Abstract
Free full text

Real-time, whole-brain, temporally resolved pressure responses in translational head impact
Abstract
Theoretical debate still exists on the role of linear acceleration (alin) on the risk of brain injury. Recent injury metrics only consider head rotational acceleration (arot) but not alin, despite that real-world on-field head impacts suggesting alin significantly improves a concussion risk function. These controversial findings suggest a practical challenge in integrating theory and real-world experiment. Focusing on tissue-level mechanical responses estimated from finite-element (FE) models of the human head, rather than impact kinematics alone, may help address this debate. However, the substantial computational cost incurred (runtime and hardware) poses a significant barrier for their practical use. In this study, we established a real-time technique to estimate whole-brain alin-induced pressures. Three hydrostatic atlas pressures corresponding to translational impacts (referred to as ‘brain print’) along the three major axes were pre-computed. For an arbitrary alin profile at any instance in time, the atlas pressures were linearly scaled and then superimposed to estimate whole-brain responses. Using 12 publically available, independently measured or reconstructed real-world alin profiles representative of a range of impact/injury scenarios, the technique was successfully validated (except for one case with an extremely short impulse of approx. 1 ms). The computational cost to estimate whole-brain pressure responses for an entire alin profile was less than 0.1 s on a laptop versus typically hours on a high-end multicore computer. These findings suggest the potential of the simple, yet effective technique to enable future studies to focus on tissue-level brain responses, rather than solely relying on global head impact kinematics that have plagued early and contemporary brain injury research to date.
1. Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in the USA that renders major socio-economic and public health burdens [1]. Indisputably, TBI is initiated through mechanical insults to the brain. Therefore, understanding the biomechanical mechanisms of TBI is critical to establish injury diagnostic criteria as well as to develop protective gear and rules to prevent and reduce the incidence, and severity of the injury.
Both linear (alin) and rotational (arot) accelerations contribute to head impact kinematics in real world. Therefore, both have been postulated as major TBI mechanisms. Elevated pressures resulting from alin-dominated translational/direct contact loading are typically related to focal brain injuries, which are also linked to skull fracture, epidural haematoma and contusion in moderate to severe brain injuries [2,3]. Early experimental studies reported that a higher peak pressure magnitude combined with a shorter duration or vice versa could both produce a concussive effect [4,5]. Subsequent studies on the significance of alin on cadavers [6] led to the use of the Wayne State Tolerance Curve (WSTC), Severity Index (SI [7]) and Head Injury Criterion (HIC [8]) to assess the risk and severity of head injury, including skull fracture and severe brain injury. Notably, HIC based on the resultant alin temporal profile is deemed as the official head injury standard, despite its well-recognized limitations for neither including arot nor considering variation in brain anatomy or impact direction [9]. Because these injury criteria are derived from a single resultant alin, which describes a point-wise rigid-body motion, they are unable to incorporate effects from non-rigid skull deformation that could also be important for brain pressures [10,11].
On the other hand, arot is considered to produce both focal and diffusive brain injuries [12]. The significance of arot was first hypothesized by Holbourn [13] and was later confirmed by experiments conducted on primates suggesting arot-induced strains responsible for concussive injury, diffuse axonal injury and subdural haematoma [14]. Because HIC does not include arot, its use in the mild/moderate spectrum of TBI has been criticized. Consequently, more recent injury metrics explicitly include arot or its derivatives for injury risk assessment. These include the generalized acceleration model for brain injury threshold (GAMBIT [15]), head impact power (HIP [16]) and the HIT severity profile (HITsp [17]). Analogous to HIC, injury metrics solely comprising arot have also been devised (e.g. the rotational injury criterion (RIC), power rotational head injury criterion (PRHIC) [18] and the brain injury criterion (BrIC [19])).
Unfortunately, these global kinematic variables and their variants do not directly relate actual brain injuries to tissue-level mechanical responses believed to initiate the injury [12,20,21]. In large part, therefore, no consensus has been reached on an appropriate kinematic injury metric to date. Further, there is still theoretical debate on the role of alin and its induced pressures on the risk of brain injury. Notably, injury metrics such as RIC, PRHIC [18] and BrIC [19] only consider arot but not alin. However, a recent study based on real-world on-field head impacts using arot and alin peak magnitudes suggests alin significantly improves a concussion risk function [22]. These controversial findings suggest a practical challenge in integrating theory and real-world experiment. Largely, this may be a result of using global head impact kinematic variables and their variants, as opposed to tissue-level brain mechanical responses [12,20,21], to assess the risk of brain injury.
Finite-element (FE) models of the human head are effective tools to estimate tissue-level responses using impact kinematics as input. However, current state-of-the-art computational schemes require a substantial computational cost (runtime and hardware) to simulate even a single impact. Therefore, they are not practical for real-world applications. Developing a computationally efficient, yet sophisticated numerical model with high predictive power remains a major challenge [23]. This challenge may be addressed using a pre-computation strategy. Using a pre-computed brain response atlas (pcBRA), (near) real-time estimation of brain strains was possible without significant loss of accuracy in the context of contact sports because of their similar temporal characteristics [24,25].
