Abstract
Free full text

Irritable bowel syndrome: Is it “irritable brain” or “irritable bowel”?
Abstract
Irritable bowel syndrome (IBS) has been recognized as one of the most common and best studied disorders among the group of functional gastrointestinal disorders. It is a functional bowel disorder in which abdominal pain or discomfort is associated with defecation or a change in bowel habit. In the Western world, IBS appears to affect up to 20% of the population at any given time but in Asian countries, the median value of IBS prevalence defined by various criteria ranges between 6.5% and 10.1%, and community prevalence of 4% is found in North India. Those attending gastroenterology clinics represent only the tip of the iceberg. The disorder substantially impairs the quality of life, and the overall health-care costs are high. IBS has therefore gained increased attention from clinicians, researchers, and pharmaceutical industries. It is often frustrating to both patients and physicians as the disease is usually chronic in nature and difficult to treat. However, the understanding of IBS has been changing from time to time and still most of its concepts are unknown. In this review we have discussed, debated, and synthesized the evidence base, focusing on underlying mechanisms in the brain and bowel. We conclude that it is both brain and bowel mechanisms that are responsible. The clinical implication of such mechanisms is discussed.
Introduction
Irritable bowel syndrome (IBS) has been recognized as one of the most common and best studied disorder among the group of functional gastrointestinal disorders.[1] It is a functional bowel disorder in which abdominal pain or discomfort is associated with defecation or a change in bowel habit.[2] In the Western world, IBS appears to affect up to 20% of the population at any given time, although the prevalence figures vary substantially depending on the definition of IBS.[3] On the contrary in Asian countries, the median value of IBS prevalence defined by various criteria ranges 6.5–10.1%.[4]
In India, IBS has been reported to be common.[5] A recent study has estimated IBS prevalence in Northern India community to be 4%.[6] Though its exact incidence is hard to assess, but it appears that those attending gastroenterology clinics represent only the tip of the iceberg.[7]
The disorder substantially impairs the quality of life, and the overall health-care costs are high. IBS has therefore gained increased attention from clinicians, researchers, and pharmaceutical industries. It is often frustrating to both patients and physicians as the disease is usually chronic in nature and difficult to treat. However, the understanding of IBS has been changing from time to time and still most of its concepts are unknown. In this review we tend to find out the various etiological mechanisms underlying IBS.
Etiological Dilemmas: Is it Irritable “Bowel” or “Brain/Mind”?
Studies on the pathophysiologic mechanisms behind IBS have always led to controversies that whether the predominant pathology lies in the bowel or in the brain. We provide here a brief overview of all such mechanisms studied so far in favor of both bowel and brain.
Evidences in favor of Irritable “bowel”
If we look into the evolution of mechanistic hypothesis in IBS, then we can formulate that initially it was thought that abnormal colonic motility was the basic pathophysiologic mechanism behind IBS. But over the years, the concept has changed. Some of the mechanisms are listed below:
Abnormal motility
Visceral hypersensitivity
Role of infection
Role of inflammation
Role of bacterial overgrowth
Role of serotonin
Role of brain-gut axis interaction.
Abnormal motility
Evidences from manometric, electromyographic, and colonic transit studies (1980s) have demonstrated that patients with IBS have: (1) Discrete clustered contractions in the small intestine which are associated with episodes of abdominal pain, (2) abnormalities in the migratory motor complex, which may lead to either delayed (constipation) or accelerated (diarrhea) intestinal transit, and (3) very high amplitude propagating contractions within the colon, especially in the postprandial period which are associated with episodes of abdominal pain.[7,8,9]
Some of the studies which have tried to find out different types of altered motility in different parts of the gastrointestinal (GI) system are enlisted in Table 1.
