found in the remote, rural village communities [69], the multi-ethnic and highly cosmopolitan population did have Pv [40].
While there is some evidence of an ape reservoir enabling zoonotic infections, this does not preclude the possibility of endemic transmission. Fig 10 makes evident the observations of Pv outside these ape natural ranges, and furthermore, the main group of travellers returning from Africa with Pv infections are visiting relatives [71,72], and less likely to visit the tourist attractions which would bring them into contact with infected vectors [73]. Therefore, while there is evidence of the zoonotic reservoir being a contributing source of infection, other mechanisms of transmission (and reservoirs of infection) must also be involved.
The multiple theories discussed so far for explaining the observations of Pv in Africa are not mutually exclusive. Instead, given the limitations to each hypothesis for explaining the overall diversity of transmission, it seems probable that all are involved to some degree. The dominant source of infection would vary between location: in remote forest areas, infections may be from the ape reservoir [64], while in more cosmopolitan areas, transmission may be being sustained by an admixed Duffy positive population. The early production of gametocytes in infections [20] means that even where the host has developed immunity to symptomatic blood-stage parasitaemia from prior exposure, short-lived sub-clinical infections could still be helping to sustain transmission. It is likely that some reported cases of Pv are in fact Po misdiagnoses, but the sheer abundance of data from various diagnostic methods means this cannot be universal. Similarly, while there is convincing evidence of Fy-Pv+ infections, these are not reflected by the widespread Pv endemicity levels that would be expected if these infections were commonplace. The epidemiology of Pv in Africa is likely to be complex and multi-faceted, driven by different mechanisms in different regions, determined by the unique host genetic, vector, and reservoir characteristics of each area. These complimentary theories together may explain the observed evidence of Pv transmission. High sensitivity molecular methods could allow further insight into the population genetics of Pv infections diagnosed in Africa and therefore their epidemiology [74], as well as helping to define true exposure and risk of exposure to infection.
Public health implications of widespread P. vivax infection risk
The non-specific clinical presentation of malaria means that diagnosis is only possible with a parasitological test [30]. The WHO T3 policy to “Test, Treat, Track” all cases of malaria is very logical in an era of resistance emergence and falling endemicity in many areas [75]. However, it becomes harmful if the “Test” component is not capable of detecting all potential parasites. In the many African countries exclusively reliant on HRP2-based RDT diagnosis (see Box 1), Pv, Po and Pm infected patients who present in clinics with fever symptoms will test negative and could leave untreated. The patients will persist in the community as sources of onward transmission, and, in the case of Pv and Po, be susceptible to the cumulative impact of clinical relapses.
In turn, therapeutic options for Pv are inadequate. While Pf-targeted control will treat Pv clinical symptoms, it will not impact on the parasite reservoir sustaining the observed blood-stage clinical infections. Even in terms of blood-stage therapy, drug policy in Ethiopia (ranked fourth largest contributor to Pv cases globally [29]) maintains chloroquine as first-line treatment despite reports of resistance [76]. A further complication to delivering Pv-specific therapy in Africa is the high prevalence of glucose-6-phosphate dehydrogenase deficiency (G6PDd) [77], causing a potentially dangerous intolerance to primaquine, the only available drug for treating the liver-stage parasites [78]. The same issues apply to Po radical cure. It has been hypothesised that the high prevalence of G6PDd may be the result of a selective advantage against malaria [79–81], meaning that G6PDd might be under-represented in Pv patients and thus primaquine therapy a lesser concern. However, given Pv’s preference for invading reticulocytes–young red cells with highest levels of G6PD enzyme activity–it is unlikely that a relatively mild G6PD variant (such as G6PDA-, the predominant African variant [82]), would provide any protective advantage against Pv infection.
Finally, the evidence presented here has two main implications for Pv mapping. First, where PvPR surveys are available, the infection rates reported are consistent with transmission only among Duffy positive hosts. This therefore supports the approach previously followed of restricting the PvPAR to Duffy positive hosts [8,83] and of using the frequency of Duffy positive hosts as an upper threshold to the maximum potential prevalence of infection [31]. Second, the PfPR surface cannot be used to predict Pv infection prevalence in Duffy positive hosts, except potentially in transmission settings up to 15% PfPR in the HoA+ region.