The goal of this study is to establish an analogous pcBRA to estimate alin-induced whole-brain pressures. Because alin-induced brain pressures are linearly related to alin magnitude and brain size [26], only the translational axis, Ω, (which is uniquely related to brain shape or anatomy) needs to be parametrized for a given head FE model, e.g. by the azimuth (θ) and elevation (α) angles. However, this requires 145 (132–12 × 2) unique atlas solutions to sample one-half of the symmetric parametric space (assuming a 15° sampling density for θ and α, and further accounting for duplicated responses when α degenerates to ±90°, analogous to that in [24]). Here, we present an optimized and much more elegant approach to pre-compute only three atlas responses to achieve real-time pressure estimation, even on a laptop, for the entire impact duration (versus approx. 6 s for element-wise interpolation at one time point on a high-end multicore computer [24]) via linear scaling and superposition.
The pre-computed pressure and strain [24,25] response atlases, when combined, may help address the current debate on the roles of alin and arot on the risk of brain injury. This is an important step towards integrating theory and experiment in the field of brain injury prediction. Because of their real-time efficiency, they may enable future studies to focus on region-specific, tissue-level mechanical responses, rather than relying solely on global kinematic variables or their variants that have plagued early and contemporary TBI research to-date.
2. Material and methods
2.1. The Dartmouth head injury model
All brain responses were obtained using the Dartmouth head injury model (DHIM; figure 1). The DHIM is a subject-specific model created with high mesh quality and geometrical accuracy based on high-resolution magnetic resonance images of an athlete. Details of the model description, material properties and validation performances were reported previously [26–28]. Briefly, DHIM is composed of solid hexahedral and surface quadrilateral elements with a total of 101.4 k nodes and 115.2 k elements (56.6 k nodes and 55.1 k elements) and a combined mass of 4.562 kg (1.558 kg) for the whole head (brain). The average element size for the whole head and the brain is 3.2 ± 0.94 mm and 3.3 ± 0.79 mm, respectively. The DHIM uses a homogeneous Ogden hyperelastic material model with rate effects incorporated through linear viscoelasticity (‘average model’ in [29]) to characterize brain mechanical responses. The DHIM achieved an overall ‘good’ to ‘excellent’ validation against relative brain–skull displacement [30,31] and intracranial pressure responses [32,33] from cadaveric experiments, as well as full-field strain responses in a live human volunteer [34].
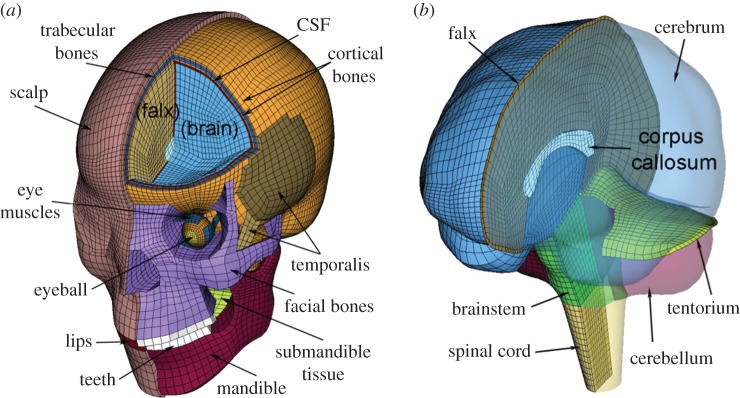
The DHIM showing colour-coded head exterior (a) and intracranial components (b), which also includes part of the spinal cord to improve model biofidelity in the inferior region. The x-, y- and z-axes of the model coordinate system corresponds to the posterior–anterior, right–left and inferior–superior direction, respectively. (Online version in colour.)
2.2. Pressure atlas responses
According to a recent dimensional analysis [26], brain pressure responses are essentially the result of contact forces generated from relative normal displacements of the brain at the brain–skull interface, which are uniquely determined by alin (magnitude and directionality), brain size and shape. Both alin and the resulting whole-brain pressure responses are functions of time. For simplicity, here we first consider a constant acceleration with a magnitude of alin and an arbitrary direction. The resulting whole-brain pressure, p, is hydrostatic. With a constant acceleration, the corresponding relative normal displacements of the brain at the brain–skull interface, d, is also a constant. It can be decomposed into three orthogonal components, dx, dy and dz, respectively, along the three major axes of the head model coordinate system:
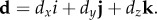
Because pressure, p, is a scalar value, we hypothesize that brain pressures corresponding to the three independent displacement components, px, py and pz, respectively, can be simply superimposed to establish the brain pressure responses corresponding to d or effectively, the given alin:

Furthermore, as pressure is linearly related to the magnitude of alin and d, a set of baseline or atlas pressures corresponding to impacts along the three major axes, 
 and
and 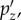 respectively, can be pre-computed. These atlas pressures (referred to as ‘brain print’, analogous to ‘finger print’) can then be individually scaled linearly to obtain pressures corresponding to dx, dy and dz, or effectively, px, py and pz, respectively. Here, we have chosen a baseline peak alin magnitude of 100 g (for convenience and relevance to injury; 1 g = 9.8 m s−2) to establish the three baseline pressures using the DHIM (see below). This leads to the following set of equations
respectively, can be pre-computed. These atlas pressures (referred to as ‘brain print’, analogous to ‘finger print’) can then be individually scaled linearly to obtain pressures corresponding to dx, dy and dz, or effectively, px, py and pz, respectively. Here, we have chosen a baseline peak alin magnitude of 100 g (for convenience and relevance to injury; 1 g = 9.8 m s−2) to establish the three baseline pressures using the DHIM (see below). This leads to the following set of equations
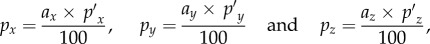
where ax, ay and az are the alin magnitude components along the three major axes (in g) at a given time. Combining equations (2.2) and (2.3), whole-brain pressure is readily obtained
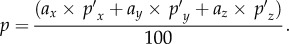
It is important to note that the above ‘dimensional analysis’ considers a constant acceleration, or alin at a given instance in time. When the same analysis is applied to an arbitrary point in time for a real-world time-varying alin temporal profile, the resulting hydrostatic pressure essentially generates a first-order approximation, purposefully neglecting any potential dynamic inertial effect. Fortunately, the hydrostatic response (equation (2.4)) would be sufficiently accurate for most real-world impacts because brain pressure reaches an equilibrium almost instantly due to its high dilatational speed (e.g. with impulse duration greater than 2 ms according to [35]).