Table 1
Studies summarizing motility studies
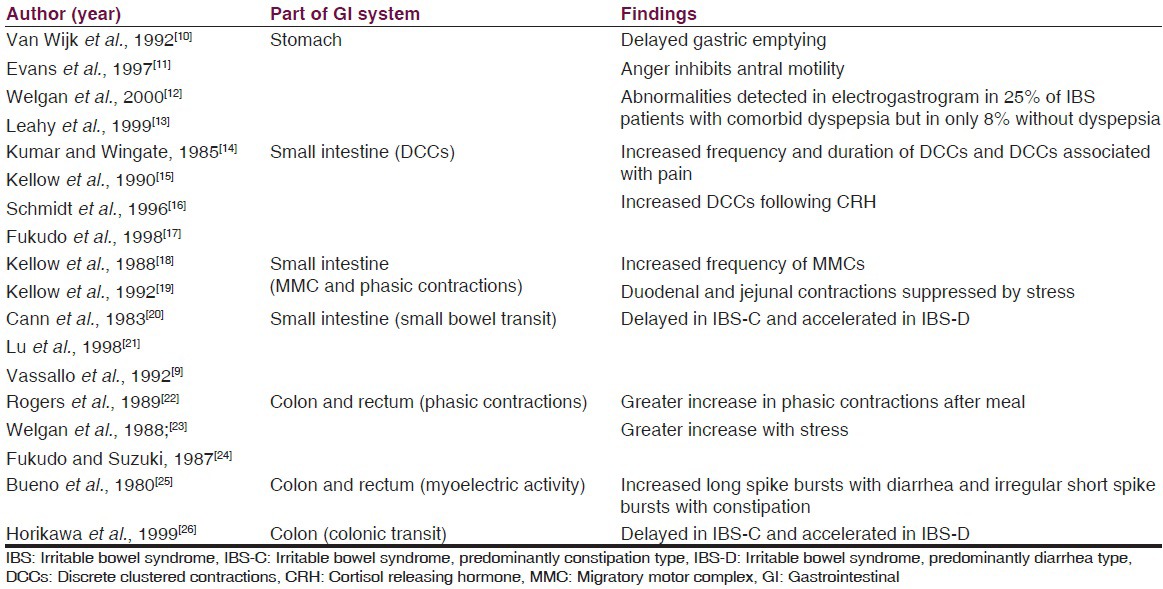
However, no single pattern of disordered motility is pathognomonic of IBS. In general, the alterations in GI motility found in patients with IBS likely reflect an exaggeration of normal patterns of GI motility.
Visceral hypersensitivity
It has been proved by the use of technique of intraluminal balloon distension that patients with IBS have altered thresholds to pain within the GI tract, compared with non-IBS patients. IBS patients perceive balloon distension at much lower levels of inflation and describe the distension as more painful, compared with normal patients.[27,28,29] They may also misinterpret normal GI function as painful.
Visceral hypersensitivity is a common and predominant pathophysiologic mechanism, responsible for triggering the motility disturbances and abdominal pain. Many researchers have proposed that it may be regarded as a “biological marker” of IBS. Nevertheless, only 60% of patients are hypersensitive to distension that is, mainly the diarrhea subtype (IBS-D) are more hypersensitive to rectal distension than patients who have constipation subtype (IBS-C).[30,31]
Although hypersensitivity is a frequent finding in patients with IBS, it does not appear to be a consistent indicator of IBS. Several external and internal factors can modulate visceral sensitivity. So, it cannot be used as a diagnostic marker for IBS. It seems to result from the sensitization of nerve afferent pathways originating from the GI tract.