Conclusions
The implications of this paper should not be misinterpreted. This is not a “call to arms” requiring huge additional resources above and beyond the considerable efforts already ongoing into control of Pf. Current epidemiological data overwhelmingly indicates that Pf is the predominant malaria pathogen across most of sub-Saharan Africa [29,31,68], and that where present, Pv prevalence remains low. Nevertheless, despite the estimated 86.6 million individuals at risk of infection, Pv is so drastically neglected across this vast and populous continent that without a commitment to add capacity for Pv in routine surveillance and reporting, this minor player will remain invisible and any quantitative burden estimates and evidence-based policy decisions impossible. Broadening the outlook beyond Pf would simultaneously also strengthen the evidence base relating to the other neglected endemic species, Po and Pm and increase access to diagnosis and treatment of these other neglected malaria species.
Supporting Information
S1 Text
Supporting methods and results: Investigation of the PvPR data in relation to host population characteristics and PfPR.(DOCX)
S1 Fig
Prevalence of infection by Pf (PfPR) in relation to PvPR among Duffy positive hosts (PvPRFy+).Panels A and B show the full dataset (n = 1,546); Panels C and D have surveys reporting zero prevalence of either species excluded (n = 249). Panels A and C represent prevalence 0–1; while B and D provide higher resolution of 0–20% prevalence. The six surveys where PvPR exceeded the proportion of Duffy positive hosts were excluded as anomalies.
(TIF)
S2 Fig
Regional summaries and relative differences in PvPRFy+ and PfPR (log transformed).Panel A boxplot summarises differences within regions, while Panel B shows relative risk of infection between species in relation to the proportion of Duffy positive hosts, and Panel C shows the relative infection risk in relation to the density of Duffy positive hosts (n = 249).
(TIF)
S3 Fig
Logistic-regression model predictions using PR values of one species to predict those of the other (n = 249).The dependent variable is plotted on the y-axis. The PvPR values are adjusted to infection rates in the subset of Duffy positive hosts (PvPRFy+). The solid line represents a significant model fit, while a dashed line indicates that there is no relationship significantly different from zero.
(TIF)
S1 Table
Reports of wild-caught Pv-infected mosquito vectors.(NR: not reported.)
(XLSX)
S2 Table
Reports of serological studies with positive reports of Pv.(NR: not reported.)
(XLSX)
S3 Table
Local case reports of Pv infection among patients in Africa.(NR: not reported.)
(XLSX)
S4 Table
Reports of Duffy negative hosts infected by Pv.(NR: not reported.)
(XLSX)
S5 Table
Summary Admin1 classifications by country.(NR: not reported.)
(XLSX)
S6 Table
National PvPAR estimates.(NR: not reported.)
(XLSX)
Acknowledgments
We acknowledge the following individuals for their assistance with accessing imported traveller data: Marian Van Esbroeck, Institute of Tropical Medicine, Antwerp and Gaëtan Muyldermans, Scientific Institute of Public Health, Brussels, Belgium; Henrik Vedel Nielsen, Statens Serum Institut, Hungary; Tim Wood, Institute of Environmental Science and Research Limited (ESR), New Zealand; Stępień Małgorzata, National Institute of Public Health—National Institute of Hygiene, Poland; Elsie Castro, Public Health Agency of Sweden; Karen Cullen, CDC/CGH/DPDM, USA. We acknowledge the dataset shared by the European Centre for Disease Prevention and Control (ECDC) extracted from The European Surveillance System–TESSy, and Encarna Gimenez and Eleanor Forbes for administrative and legal support with the request. We acknowledge the Australian Government Department of Health for supplying National Notifiable Diseases Surveillance System (NNDSS) data on behalf of the Communicable Diseases Network Australia, and Cathy Boyle for administrative support. We are also grateful to Giacomo Maria Paganotti and Isaac Kweku Quaye for help with prevalence data from Botswana. Finally, we thank Maria Devine for help identifying the sources and proof-reading, Andy Henry for help with the MAP databases, and Alexander Rhodes for proof-reading.
The views and opinions of the authors expressed herein do not necessarily state or reflect those of the ECDC. The accuracy of the authors’ statistical analysis and the findings they report are not the responsibility of ECDC. ECDC is not responsible for conclusions or opinions drawn from the data provided. ECDC is not responsible for the correctness of the data and for data management, data merging and data collation after provision of the data. ECDC shall not be held liable for improper or incorrect use of the data.