To establish the atlas hydrostatic pressures (i.e. 
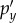 and
and  in equations (2.3) and (2.4)), a ‘ramp-and-hold’ alin profile (figure 2) was applied to the rigid skull along the three major axes. This allowed element-wise brain pressures within the 15–20 ms window to be averaged along the temporal direction to serve as the atlas responses. The choice of this particular temporal profile was to ensure a hydrostatic response and to avoid perturbations from the dynamic inertial effects (arrow in figure 2), as explained. The peak magnitude of alin (i.e. 100 g) was not important here, because the pressure responses would be linearly scaled during the stable ‘hold’ period (15–20 ms). Importantly, the resulting element-wise, hydrostatic pressures or ‘brain print’ served as atlas ‘modes’ for linear scaling and superposition (figure 3; equation (2.4)).
in equations (2.3) and (2.4)), a ‘ramp-and-hold’ alin profile (figure 2) was applied to the rigid skull along the three major axes. This allowed element-wise brain pressures within the 15–20 ms window to be averaged along the temporal direction to serve as the atlas responses. The choice of this particular temporal profile was to ensure a hydrostatic response and to avoid perturbations from the dynamic inertial effects (arrow in figure 2), as explained. The peak magnitude of alin (i.e. 100 g) was not important here, because the pressure responses would be linearly scaled during the stable ‘hold’ period (15–20 ms). Importantly, the resulting element-wise, hydrostatic pressures or ‘brain print’ served as atlas ‘modes’ for linear scaling and superposition (figure 3; equation (2.4)).

A sample alin profile and the resulting Pcoup and Pc_coup (pressure at the coup and contrecoup sites, respectively) for a frontal impact (i.e. along the x-axis). Element-wise pressure values within the 15–20 ms window (shaded area) were averaged along the temporal direction to serve as the atlas response. Pressure perturbations due to the dynamic inertial effects are evident (arrow). (Online version in colour.)
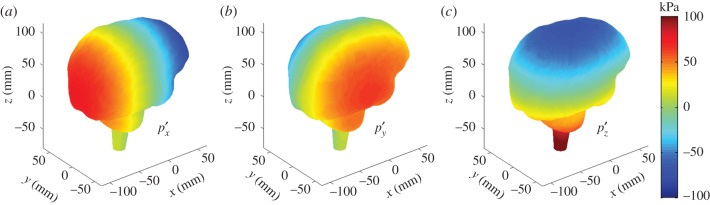
(a–c) The three hydrostatic baseline or atlas pressures (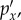
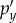 and
and 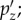 referred to as ‘brain print’) corresponding to translational head impacts along the three major axes. These atlas pressures are linearly scaled and superimposed to directly assemble brain pressure responses at an arbitrary given time. (Online version in colour.)
referred to as ‘brain print’) corresponding to translational head impacts along the three major axes. These atlas pressures are linearly scaled and superimposed to directly assemble brain pressure responses at an arbitrary given time. (Online version in colour.)
2.3. Performance evaluation
Twelve 3 d.f. alin profiles from cadaveric head impacts, reconstructed traffic accidents, anthropomorphic test dummies (ATD) and helmeted contact sports were used for evaluation. Their alin temporal profiles were manually digitized from their respective published sources. The alin magnitudes ranged 73.9–680.2 g and the HIC ranged 163.9–6606.7 (table 1). The lower impact severities relevant to contact sports were included here because alin was found to significantly improve a concussion risk function [22] and because HIC continues to be assessed in contact sports [17,18,29,38,40].
Table 1.