Role of infection
Chaudhry and Truelove (1962) reported that a percentage of patients with irritable colon developed typical IBS symptoms after an acute infective enteritis. Epidemiologic studies have shown that GI infection is the strongest environmental risk factor for the development of IBS.[32] A recent systematic review and meta-analysis identified that the pooled incidence for IBS development after infectious gastroenteritis was 10%.[33] Salmonella, Shigella, and Campylobacter are among the most frequently isolated infectious agents, but viral infection has also been documented as a trigger of IBS.[34,35] Several risk factors increase the risk of postinfectious IBS (PI-IBS) development, including prolonged duration of the initial illness, toxicity of infecting bacterial strain, smoking, degree of mucosal inflammation, female gender, presence of psychological disorders, such as depression and anxiety, and treatment with antibiotics during the acute gastroenteritis episode.[36,37,38] Studies showed a continuing presence of CD3 and CD8 T lymphocytes and increased expression of interleukin-1 (IL) beta in patients progressing to PI-IBS after acute infection with Shigella or Campylobacter jejuni.[39]
Though there is growing evidence in favour of infection as an etiologic model for IBS, but the point against it is that neither all patients have history of infection prior to developing IBS symptoms nor treatment by antibiotics helps in improvement of symptoms in all IBS patients.
Role of mucosal inflammation
Normal intestine is always in a state of inflammation, with a balance between commensal enteric organisms and the immune system. Mast cells play a critical role in normal immune function and respond to antigen stimuli through degranulation resulting in release of the inflammatory mediators histamine and tryptase. Degree of cellularity of mucosal mast cells and proximity to sensory nerves have been found to be correlated with abdominal pain in IBS.[40] Studies have reported that increased number of mast cells throughout the GI tract in patients with IBS with no history of gastroenteritis.[41] It has also been seen that biopsy specimens contained increased numbers of neutrophils and mast cells in the colonic mucosa, and chronic inflammatory infiltrate with neuronal degeneration in the myenteric plexus of the jejunum.[42,43]
These findings have resulted in interest not only in the role of mast cells in IBS, but also the potential therapeutic role of mast cell stabilizers in IBS. However, the results are arbitrary with no consistent findings.
Role of bacterial overgrowth
Numerous theories have been implicated regarding the role of small intestinal bacterial overgrowth (SIBO) in the pathogenesis of IBS, however definite evidence is still lacking. SIBO has been defined based on jejunal aspirate and culture demonstrating more than 105 CFU/mL of coliform bacteria in the small intestine.[44] Methodological issues in diagnosing SIBO by lactulose breath test or by glucose breath test has resulted in controversies.
However, factors which support role of bacteria in IBS are:[45]
Excessive fermentation
Increased small bowel gas formation
Abnormal breath tests compared with healthy controls
Excessive coliform bacteria in proximal small bowel of IBS subjects
Improvement of IBS symptoms with antibiotic therapy.
The causal link of SIBO with IBS is somewhat argued due to the evidence of treatment of SIBO with nonabsorbable antibiotics like rifaximin which has been shown to result in improvement of symptoms in patients with IBS. But still, it remains yet to be clarified whether SIBO is an essential factor in the pathophysiology of IBS, or is just linked by serendipity.
Role of serotonin
Serotonin (5-hydroxytryptamine [5-HT]) is a neurotransmitter vital to normal GI function. Studies have shown that 5-HT plays a critical role in GI motility, visceral sensitivity, GI immune function, and blood flow.[46] At the molecular level, abnormalities in the serotonin reuptake transport system have been found in IBS patients. Polymorphism of the 5-HT2A receptor gene may be associated with the development of IBS[47] has also been suggested. In a recent study done in India, a significant association was found between the SS genotype of serotonin re-uptake transporter polymorphism (SERT-P) and constipation predominant type of IBS.[48]
Serotonin released as neurotransmitter from both the afferent and efferent neurons of the myenteric plexus present in layers of intestinal wall increases intestinal motility and secretion by increasing the firing rate of secretomotor neurons. These secretions from the crypts of Lieberkuhn in turn induce vasodilation of arterioles to increase blood flow in support to stimulated secretion. In case of IBS patients with predominant diarrhea subtype, there is increased serotonin production leading to increase in secretions. Thus, serotonin plays a major role in the pathophysiology of IBS-D type. Evidence in favour of this mechanism can be linked to the efficacy of the 5-HT3 receptor antagonist (Alosetron) blockade in women with diarrhea predominant IBS which suggests an overstimulation of secretomotor neurons by serotonin.[49,50]
It has also been investigated and found that in patients with constipation predominant IBS there occurs an increased serotonin concentration in colonic mucosa when compared with individuals with diarrhea predominant IBS, reflecting impaired release of serotonin.[51] This further strengthens the role of serotonin in IBS.