Funding Statement
REH is funded by a Wellcome Trust (www.wellcome.ac.uk) Senior Research Fellowship to SIH (095066) and an NIH (www.nih.gov) grant to PAZ (R01 AI097366). The Wellcome Trust Senior Research Fellowship to SIH (095066) also supports KEB and JL. AJT is funded by grants from the Bill & Melinda Gates Foundation (#OPP1106427, #1032350) (www.gatesfoundation.org). CD is funded by the Wellcome Trust (091924). PWG is a Career Development Fellow (K00669X) jointly funded by the UK Medical Research Council (MRC; www.mrc.ac.uk) and the UK Department for International Development (DFID; www.gov.uk/government/organisations/department-for-international-development) under the MRC/DFID Concordat agreement and receives support from the Bill and Melinda Gates Foundation (OPP1068048, OPP1106023), which also supports BM. DLS is funded by a grant from the Bill & Melinda Gates Foundation (OPP1110495), which also supports RCR. RCR, AJT, DLS and SIH also acknowledge funding support from the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health, USA (www.fic.nih.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
Articles from PLOS Neglected Tropical Diseases are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pntd.0004222
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosntds/article/file?id=10.1371/journal.pntd.0004222&type=printable
Citations & impact
Impact metrics
Article citations
Contrasting genomic epidemiology between sympatric Plasmodium falciparum and Plasmodium vivax populations.
Nat Commun, 15(1):8450, 30 Sep 2024
Cited by: 0 articles | PMID: 39349478 | PMCID: PMC11442626
Prevalence and Characteristics of Plasmodium vivax Gametocytes in Duffy-Positive and Duffy-Negative Populations across Ethiopia.
Am J Trop Med Hyg, 110(6):1091-1099, 16 Apr 2024
Cited by: 0 articles | PMID: 38626749 | PMCID: PMC11154031
Presence of Plasmodium vivax in Anopheles gambiae and absence in other malaria vectors in Cove-Zagnanando-Ouinhi health zone in southern Benin, West Africa.
Malar J, 23(1):20, 15 Jan 2024
Cited by: 0 articles | PMID: 38225627 | PMCID: PMC10790420
Malaria infection among adults residing in a highly endemic region from the Democratic Republic of the Congo.
Malar J, 23(1):82, 18 Mar 2024
Cited by: 2 articles | PMID: 38500094 | PMCID: PMC10946143
Determination of Plasmodium vivax and Plasmodium falciparum Malaria Exposure in Two Ethiopian Communities and Its Relationship to Duffy Expression.
Am J Trop Med Hyg, 109(5):1028-1035, 16 Oct 2023
Cited by: 3 articles | PMID: 37918005 | PMCID: PMC10622468
Go to all (71) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Growing evidence of Plasmodium vivax across malaria-endemic Africa.
PLoS Negl Trop Dis, 13(1):e0007140, 31 Jan 2019
Cited by: 92 articles | PMID: 30703083 | PMCID: PMC6372205
The international limits and population at risk of Plasmodium vivax transmission in 2009.
PLoS Negl Trop Dis, 4(8):e774, 03 Aug 2010
Cited by: 299 articles | PMID: 20689816 | PMCID: PMC2914753
The Pan African Vivax and Ovale Network (PAVON): Refocusing on Plasmodium vivax, ovale and asymptomatic malaria in sub-Saharan Africa.
Parasitol Int, 84:102415, 01 Jul 2021
Cited by: 7 articles | PMID: 34216801
[Role of primaquine in malaria control and elimination in French-speaking Africa].
Bull Soc Pathol Exot, 110(3):198-206, 17 Apr 2017
Cited by: 3 articles | PMID: 28417346
Review
Funding
Funders who supported this work.
Medical Research Council
NCATS NIH HHS (1)
Grant ID: UL1 TR001108
NIAID NIH HHS (1)
Grant ID: R01 AI097366
Wellcome Trust (2)
An evidenced based platform for the wider utility of serological analysis in malaria control and elimination.
Professor Chris Drakeley, London School of Hygiene & Tropical Medicine
Grant ID: 091924
Defining the population at risk and burden of disease of Plasmodium vivax malaria.
Prof Simon Hay, University of Oxford
Grant ID: 095066