Summary of impact scenarios, injury details, HIC and the alin peak magnitudes for the cases evaluated. HIC, Head Injury Criterion; AIS, Abbreviated Injury Scale; GCS, Glasgow Coma Scale.
| case no. | impact scenario | injury detail | HIC | alin peak (g) | reference |
|---|---|---|---|---|---|
| 1 | cadaveric experiments | no injury | 786.2 | 199.2 | [32] |
| 2 | N/A (not reported) | 226.2 | 91.0 | [33] | |
| 3 | reconstructed traffic accidents | AIS 4: GCS = 11 (moderate)a | 6606.7 | 680.2 | [36] |
| 4 | AIS 5: extradural haematoma; contusion, GCS = 3 (severe)a | 1782.2 | 217.6 | ||
| 5 | AIS multiple: extradural and subdural haematoma; cerebral swelling and contusion | 2221.1 | 293.5 | ||
| 6 | ATD test | N/A | 465.5 | 84.7 | [37] |
| 7 | reconstructed on-field impacts | N/A (not reported) | 210.5 | 76.4 | [38] |
| 8 | concussion | 281.3 | 80.8 | [39] | |
| 9 | struck, concussion | 450.1 | 98.7 | [29] | |
| 10 | striking, no injury | 163.9 | 75.7 | ||
| 11 | measured on-field impacts | diagnosed concussion | 165.0 | 73.9 | [28] |
| 12 | loss of consciousness | 770.2 | 105.0 | [40] |
aAccording to [41].
The estimated whole-brain, temporally resolved pressures were compared with the directly simulated counterparts. Element-wise pressure values at each time point were directly extracted from the simulation. Brain elements with maximum coup and (absolute) contrecoup pressures (Pcoup and Pc_coup, respectively) over time were localized based on the directly simulated responses (excluding the spinal cord). Then, the Pcoup and Pc_coup values were averaged with those from their respective eight neighbouring surface elements to compute the peak and time-varying Pcoup and Pc_coup for both the estimated and directly simulated pressures. The local averaging at the coup/contrecoup sites was meant to mitigate any influence from spuriously high Pcoup or absolute Pc_coup values due to potentially excessive mesh distortions (which could occur with a poor mesh quality, e.g. around sharp corners), as similarly applied before (e.g. average of three selected elements in the region where the maximum pressure occurred [38]). For all the 12 cases evaluated here, the directly extracted values of maximum Pcoup or absolute Pc_coup differed from their locally averaged values by 0.65 ± 0.50%, suggesting no excessive mesh distortion occurred.
The estimation performance was evaluated against the directly simulated counterpart according to the relative percentage errors of the peak Pcoup and Pc_coup. For the time-varying Pcoup and Pc_coup, the performance was evaluated in terms of root mean squared errors (normalized by the directly simulated peak to compare across cases; nRMSE in percentages) and correlation scores (in terms of phase, amplitude and shape, or CSN−phase, CSN-amp and CSN-shape, respectively [42]) for the entire impact as well as for the temporal window corresponding to HIC. While these criteria assessed the estimation accuracy locally at the coup/contrecoup sites, a ‘double-10%’ global criterion was further used to assess the accuracy of whole-brain pressure distributions when Pcoup reached its peak. The ‘double-10%’ criterion was designed to compare two scalar fields on an element-wise basis, i.e. to test whether the volume fraction of large (10%, relative to the directly simulated response value at each element) pressure differences was greater than 10% of the whole brain. This was used previously to compare two whole-brain strain fields generated from estimation and direct simulation [24]. For each case, the estimation was considered sufficiently accurate when all the percentage metrics were less than 10% and that the correlation scores were rated ‘excellent’ [43].
2.4. Data analysis
All brain pressure response simulations were obtained from the DHIM via Abaqus/Explicit (v. 6.12; Dassault Systèmes, France) with a temporal resolution of 0.1 ms. The typical runtime for a 40 ms impact was approximately 50 min plus approximately 5 min for post-processing on a high-end multicore Linux cluster using eight CPUs (Intel Xeon X5560, 2.80 GHz, 126 GB memory). With the pcBRA, whole-brain pressure estimation for an entire alin profile took less than 0.1 s on a laptop (essentially via a simple matrix multiplication). A range of global and local accuracy criteria was used to assess the pcBRA performance, as described previously. All data analyses were performed in MATLAB (R2014a; Mathworks, Natick, MA, USA).
3. Results
Figure 4 compares the estimated peak Pcoup and Pc_coup with their directly simulated counterparts for all the cases combined. Except for case 3 (with an underestimation of 27.8% and 23.4% in peak Pcoup and Pc_coup, respectively), the estimated and the directly simulated peak values nearly aligned. More quantitatively, table 2 summarizes the estimation performances in terms of the various metrics for Pcoup (results for Pc_coup were similar and are not repeated). One representative case was chosen to illustrate the pressure estimation process (figure 5). Figure 6 graphically compares pressure distributions between the estimated and the directly simulated responses for three additional cases. For the remaining cases, the estimated Pcoup temporal profiles are compared with their directly simulated counterparts, along with the resultant alin profiles (figure 7).

Comparison between the estimated and the directly simulated peak Pcoup and Pc_coup for all cases evaluated, demonstrating an excellent performance overall as most closely aligned with the diagonal line (except for one case with an underestimation of 27.8% and 23.4% in peak Pcoup and Pc_coup, respectively; arrow). (Online version in colour.)