Brain – gut axis interactions in irritable bowel syndrome
“Brain-gut axis” refers to the bi-directional continuous communication between the gut (enteric nervous system [ENS], luminal wall) and the central nervous system (CNS), including the hypothalamic-pituitary-adrenal axis (HPA). It plays a prominent role in modulation of gut function in health and disease.
The emotional motor system (EMS) in the brain - a revised name for limbic system and some paralimbic structures (including the medial prefrontal cortex, amygdala, and hypothalamus) communicate emotional changes via the autonomic nervous system to the gut. The ENS, also called the “brain-in-the-gut,” organizes and coordinates activity of musculature, mucosal epithelium, and vasculature to generate functionally significant patterns of behavior.[52,53]
Pathophysiological consequences of disruption of brain-gut axis has been linked to many disorders of GI tract like gastroesophageal reflux disease, peptic ulcers, functional dyspepsia, IBS, inflammatory bowel disease, food allergy etc.[54,55]
In IBS, it is hypothesized that stress acts on the emotional limbic system and leads to increased release of adrenocorticotropic hormone and cortisol which act upon the ENS causing the symptoms of IBS that is, abdominal pain, loose stools etc. There is also a role of several immune mediators like IL-6, IL-8 etc.
Evidence in this favor are from the various neuroimaging studies (positron emission tomography, functional magnetic resonance imaging) on IBS patients which have suggested that there is a dysregulation of central and ENS which induces dysmotility or visceral sensitivity and all these processes are modified by psychosocial processes.[56,57]
However, still many of these mechanisms are yet to be understood and mechanisms related to bowel to be the only pathophysiology of IBS is still debatable.
Now let us discuss the various evidences and mechanisms which favor that brain and the psyche has a predominant role in the pathophysiology of IBS.
Evidences in Favor of “Irritable Brain and/or Mind”
For better understanding, let us conceptualize the bio-psycho-social model of IBS which includes
Biological factors: Neuroanatomical factors, neuroimmunological factors
Role of psyche
Social factors: Environmental influences and role of stress.
Biological factors
Neuroanatomical factors
As discussed above, the EMS is concerned with the modulation at CNS level. In IBS patients, it has been seen that there is selective activation of dorsal cingulate cortex which is concerned with attentional processes and response selection. Activation of dorsolateral prefrontal cortex concerned with emotional and autonomic response to stimuli has also been seen. IBS patients showed greater degree of activation in these regions than controls.[58]
Several studies have been carried out to unpin the details of brain pathways behind IBS, but still whether it is selective attention at cortical level or there is increased afferent processing of the pathways ascending to brain remains unclear.
Neurophysiological factors
CNS communicates with ENS via three pathways that is, autonomic pathway, neural pathway, and neuroendocrine pathway.
As far as autonomic pathway is concerned, data from various studies have not been consistent. Some studies have suggested that sympathetic activity may be increased or reduced[59] while some other studies have suggested diminished or enhanced parasympathetic tone.[60,61] Overall it has been observed that patients with IBS have altered autonomic activity associated with symptom exacerbation.
Neural pathways play a major role in modulating symptoms of IBS especially the experience of pain. Spinothalamic tracts provide information that is largely directed to the primary somatosensory cortex and functions to localize and discriminate visceral stimuli. Spinoreticular pathways do not function primarily to localize stimuli but are important in the reflexive, affective, and motivational aspects of sensation. The brain modulates afferent pain signals by dispersing inhibitory signals to the spinal cord. The inhibitory efferent signals then travel by way of the opioidergic, serotoninergic, and noradrenergic systems to the dorsal horn of the spinal cord where they presynaptically inhibit the afferent pain signals.