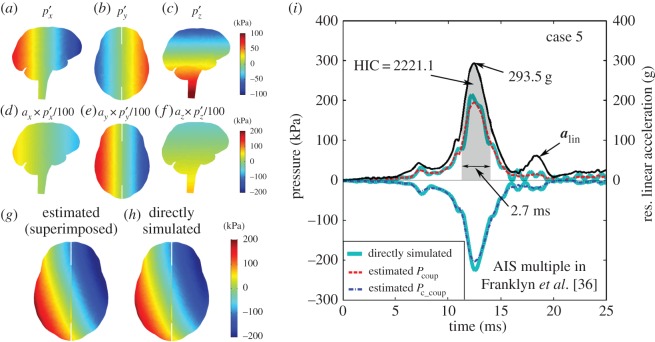
Illustration of using three baseline or atlas responses (
 and
and 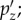 a–c) to construct a pressure distribution map for an arbitrary given time in a selected alin profile (case 5). The atlas responses are first linearly scaled according to equation (2.3) using the three alin magnitude components along the three major axes (d–f) and then superimposed (g), resulting in a virtually identical response relative to the directly simulated counterpart (h). The estimated Pcoup and Pc_coup temporal profiles are compared with their directly simulated counterparts (i; time interval corresponding to HIC shaded). To improve visualization, only the resultant alin profile is shown (versus 3 d.f. used in pressure simulation and estimation). (Online version in colour.)
a–c) to construct a pressure distribution map for an arbitrary given time in a selected alin profile (case 5). The atlas responses are first linearly scaled according to equation (2.3) using the three alin magnitude components along the three major axes (d–f) and then superimposed (g), resulting in a virtually identical response relative to the directly simulated counterpart (h). The estimated Pcoup and Pc_coup temporal profiles are compared with their directly simulated counterparts (i; time interval corresponding to HIC shaded). To improve visualization, only the resultant alin profile is shown (versus 3 d.f. used in pressure simulation and estimation). (Online version in colour.)
Comparison between the estimated (a,d,g) and the directly simulated (b,e,h) whole-brain pressure distributions when Pcoup reached its peak for three selected cases. The estimated pressure response temporal profile is nearly identical to the directly simulated counterpart, and is compared with that of the resultant alin (c,f,i). (Online version in colour.)
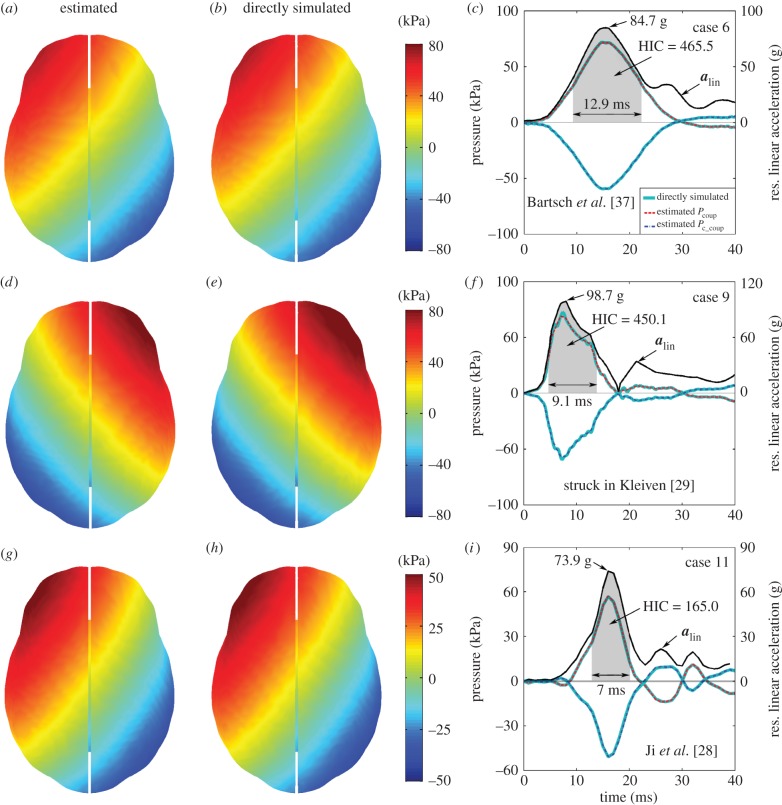
Comparison between estimated pressure temporal profiles and their directly simulated counterparts for the remaining cases, along with the resultant alin profiles. (Online version in colour.)
Table 2.
Summary of estimation accuracy performances for Pcoup for all cases evaluated. Performances for Pc_coup are largely similar and are not repeated here.
| case no. | % error | nRMSE % | CSN-phase | CSN-amp | CSN-shape | V0.1c (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wa | Ib | W | I | W | I | W | I | |||
| 1 | 5.1 | 6.4 | 6.8 | 98.2 | 95.4 | 99.9 | 99.8 | 99.7 | 95.5 | 0.1 |
| 2 | 9.2 | 4.3 | 6.6 | 99.4 | 97.0 | 100.0 | 100.0 | 99.8 | 97.7 | 5.4 |
| 3 | 27.8 | 7.4 | 21.8 | 99.2 | 83.3 | 97.9 | 97.3 | 90.7 | 86.9 | 66.0 |
| 4 | 8.6 | 2.0 | 4.5 | 100 | 100 | 100.0 | 100.0 | 99.8 | 99.8 | 0.7 |
| 5 | 8.8 | 2.7 | 6.9 | 99.6 | 95.9 | 100.0 | 100.0 | 98.8 | 96.3 | 6.2 |
| 6 | 0.5 | 0.3 | 0.3 | 100 | 100 | 98.7 | 96.9 | 98.7 | 96.9 | 0.1 |
| 7 | 1.1 | 1.9 | 0.9 | 100 | 100 | 100.0 | 100.0 | 99.9 | 100.0 | 0 |
| 8 | 1.2 | 1.5 | 1.3 | 99.6 | 99.2 | 100.0 | 100.0 | 99.7 | 99.8 | 0.0 |
| 9 | 4.9 | 1.7 | 2.7 | 99.5 | 97.6 | 100 | 100 | 99.6 | 97.6 | 0.4 |
| 10 | 2.7 | 2.1 | 3.9 | 99.5 | 97.3 | 100.0 | 100.0 | 99.7 | 97.4 | 7.5 |
| 11 | 0.7 | 0.5 | 0.7 | 100 | 100 | 100.0 | 100 | 100.0 | 100.0 | 0.0 |
| 12 | 0.6 | 4.4 | 5.0 | 99.8 | 99.8 | 99.9 | 99.9 | 99.8 | 99.8 | 0.4 |
| Avg.d | 3.9 | 2.5 | 3.6 | 99.6 | 98.4 | 99.9 | 99.7 | 99.6 | 98.3 | 1.9 |
aW, whole temporal profile.
bI, interval corresponding to HIC.
cV0.1 whole-brain volume fraction of element-wise differences relative to those directly simulated greater than 10%.
dAvg. excluding the failed case no. 3.
4. Discussion
Using tissue-level brain responses may help resolve the current debate on the roles of alin and arot on the risk of brain injury. As tissue mechanical responses are believed to initiate the injury [12,20,21], they may enable a more reliable injury diagnosis than head impact kinematics alone. Obviously, this requires an efficient estimation of tissue responses in the first place for practical deployment, which is beyond the capability of current direct simulations using FE head models. In this study, we established a simple, yet effective technique to achieve real-time estimation—even on a laptop—of whole-brain, temporally resolved (i.e. whole alin profile) pressure responses in translational head impact. Essentially, this technique generates a ‘brain print’ along three orthogonal directions by pre-computing hydrostatic brain pressures corresponding to impacts along the three major axes to permit a direct superposition of whole-brain pressure responses. As only three baseline responses are required, individualized pressure response atlases can be easily generated without the need for mass or geometrical scaling in order to account for anatomical variations. The notion that an FE model is not needed when computing alin-induced brain pressures is not new (e.g. ‘hand calculation’ in [35]), albeit, baseline pressure responses from an FE model are still necessary. Because dynamic inertial effects are purposefully neglected, the pcBRA effectively generates a first-order approximation.
Nevertheless, the estimation scheme was sufficiently accurate for 11 out of 12 real-world 3 d.f. alin profiles according to a range of criteria (table 2). For the spatially local metrics measuring the accuracies of Pcoup and Pc_coup, the average percentage errors for the peak values were within 4%. For their time-varying profiles, similar errors were found for the average nRMSE, either for the entire impact duration or the interval corresponding to HIC (i.e. temporally global or local). Their estimations were also considered ‘excellent’ based on correlation scores [43], regardless of the temporal window. The excellent performance was further confirmed by the spatially global ‘double-10%’ criterion that measured the accuracy of full-field pressure distributions (average V0.1 of 1.9%, i.e. 1.9% of the whole brain experienced greater than or equal to 10% differences on average). Notably, these errors were far below those in real-world alin measurement (e.g. up to 17–31% according to [44,45]). We have also confirmed that the reference alin magnitude to pre-compute the atlas pressures was not important. When using 1 g instead of 100 g to pre-compute, the estimated peak Pcoup (Pc_coup) pressure increased (decreased) by only 1.5% (0.6%) for case 5.
The failed case (case 3; table 2) had a much shorter impulse (approx. 1 ms) than the others (greater than or equal to 3–4 ms; figure 7c), although it also corresponded to the largest alin and HIC magnitudes. Significant underestimation was evident for both peak Pcoup and Pc_coup (by 27.8% and 23.4%, respectively; albeit, still below the maximum alin measurement error; figure 4) or the entire brain (V0.1 of 66%; table 2). Obviously, the underestimation was a result of the much more pronounced dynamic inertial effects activated by the short impulse (analogous to a mathematical ‘singularity’) not captured by the atlas responses. To verify this, we temporally scaled the 3 d.f. alin profile by five times while maintaining the same alin magnitude (leading to an impulse of approx. 5 ms with HIC of 33 093). Once again, the pcBRA achieved an excellent performance as the peak Pcoup and Pc_coup percentage errors became 4.1% and 1.2% with V0.1 of 0.0%. These results confirm previous reports that hydrostatic equilibrium predominates and wave effects are of no consequence in most real-world head impacts [46] (when impulse duration greater than 2 ms according to [35]). Additionally, the resulting peak Pcoup and Pc_coup for the scaled profile (462.5 kPa and −489.4 kPa, respectively) far exceeded the thresholds for contusion (e.g. 173 kPa or 234 kPa corresponding to moderate or severe brain injury, i.e. AIS 3–4 or AIS 5–6, respectively [47]) or contrecoup cavitation (e.g. negative dilatational pressure of –100 kPa according to [48]). These findings suggest that the applicability of pcBRA does not necessarily depend on the magnitudes of alin or HIC, or the impact severity.