It was seen in studies that the dorsal subregion of the anterior cingulate cortex is an area that is selectively activated to a greater degree in patients with IBS compared with controls.[56,62] Evidence in this regard is seen in the effectiveness of tricyclic antidepressants in pain relief, at doses less than that used in depression.[63,64,65] Brain imaging studies have shown compromised activation of corticopontine pain inhibitory circuits. Patients with IBS may fail to use CNS downregulating mechanisms in response to incoming or anticipated visceral pain.[66] It seems that differences exist between patients with IBS and healthy subjects with respect to regional brain activation. But, debate still continues whether it is the central activation or the peripheral pathways responsible for pathogenesis.
If we consider the neuroendocrine pathways abnormalities, alteration in the HPA has been reported in IBS patients. Studies have reported an increased level of corticotropin releasing hormone which in turn affects the motility and sensitivity of the gut.[67] However, it is unsure whether the change is primary or in response to other stressors. Studies have reported that increased levels of cortisol in IBS patients compared to controls, either at baseline or in response to stress.[68]
Role of the psyche
There is upcoming evidence between the relationship of IBS and “the psyche.” Psychiatric disorders have been found to coexist with IBS patients as evident from various studies. Human psyche is affected by several factors like personality factors, altered health beliefs, and coping skills.
Psychiatric disorders and irritable bowel syndrome
Several studies show that among patients who seek medical attention for IBS, around 70% have psychiatric comorbidity.[69,70,71] Rates of psychiatric disorders are depression - 46%, generalized anxiety disorder - 34%, panic disorder - 31%, and somatization - 26%.[72] Psychiatric disturbances among persons with IBS may represent a reaction to stress of chronic GI illness.[73,74] Severity of anxiety and depressive symptoms correlated with IBS symptoms.[75] Patients with co-morbid anxiety had more pronounced cortical evoked potential indicating selective attention compared with noncomorbid IBS.[76] Also it has been reported that the prevalence and role of psychiatric comorbidity in individuals with IBS is higher than in nonulcer dyspepsia.[77]
It has also been seen that psychiatric disorders and IBS share common genetic predisposition. For example, SERT-P gene is associated with a subtype of IBS and the same polymorphism in promoter region of SERT gene is also seen in depression.[78,79] Another study has reported that C/C genotype polymorphism in 5HT3A receptors is associated with increased anxiety and severity of IBS symptoms.[79,80] A positive association between psychological stress and abnormal immunity has been implicated as evident from a study in which it was seen that IBS patients with high level of IL-6 was associated with the presence of psychological co-morbidity.[81]
In summary, still there is little data to support the etiological role for psychiatric disturbance in IBS. However, it has significant impact on severity as well as outcome. Treatment of psychiatric disturbances can result in improvement of symptoms. Hence, it is important to recognize and treat them.
Personality factors
Majority of studies have supported that IBS patients have high level of neuroticism.[82] Neuroticism might influence coping strategies like catastrophizing and somatization.[83] Neuroticism is also a significant predictor of illness perception and treatment beliefs in IBS.[84] Several studies have found that many IBS sufferers score high in the personality trait alexithymia[85,86] which is associated with increased symptom severity in IBS. However, these personality traits are not unique to IBS, but it reflects an important intervening variable.
Health beliefs and coping skills
Patients having IBS often believe that their chronic gut symptoms indicate a serious illness. They report a sense of losing freedom, spontaneity, social contacts and feeling of fearfulness, shame and embarrassment.[87] These in turn lead to change in their behavior like avoidance of activities and adaptations in routine in an effort to gain control.[88]
Patients with IBS have high scores on bodily preoccupation, hypochondriacal beliefs and disease phobia.[89] They are more likely to catastrophize than patients with organic disorder. It has been noticed that patients with IBS with a high degree of catastrophizing, have a tendency to report more severe pain.[90]
It is difficult to draw conclusions in generalizing coping skills in patients with IBS. Health beliefs are irrational leading to hypochondriacal attitudes and various maladaptive coping strategies such as catastrophizing are used.
Role of social factors
Role of social learning
Social learning has been implicated as one of the important social factors in the biopsychosocial model of IBS. Many patients with moderate to severe IBS have a coexisting somatization disorder, and it is quite likely that excessive illness behavior could be transmitted from parents to children in an intergenerational transmission of illness behavior.[91] Parental modeling and reinforcement of illness behavior contributes to the causes of illness. It has been suggested that contribution of social learning is as great as the contribution of heredity.
Environmental stressors
Environmental stressors have been classified as early life stressors and psychosocial stressors. Some of these factors implicated in IBS are as described below:
Early life stressors like prenatal traumatic events (poor nutrition in fetal life), early stressful life events (surgery, emotional, physical or sexual abuse), family dysfunctioning (divorce of parents), family history of abdominal pain, bowel dysfunction, and inflammatory bowel diseases.[87]
Psychosocial stressors like major life events (divorce, unemployment, death of a relative), major social events (social changes, revolution), and daily hassles.
Abuse history either in childhood or during adult life needs a special mention in this aspect. Abuse has been reported by patients with other chronic and painful non-GI functional conditions as well.[92] But the constant finding is that abused individuals experience high level of psychological distress and somatization, both being risk factors for development of IBS.[93] Two recent studies have concluded that lifetime history of broad range of trauma and abuse are independent risk factors for development of IBS.[94,95] The findings suggest that physicians should become aware of various risk factors among patients with chronic or severe refractory symptoms and some sort of psychological treatment is necessary for such patients.
Role of stress
Stress is defined as acute threat to the homeostasis of an organism, real (physical) or perceived (psychological), and posed by events in the outside world or from within.[96] In the genetically predisposed individual, both early life stress and severe life threatening stress referred to as pathological stress can result in permanent irreversible enhancement of responsiveness of central stress circuits and vulnerability to development of functional disorders later in life. Later on, fear conditioning plays an important role in triggering stress responses to situations and context.
In IBS patients, it has been reported from many studies that stress is associated with symptom severity.[97] Nearly 51% of IBS patients report that a stressful event preceded the onset of the disorder.[98] Patients with IBS have greater reactivity to stress. The identification of a specific stressor helps in planning treatment through psychological and pharmacological treatment.
A recent study which aimed to investigate the effect of stress on intestinal permeability in humans revealed that when the subjects were subjected to acute psychological stress there was increase in small intestinal permeability.[99] Also when the link between posttraumatic stress disorder and IBS was investigated in an urban African Americans population, the former was found to be independently associated with IBS, thereby further strengthening the role of psychosocial stress in IBS.[100]
Acute psychological stress, as well as administration of corticoliberin (also known as corticotrophin releasing factor) to mimic the stress response, have both been found to increase intestinal mucosal permeability in healthy volunteers.[99] It is also well known that stress affects the immune system.[54] A number of studies have supported that the increase in intestinal permeability in patients with IBS is linked to enhanced activity of the immune system via release of proinflammatory cytokines such as tumor necrosis factor and interferon-gamma.[101,102]
Some landmark studies that have evaluated the role of stress in IBS are described in Table 2.