An important limitation of our study was the omission of brain dynamic inertial effects, which could be an important contributor to brain pressure responses with a very short alin impulse (e.g. less than 3–4 ms, figure 5i, figure 7a and c). While the majority of real-world impacts has an impulse duration greater than 2 ms [35] and the resulting brain pressures are essentially hydrostatic [46], it appears that alin-induced focal brain injuries such as subdural haematoma, epidural haematoma, subarachnoid haemorrhage and contusion often occur with relatively short duration (less than 5 ms) direct impacts [49]. For extremely short duration impacts (e.g. less than 1 ms, resulting from light projectile impacts), the brain pressures can increase significantly above the quasi-static estimates with large fluctuations at both the coup and contrecoup sites [50]. These observations suggest the importance to further compensate for dynamic inertial effects under these impact conditions in order to improve the estimation accuracy. This appears possible, though, e.g. by systematically probing the differences between the estimated and the directly simulated brain pressures subject to a number of pre-defined input variations (e.g. impulse duration and impact direction). The deterministic causal relationship could be explicitly modelled, making the compensation straightforward.
It should be recognized that the estimated pressures, even after compensating for the dynamic inertial effects, do not consider the potentially important non-rigid skull deformation, which was another limitation of our study. However, it should be noted that none of the existing injury metrics is able to incorporate effects from non-rigid skull deformation. Nevertheless, while non-rigid skull deformation does influence pressure responses [26,51–53], brain pressures are predominantly induced by the skull rigid-body acceleration when head protective gear (e.g. airbags and helmets) is used to extend impact duration, absorb impact energy and to minimize skull deformation [54]. On the other hand, when non-rigid skull deformation becomes more significant, a distributed impact force on the head or an impactor is necessary to explicitly model a deformable skull.
Interestingly, the pressure pcBRA derived from a rigid skull assumption may still serve as an upper bound for brain pressures when significant non-rigid skull deformation occurs. This is because the increased local contact area as a result of the skull deformation would lower Pcoup (i.e. ‘over-estimation’; with an identical alin magnitude; albeit, uniquely determining alin magnitude may become challenging). The pressure over-estimation was parametrically investigated by iteratively adjusting the skull Young's modulus and impact force to maintain an identical alin magnitude in frontal impacts [26]. On the other hand, when significant non-rigid skull deformation is coupled with an extremely short impulse (e.g. case 3 in figure 7c), their respective significances (i.e. overestimate and underestimation, respectively) would compete. The resulting overall effects on pressure responses require further exploration.
Another limitation was that the pressure pcBRA only considers translational impacts but does not include arot-induced pressures, while both alin and arot contribute to head impact kinematics in the real world. This may explain the large differences in peak Pcoup and Pc_coup compared with those found previously in which both alin and arot were applied, especially with large arot magnitudes (e.g. peak Pcoup of 214.6 kPa reported here versus 138 kPa in [36] for case 5 with arot > 20 krad s−2). For smaller arot magnitudes (e.g. less than 5 krad s−2 in helmeted contact sports), perturbations from arot-induced pressure appeared insignificant, and our study reported similar peak Pcoup magnitudes (figures 6f and and7g;7g; 87.7 kPa and 73.9 kPa for cases 9 and 10 versus 81.5 kPa in [29]). These findings suggest that the pressure pcBRA may be directly applicable for translation-dominated impacts but a pressure compensation scheme may be necessary for impacts with large arot magnitudes. A systematic investigation of arot-induced pressures appears not to have been performed before, although a preliminary study suggests pressures from alin and arot can be superimposed [55]. However, the applicability of the pressure pcBRA when a significant arot component is present is probably debatable. This is because many consider arot or its resulting strain responses as the major injury mechanism in these cases [12], despite that combining both alin and arot peak magnitudes (presumably, the resulting pressure and strain responses) was found to improve the performance of an injury risk curve [22]. Therefore, it is conceivable that the pressure and strain pcBRAs be combined for optimal use in practice, especially for contact sports [24,25].