Table 2
Studies evaluating the role of stress in IBS
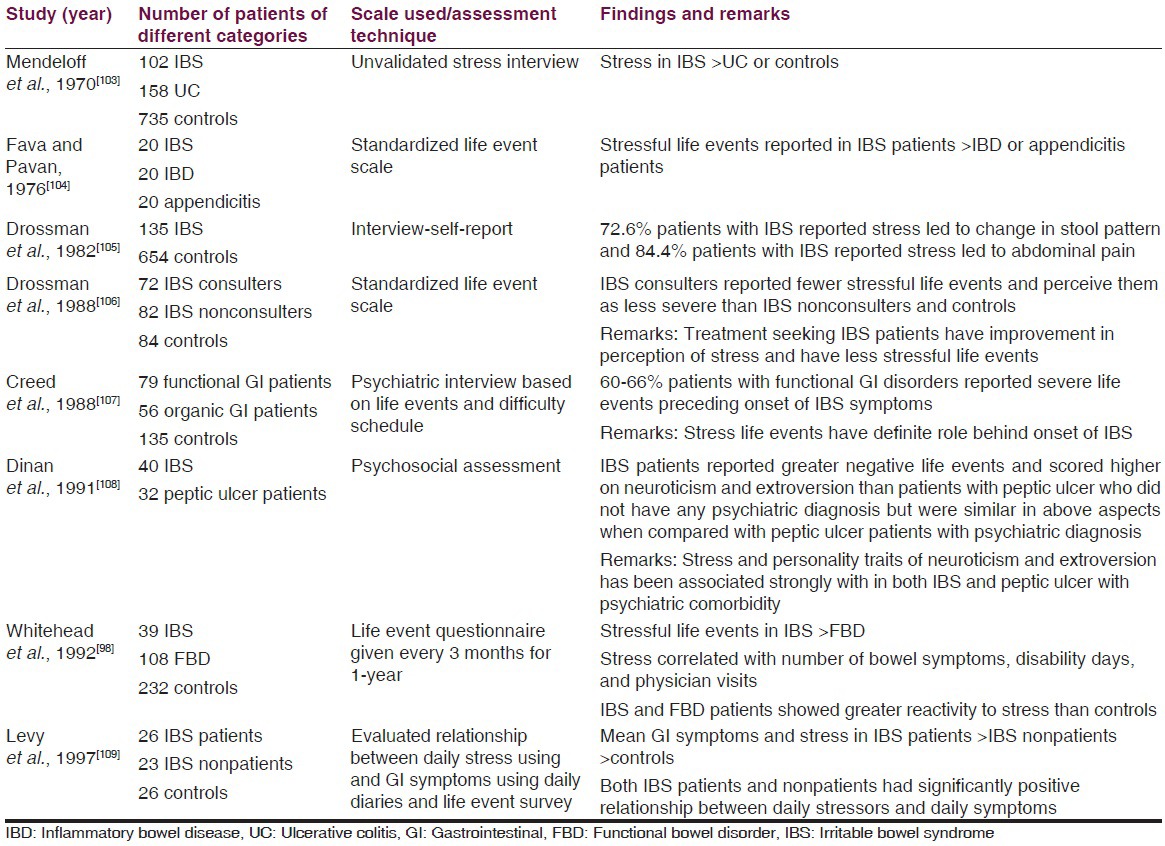
Summarizing all the psychosocial factors, we can say that direct cause and effect relationship has not been found. Various psychological factors have an effect on outcome as well as they are associated with each other. Each one of the factor plays an important role as far as management is concerned and all these should be kept in the management protocol while managing an IBS patient.
Lastly, we summarize, the etiopathogenesis in IBS in Table 3.
Table 3
Summary of etiopathogenesis of IBS
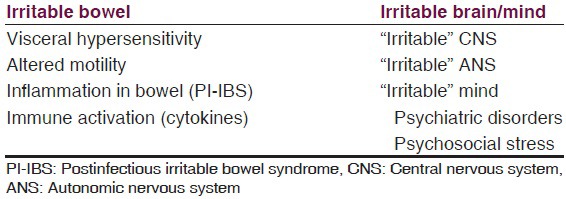
Mostly the evidence has been found on these above mentioned aspects/areas of research. There have always been controversies in almost all the theories put forward to explain IBS. We can rather say that it is the “irritable” brain-gut axis which plays a significant role in the genesis of IBS.