Certainly, these pressure response behaviours may be model dependent. Different CSF modelling strategies [56] and brain–skull boundary conditions [57] are known to significantly influence brain pressure responses. Therefore, it is unclear whether the real-time pressure estimation technique developed here is applicable to other head models (e.g. with an equation of state to simulate CSF [58]). However, brain pressure responses are insensitive to the brain nonlinear shear property as long as a sufficiently high bulk modulus is used to ensure a near incompressibility, which is well known [21] and was recently confirmed [26]. Regardless, the DHIM achieved an overall ‘good’ to ‘excellent’ performance when validating against cadaveric pressure responses, in addition to a similar performance validating against relative brain–skull displacements from cadaveric impacts and strain responses from a live human volunteer [26,28]. In comparison, many other models are typically not rated or validated against strain responses in a live human. Further, the DHIM also successfully confirmed an approximately 0.5 ms time delay of the pressure peak response relative to the alin peak magnitude (figure 7a) as experimentally observed [26,32], which, to the best of our knowledge, has not been reported before. These observations suggest high confidence in our findings.
In modern brain injury studies, theoretical debates still exist regarding the importance of alin and its resulting pressures on the risk of brain injury. Recent brain injury metrics such as RIC, PRHIC [18] and BrIC [19] are only composed of arot but do not include alin. However, on-field data based on real-world injuries using arot and alin peak magnitudes suggests alin does significantly improve the performance of a concussion risk function [22]. These controversies highlight the challenges when integrating theory and experiment in real-world applications. Our ‘brain print’ technique may serve as a useful and important tool to enable future brain injury studies to address this controversy using tissue-level brain responses as opposed to kinematic variables alone, even on a subject-specific basis. To allow further exploration of the use of the pre-computed ‘brain print’, we have made it freely available along with the associated analysis software for research use (see appendix A).
5. Conclusion
We have successfully established and validated a simple, yet effective technique based on three pre-computed atlas pressure responses for real-time, whole-brain, temporally resolved pressure estimation in translational head impact. The technique appeared sufficiently accurate and robust for a range of real-world impact/injury scenarios (with impulse duration greater than or equal to 3–4 ms). Therefore, it may allow future studies to focus on tissue-level brain responses, as opposed to global kinematic variables alone, to better understand the mechanism of brain injury.
Appendix A
The pressure pcBRA and a subset of strain pcBRA [24,25] were spatially resampled, and are freely available along with the associated analysis software for research use and evaluation, at http://www.thayer.dartmouth.edu/~songbai_ji/DHIM/.
Funding
This work was sponsored by the NIH via grant nos. R01 NS092853, R21 NS088781 and R21 NS078607, and the Dartmouth Hitchcock Foundation.
References
Articles from Interface Focus are provided here courtesy of The Royal Society
Full text links
Read article at publisher's site: https://doi.org/10.1098/rsfs.2015.0091
Read article for free, from open access legal sources, via Unpaywall:
https://royalsocietypublishing.org/doi/pdf/10.1098/rsfs.2015.0091
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1098/rsfs.2015.0091
Article citations
An anatomically detailed and personalizable head injury model: Significance of brain and white matter tract morphological variability on strain.
Biomech Model Mechanobiol, 20(2):403-431, 10 Oct 2020
Cited by: 40 articles | PMID: 33037509 | PMCID: PMC7979680
White Matter Anisotropy for Impact Simulation and Response Sampling in Traumatic Brain Injury.
J Neurotrauma, 36(2):250-263, 10 Aug 2018
Cited by: 22 articles | PMID: 29681212 | PMCID: PMC6338585
Material properties of the brain in injury-relevant conditions - Experiments and computational modeling.
J Mech Behav Biomed Mater, 80:222-234, 06 Feb 2018
Cited by: 24 articles | PMID: 29453025 | PMCID: PMC5841256
Brain strain uncertainty due to shape variation in and simplification of head angular velocity profiles.
Biomech Model Mechanobiol, 16(2):449-461, 19 Sep 2016
Cited by: 18 articles | PMID: 27644441 | PMCID: PMC5352487
Integrated multiscale biomaterials experiment and modelling: a perspective.
Interface Focus, 6(1):20150098, 01 Feb 2016
Cited by: 0 articles | PMID: 28981126 | PMCID: PMC4686254
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Brain pressure responses in translational head impact: a dimensional analysis and a further computational study.
Biomech Model Mechanobiol, 14(4):753-766, 21 Nov 2014
Cited by: 17 articles | PMID: 25412925 | PMCID: PMC4440857
A Pre-computed Brain Response Atlas for Instantaneous Strain Estimation in Contact Sports.
Ann Biomed Eng, 43(8):1877-1895, 02 Dec 2014
Cited by: 23 articles | PMID: 25449149 | PMCID: PMC4452476
Assessment of Kinematic Brain Injury Metrics for Predicting Strain Responses in Diverse Automotive Impact Conditions.
Ann Biomed Eng, 44(12):3705-3718, 19 Jul 2016
Cited by: 39 articles | PMID: 27436295
Concussion in professional football: biomechanics of the struck player--part 14.
Neurosurgery, 61(2):313-27; discussion 327-8, 01 Aug 2007
Cited by: 126 articles | PMID: 17762744
Funding
Funders who supported this work.
NINDS NIH HHS (3)
Grant ID: R21 NS078607
Grant ID: R01 NS092853
Grant ID: R21 NS088781
National Institutes of Health (3)
Grant ID: R01 NS092853
Grant ID: R21 NS078607
Grant ID: R21 NS088781