Conclusions and Future Directions
Our understanding of the pathophysiology of IBS is still in its evolution level. Psychosocial factors contribute to predisposition, precipitation, and perpetuation of IBS symptoms. Neuroimaging studies have tried to delineate the relationship between psychosocial factors and emotions, gut physiology and clinical symptoms.
Screening subjects for psychosocial stressors may be an effective intervention strategy. The potential role that inflammation may play in IBS, genes involved in cytokine production and/or receptors is an important avenue for future research.
Evidence is growing that IBS can no longer be purely regarded as a functional disorder. Neither of the notions, about IBS that, “It's all in the head” or “it's all in the mind”, is right, in its entirety. Rather, psychiatrists and physicians/gastroenterologists should work in close liaison and manage it comprehensively. Essentially, it is the person as well as the GI symptoms to be taken care of, along with attempts to improve the quality of life as well. Therefore, perhaps, it is the combination of irritable “brain” and “bowel” is what constitutes the disease entity.109
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
Articles from Journal of Neurosciences in Rural Practice are provided here courtesy of Scientific Scholar
Full text links
Read article at publisher's site: https://doi.org/10.4103/0976-3147.169802
Read article for free, from open access legal sources, via Unpaywall:
http://www.thieme-connect.de/products/ejournals/pdf/10.4103/0976-3147.169802.pdf
Citations & impact
Impact metrics
Article citations
Causative Factors, Clinical Manifestations, and Therapeutic Strategies for Irritable Bowel Syndrome.
Cureus, 16(4):e58728, 22 Apr 2024
Cited by: 2 articles | PMID: 38779277 | PMCID: PMC11110641
Review Free full text in Europe PMC
Restraint stress-associated gastrointestinal injury and implications from the Evans blue-fed restraint stress mouse model.
Tzu Chi Med J, 36(1):23-29, 07 Sep 2023
Cited by: 4 articles | PMID: 38406572 | PMCID: PMC10887336
Review Free full text in Europe PMC
Somatization in patients with predominant diarrhoea irritable bowel syndrome: the role of the intestinal barrier function and integrity.
BMC Gastroenterol, 21(1):235, 22 May 2021
Cited by: 3 articles | PMID: 34022802 | PMCID: PMC8141183
FODMAP Fingerprinting of Bakery Products and Sourdoughs: Quantitative Assessment and Content Reduction through Fermentation.
Foods, 10(4):894, 19 Apr 2021
Cited by: 0 articles | PMID: 33921672 | PMCID: PMC8074121
How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome.
Cureus, 12(8):e9871, 19 Aug 2020
Cited by: 16 articles | PMID: 32968548 | PMCID: PMC7505258
Review Free full text in Europe PMC
Go to all (18) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recent advances in pharmacological treatment of irritable bowel syndrome.
World J Gastroenterol, 20(27):8867-8885, 01 Jul 2014
Cited by: 14 articles | PMID: 25083060 | PMCID: PMC4112893
Review Free full text in Europe PMC
Are bowel symptoms and psychosocial features different in irritable bowel syndrome patients with abdominal discomfort compared to abdominal pain?
World J Gastroenterol, 28(33):4861-4874, 01 Sep 2022
Cited by: 4 articles | PMID: 36156921 | PMCID: PMC9476853
Irritable Bowel Syndrome: Is it Rare in Children?
Mymensingh Med J, 27(1):216-221, 01 Jan 2018
Cited by: 1 article | PMID: 29459618
The overlap of upper functional gastrointestinal disorders with irritable bowel syndrome in Chinese outpatients: A multicenter study.
J Gastroenterol Hepatol, 31(9):1584-1593, 01 Sep 2016
Cited by: 14 articles | PMID: 26875585





